全文HTML
--> --> --> 水体重金属污染是严重威胁生态、环境和人类健康的全球性问题之一[1]。镉是?类致癌物,具有生物积蓄性大、迁移能力强、半衰期长等特点[2]。其主要来源于人为活动(如采矿、电镀、颜料、电池等行业和冶炼排放的废渣、废气等),其排放造成水污染,严重影响陆地生态系统[3-4]。因此,迫切需要安全高效去除水体中的镉。吸附法因其简单易操作被认为是去除水中镉污染的有效方法之一。其中,生物炭作为吸附剂具有廉价易得、物理化学性质稳定、官能团较多等特点,常被应用在重金属污染土壤和水体修复中[5-6]。但原始生物炭的分散性差、吸附能力有限,需通过活化或负载等手段提高吸附能力[7]。活化的制备工艺相对简单,可分为物理活化和化学活化。物理活化一般以CO2、H2O为活化剂,其操作简单,但活化后孔道分布不均,且能耗高。化学活化以ZnCl2[8]、KOH[9-10]、H3PO4[11]等为常用活化剂,其能耗低,但有些活化剂(如ZnCl2)会产生有毒气体。采用KOH活化可以有效改善炭材料孔道结构和比表面积,并且能够形成碳的官能团[12-13],不易产生二次污染。2步KOH活化法是利用已经炭化的前驱体与KOH混合均匀后共同热解制备得到的炭材料,其相对于1步活化法的优势在于能更好改善炭材料孔隙结构。目前大部分生物炭主要由小麦、玉米、水稻等秸秆制备而成,关于蚕沙生物炭吸附去除水体中镉的研究相对较少。2步KOH(浸渍-热解)活化法中每步对于炭材料的理化性质的影响及吸附贡献率也鲜见报道。
蚕沙是农业废弃物,其来源广泛,富含氨基酸、粗蛋白质和叶绿素等有机化合物,因此,在炭化后其可能含有较多活性基团。本研究以蚕沙生物炭为原料,以KOH为活化剂制备蚕沙基生物炭,分步探究了浸渍-热解活化法对炭材料理化性质的影响,并通过一系列的单因素实验考察了其对重金属Cd2+的吸附性能,以期为利用蚕沙制备高吸附性能生物炭提供参考。
1.1. 仪器与试剂
蚕沙来自于广西农业技术推广所(5龄蚕沙),氢氧化钾(KOH)、硝酸镉(Cd(NO3)2·4H2O)、硝酸钙(Ca(NO3)2·4H2O)、硝酸镁(Mg(NO3)2·6H2O)、氯化钾(KCl)、氯化钠(NaCl)等试剂均为分析纯;实验用水为超纯水。主要仪器包括管式炉(KGF1600-80,河南酷斯特仪器科技有限公司)、原子吸收分光光度计(AA-7000,日本岛津SHIMADZU株式会社)、水浴恒温振荡器(SHA-BA,常州国华仪器有限公司)。1.2. 生物炭的制备与表征
将蚕沙中的杂质挑除干净,烘干后用粉碎机粉碎,过60目筛,放置于刚玉舟中压实并密封,通入N2,于真空管式炉中缓慢升温(5 ℃·min?1)至500 oC,保温2 h,用去离子水洗涤至中性,标记为BC。待冷却后取出样品,并将生物炭进行研磨、过100目筛,放入干燥器中备用。1)浸渍活化炭材料的制备。BC和KOH按照质量比1∶1的比例置于烧杯中混合搅拌5 h,用超纯水洗至中性,于105 ℃下烘干12 h,将得到的生物炭进行研磨、过100目筛,储存在干燥器中。标记为KBC。
2)浸渍-热解活化炭材料的制备。浸渍步骤同上,于105 ℃下烘干12 h,再置于25 mL的刚玉舟中压实,密封,在N2氛围下于真空管式炉中升温至400 oC,保温1 h。热解完成后用水洗至中性,于105 ℃下烘干12 h。将得到的生物炭进行研磨、过100目筛,储存在干燥器中。标记为KBC400。
采用Sigma300型场发射扫描电镜SEM-EDS(卡尔蔡司公司,德国)分析样品的表面形貌。采用ASAP 2460型多站全自动比表面积分析仪BET(麦克默瑞提克公司,美国)分析样品的比表面积。采用inVia Reflex型激光拉曼光谱仪(雷绍尼公司,英国)分析样品晶格振动。采用IRTracer-100型傅里叶变换红外光谱仪(岛津公司,日本)对样品进行表面官能团分析。采用NanoBrook Omni型多角度粒度及高灵敏Zeta电位分析仪(布鲁克海文公司,美国)对样品所带电负性分析。
1.3. 吸附实验
本实验所用溶液均使用去离子水配制,用硝酸镉(Cd(NO3)2·4H2O)配制1 g·L?1的Cd2+标准储备液,以0.01 mol·L?1 NaNO3作为溶液背景电解质。后续实验所需Cd2+溶液通过稀释储备液制得。每组实验设置3个重复和1个空白对照。蚕沙基生物炭投加量对溶液Cd2+吸附的影响。取50 mL质量浓度为30 mg·L?1 Cd2+溶液置于100 mL离心管中,调节pH为5.0,分别称取BC、KBC、KBC400样品0.01、0.015、0.02、0.025、0.03、0.04、0.05 g加入其中,在25 ℃水浴中,以200 r·min?1振荡24 h后取样,使用0.45 μm聚醚砜针筒滤器过滤,采用原子吸收分光光度计(AAS)测定滤液中Cd2+的浓度。
不同pH对Cd2+吸附效果影响。取50 mL质量浓度为30 mg·L?1 Cd2+溶液置于100 mL离心管中,用0.1 mol·L?1 NaOH或HCl调节pH为2.0、3.0、4.0、5.0、6.0,称取BC、KBC、KBC400样品0.02 g加入其中,在25 ℃、200 r·min?1下振荡24 h,测定滤液中Cd2+的浓度。
共存离子对Cd2+吸附效果影响。取50 mL浓度为0.1 mmol·L?1 Cd2+溶液置于100 mL离心管中,分别配制0.1 mmol·L?1的K+、Na+、Ca2+、Mg2+溶液作为背景液,pH为5.0,加入BC、KBC、KBC400样品0.02 g,在25 ℃、200 r·min?1下振荡24 h,测定滤液中Cd2+的浓度。
吸附平衡实验。1)动力学实验:取50 mL质量浓度为30 mg·L?1 Cd2+溶液置于100 mL离心管中,调节pH为5.0,称取BC、KBC、KBC400样品0.02 g加入其中,在预定的反应时间(5、10、30、60、120、360、720、1 440、2 880 min)取样,测定滤液中Cd2+的浓度。2)吸附等温线:分别称取BC、KBC、KBC400样品0.02 g,设置一系列Cd2+溶液质量浓度梯度(5、10、20、30、40、60、80、100 mg·L?1),调节pH为5.0,振荡24 h取样,测定滤液中Cd2+的浓度。
1.4. 分析方法
蚕沙基生物炭的吸附量(qe)和去除率(η)通过式(1)和式(2)计算。式中:C0为镉溶液的初始质量浓度,mg·L?1;Ce为镉溶液吸附平衡时的质量浓度,mg·L?1;V是镉溶液的体积,L;m是吸附剂的质量,g。
吸附动力学通过拟合准一级动力学、准二级动力学和Weber-Morris颗粒内扩散模型来分析镉离子的吸附过程。计算模型如式(3)~式(5)所示。
式中:qt和qe分别为t时刻和吸附平衡时的吸附量,mg·g?1;k1和k2表示准一级和准二级动力学的速率常数,min?1和g·(mg·min)?1;Kdi是粒子内扩散的速率常数,g·(min·mg1/2)?1;Ci是与边界层厚度相关常数,mg·g?1。
吸附等温线通过拟合Langmuir模型和Freundlich模型分析镉离子的吸附机理,具体如式(6)和式(7)所示。
式中:qm是最大吸附量,mg·g?1;Ce是吸附平衡时镉溶液质量浓度,mg·L?1;KL是Langmuir等温吸附常数,L·mg?1;KF是Freundlich吸附常数;n是与Freundlich等温模型有关的吸附剂表面作用强度;qe是平衡吸附量,mg·g?1。
活化步骤贡献率根据式(8)和式(9)进行计算。
式中:QKBC是KBC吸附Cd2+的吸附量,mg·g?1;QBC是BC吸附Cd2+的吸附量,mg·g?1;QI是浸渍活化处理的吸附量,mg·g?1;QP是热解活化处理的吸附量,mg·g?1。
2.1. 生物炭的基本性质
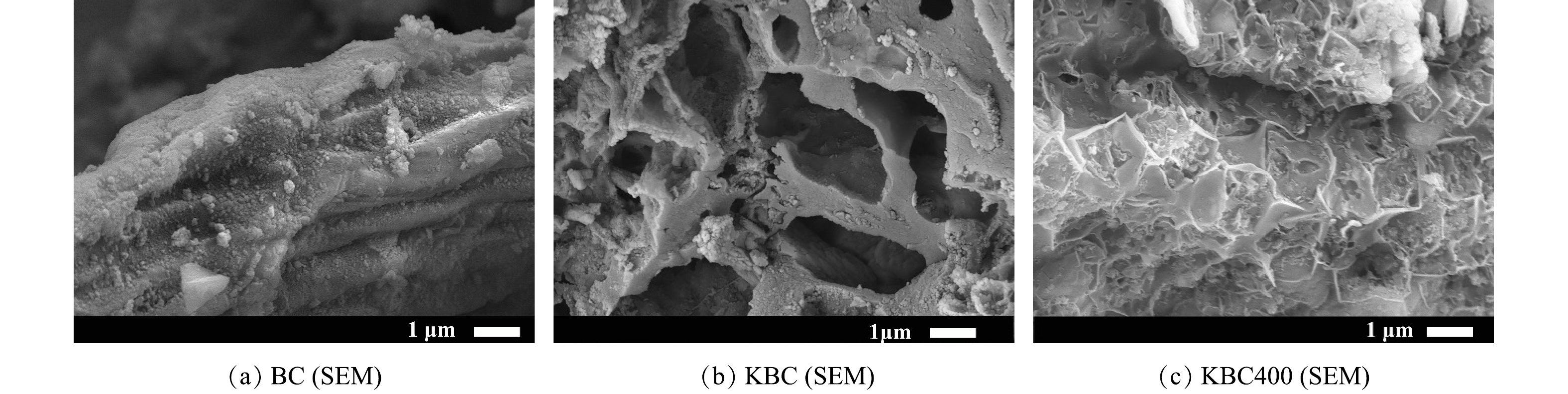
1)表面形貌(SEM)与比表面积(BET)分析。活化前后生物炭的微观表面形貌如图1所示。未经KOH活化的BC具有粗糙、致密的表面特征,且观察不到明显的孔结构(图1(a)),说明仅500 ℃碳化对生物炭的造孔能力有限。经过KOH浸渍的KBC表面变得孔隙结构丰富起来,主要以中孔为主,并具有较为清晰的纤维状结构(图1(b))。这是由于浸渍KOH可以去除一部分灰分以及促进孔结构膨胀,说明KOH在孔结构的形成中起着重要作用[14-15]。加入活化剂KOH浸渍并再次热解后,KBC400的表面出现了许多不均匀的凹陷(图1(c))。这可能是KOH在400 ℃下与碳原子反应并转化为易溶于水的K2CO3,形成大量孔隙[16-17]。其具体反应过程如式(10)~式(13)所示[18]。在浸渍-热解活化过程中,浸渍时KOH进入生物炭内部,K+起到支撑作用,随后K+在孔隙中刻蚀掉不稳定的C、N、H等原子,不稳定的C原子会变成CO、CO2等气体挥发掉。但当热解温度较低(≤600 ℃)时,生物炭内部的K+没有释放出来,导致不能形成致密的孔结构。
活化后的生物炭比表面积和孔隙结构如表1和图2所示。KBC400的比表面积比KBC增加了1倍,孔容和微孔面积也有所增加。经过KOH活化的炭材料平均孔径位10~14 nm,主要以中孔为主。从孔径分布(图2(b))可以发现,浸渍-热解活化生物炭中一部分中孔是由微孔裂变而来的。这些丰富的中孔结构有利于吸附质的扩散。
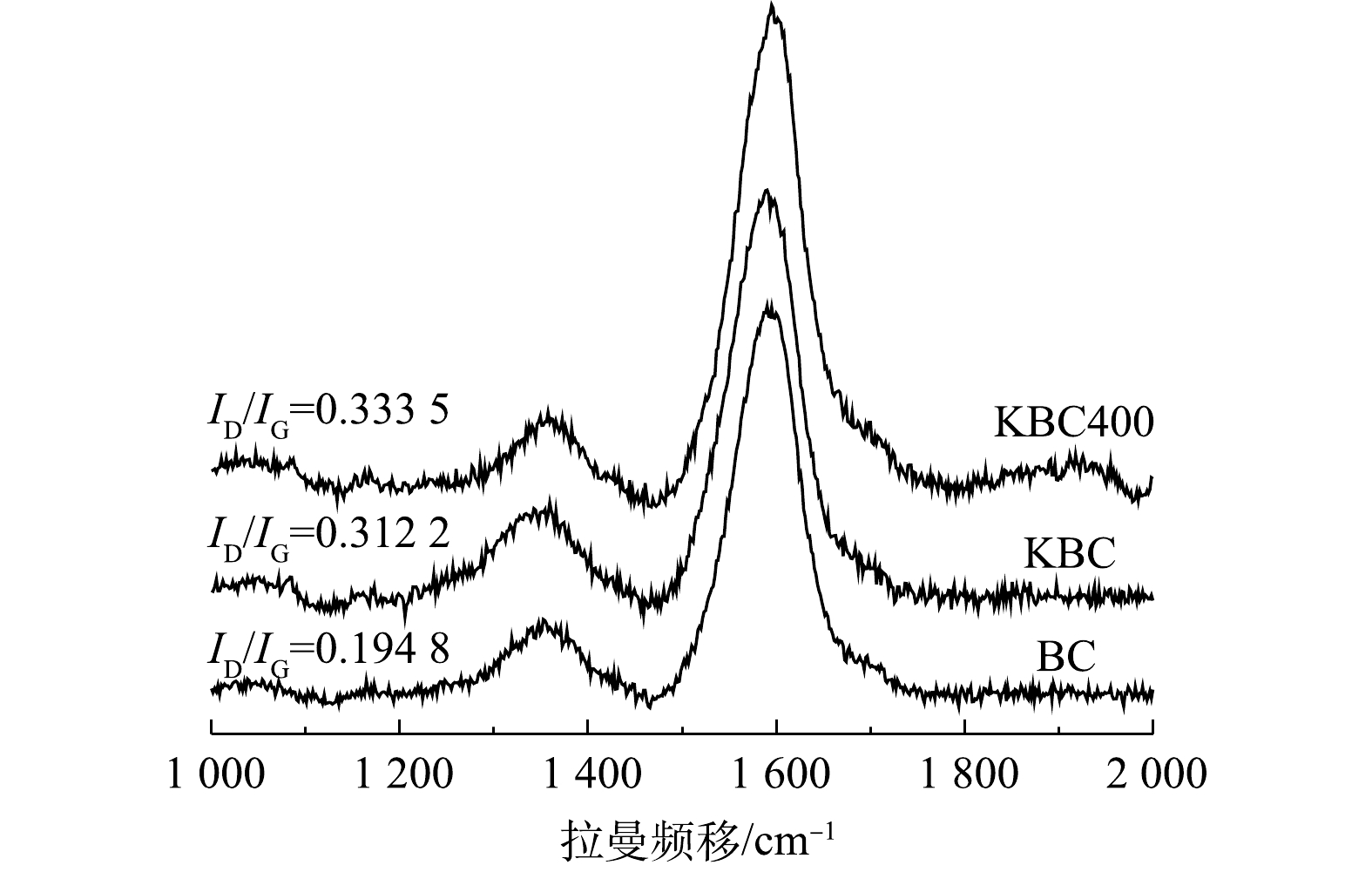
2)拉曼(Raman)分析。图3为活化前后生物炭的拉曼光谱图。可见,在1 359 cm?1和1 593 cm?1有2个明显的吸收峰,分别代表D峰和G峰。D峰表示炭材料的面内缺陷结构(SP3杂化碳原子)[19],与无序化程度有关;G峰表示炭材料的类石墨微晶的面内振动以及稠芳环结构(芳香结构骨架中SP2碳原子)[20],与内部结晶度和对称性相关(石墨化程度)。D峰和G峰的强度表明,蚕沙基生物炭是包括SP2和SP3杂化的不同碳结构的混合体,且G峰强度较高,表明其内部的结晶度和对称性较好,且表明其可以形成π电子供体,有利于形成重金属Cd2+-π键[21](图3)。基于D峰和G峰的物理意义,可以用R来表示炭材料的无序化度,即R=ID/IG[22-23]。对比R值可以得出生物炭的无序化程度,即BC<KBC<KBC400,表明KOH活化可以增加材料的无序化程度,且浸渍-热解方式更能增加无序化程度,这可能是由比表面积和孔体积增大导致的,有利于提高Cd2+的吸附量[24]。
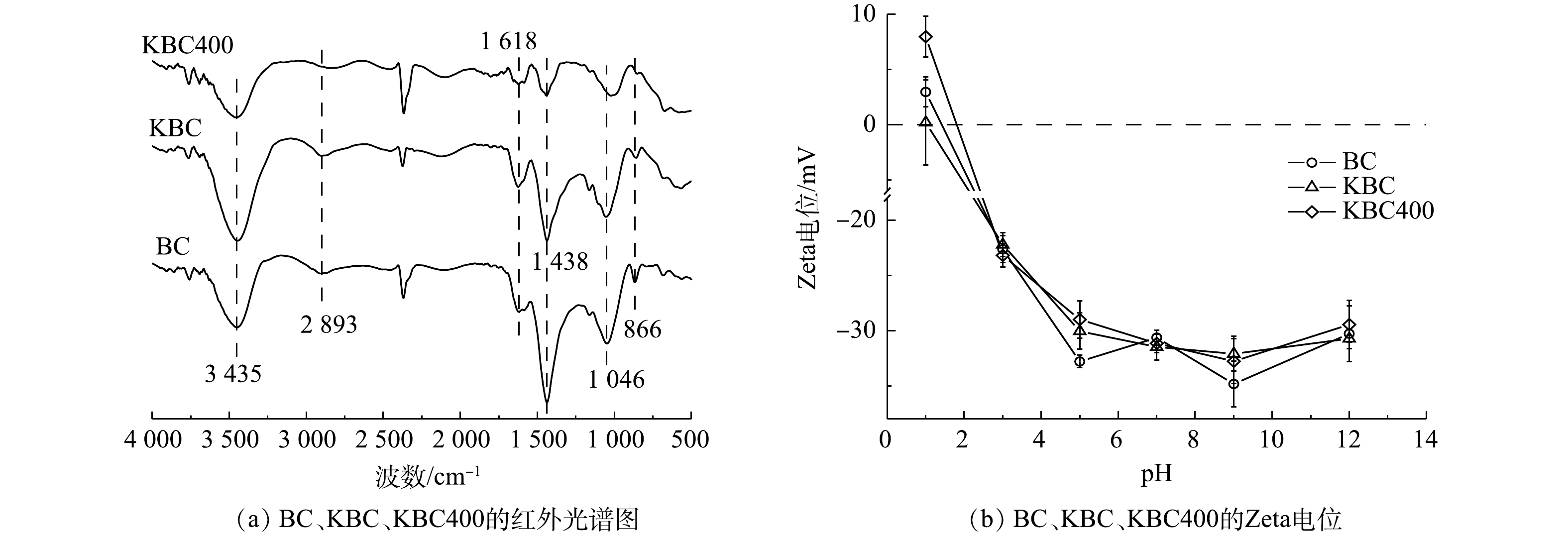
3)傅里叶红外光谱(FT-IR)分析。炭材料在500~4 000 cm?1内的傅里叶红外光谱图如图4(a)所示。3 452 cm?1附近出现的特征峰对应于醇类、酚类或者水的分子间的O—H的伸缩振动[25]。在此区域由生物炭的O—H伸缩振动引起的吸收峰强度为KBC>BC>KBC400。这说明在KOH浸渍的过程中,氢氧根会结合到生物炭表面形成羟基,但随着裂解温度的升高,O—H峰的振动强度逐渐减弱[26]。2 893 cm?1处出现的峰为脂肪族的(甲基(—CH3)或者次甲基(—CH))C—H键伸缩振动引起的[13];1 046、1 438和1 618 cm?1附近出现的峰分别表示C—O—C的伸缩振动、—O—CH3(甲氧基)和芳香族的C=C骨架振动或C=O键分子面的伸缩振动,是原料木质素最典型的红外特征带[27]。此区域生物炭的吸收峰强弱为KBC>BC>KBC400。其中KBC400在1 046 cm?1处的峰有所偏移,说明KOH能够影响碳骨架;1 618 cm?1处的峰强度有所减弱,可能是KOH在高温下的脱水作用[28-30]。866 cm?1处出现的峰为芳香族的C—H键面外弯曲振动引起的[31]。综上所述,可能发生如式(14)所示的反应[32]。
由此可见,KBC和KBC400主要含有羟基、羧基等含氧官能团,能与Cd2+结合,有利于吸附的进行。
4) Zeta电位分析。Zeta电位分析结果表明(图4(b)),蚕沙基生物炭的等电点(PZC)均在1~1.5,且Zeta电位均随着pH的升高而降低。这是由于生物炭表面的含氧官能团(羧基、羟基等)在不同H+浓度的溶液中发生的电离作用引起的[33]。BC与2种活化生物炭对比显示Zeta电位变化不大,说明不同的KOH活化方式对生物炭表面的电负性影响较小[34]。
2.2. 投加量对吸附效果的影响
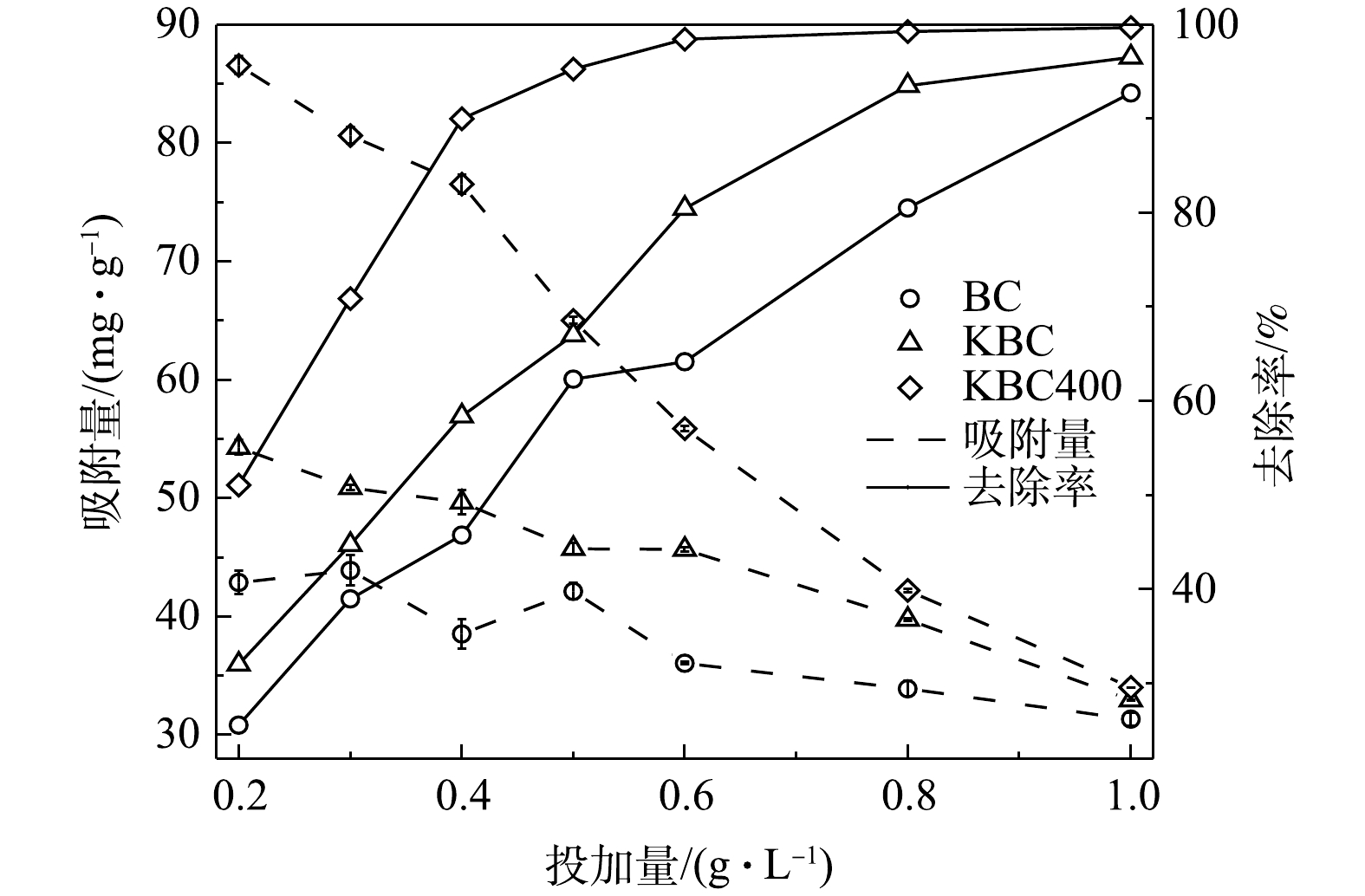
活化前后生物炭的投加量对吸附Cd2+的影响(图5)。在本研究条件下,BC、KBC和KBC400对Cd2+的最大去除率分别为92.73%、96.54%和99.68%,可见,在同等条件下KBC400去除率最高。其中,KBC400投加量由0.2 g·L?1增加至0.4 g·L?1,对Cd2+的去除率增加显著,去除率由51.06%增加至90.00%;之后再增大投加量,去除率变化不大。KBC投加量由0.2 g·L?1增加至0.8 g·L?1时,Cd2+的去除率从由32.02%增加至93.50%。BC在投加量由0.2 g·L?1增加至1 g·L?1时,Cd2+的去除率仅由25.54%增加至92.73%。各材料的吸附量随着投加量的增加而减少。在投加量为0.4 g·L?1时,BC、KBC、KBC400的吸附量分别为38.53、49.65、76.53 mg·g?1,而投加量超过0.4 g·L?1,其吸附量分别降低至31.33、32.94、34.01 mg·g?1。这是因为生物炭投加量增加时,生物炭表面的吸附位点和Cd2+的去除率均呈增加趋势,由于溶液中的Cd2+数量有限,其表面的吸附位点未达到饱和,所以对Cd2+的吸附量降低。考虑到去除效果、经济和一致性原则,在后续研究中选择0.4 g·L?1作为的生物炭最优投加量。2.3. 溶液pH对吸附效果的影响
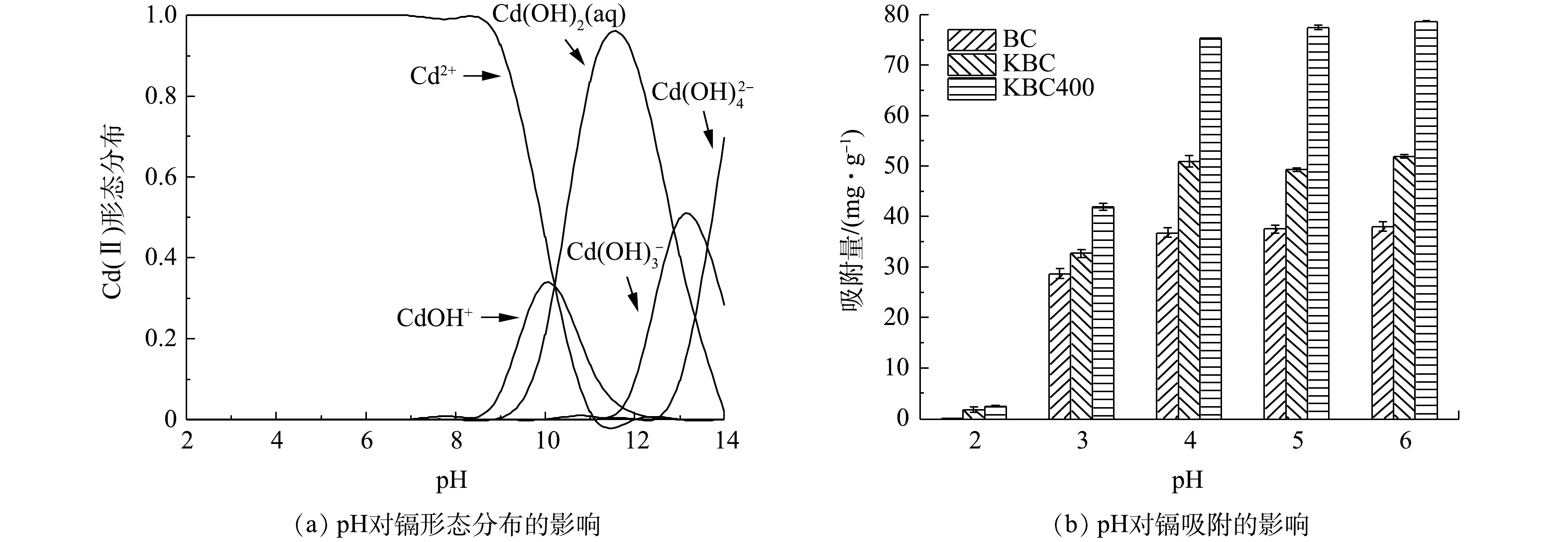
初始pH对重金属的存在形态和吸附剂表面的电荷情况来说意义重大[35],会直接影响吸附剂对水中重金属的吸附。由Visual MINTEQ 3.1计算得到纯水中Cd2+在不同pH下的水解情况(有效质量浓度为30 mg·L?1)如图6(a)所示。Cd(Ⅱ)在水中主要以Cd2+、Cd(OH)+、Cd(OH)2(aq)、Cd(OH)3?和Cd(OH)42?4种形态存在。在pH<8.0时,镉在水中的存在形态以Cd2+为主;在pH为8.0~10.0时,有部分少量的水解产物(CdOH+)和沉淀Cd(OH)2;在pH为10.0~14.0时,Cd2+形成的水解产物组成比较复杂,但主要以沉淀形式存在。因此,在pH<8.0的环境中,水中Cd2+的去除几乎均由生物炭的吸附作用完成,而不是水解形成的难溶产物的沉淀。由图6(b)可以观察到,BC、KBC、KBC400对Cd2+去除率和吸附容量随着pH的增加呈现先迅速增大后趋于平缓。由于BC所含的碱性矿物较多,会显著提高溶液pH,若pH≥8.0,Cd2+有聚沉的趋势,故只考察吸附剂pH在2.0~6.0内对Cd2+吸附的影响。在pH=3.0时,BC、KBC和KBC400对Cd2+的吸附量提高最显著,分别由低于3 mg·g?1提高至28.64、33.20和41.90 mg·g?1。在弱酸性条件下(pH=4.0~6.0),与BC相比,KBC和KBC400对Cd2+的吸附容量由33.20 mg·g?1和41.90 mg·g?1提高至51.90 mg·g?1和78.76 mg·g?1,分别提高了56.33%和87.97%。综合上述结果可知,浸渍-热解活化制备的炭材料(KBC400)能适用于较广的pH范围,且吸附量也显著高于浸渍活化制备的生物炭。
蚕沙基生物炭表面都含有丰富的—OH等官能团,电离后使其表面带有负电荷,Cd2+在生物炭表面发生吸附主要是静电引力的作用,与其本身的电负性密不可分。当pH较低时,溶液中存在大量H+、H3O+离子会占据生物炭表面的吸附位点,使得Cd2+的吸附受到抑制。同时,生物炭表面的—OH也会与溶剂中的H+发生质子化反应[36],抑制炭材料表面的—COOH等官能团解离,从而阻碍Cd2+与官能团结合。此外,若溶剂中存在大量的质子,则会导致炭材料表面带正电荷,使带正电荷的Cd2+难以与之结合。随着pH的增加,生物炭表面官能团(—COOH、—OH)等开始去质子化,所带的负电荷数量的增加能提高与Cd2+间的静电引力,利于发生离子交换作用,所以Cd2+的去除率逐渐增加[37]。
2.4. 共存离子对吸附的影响
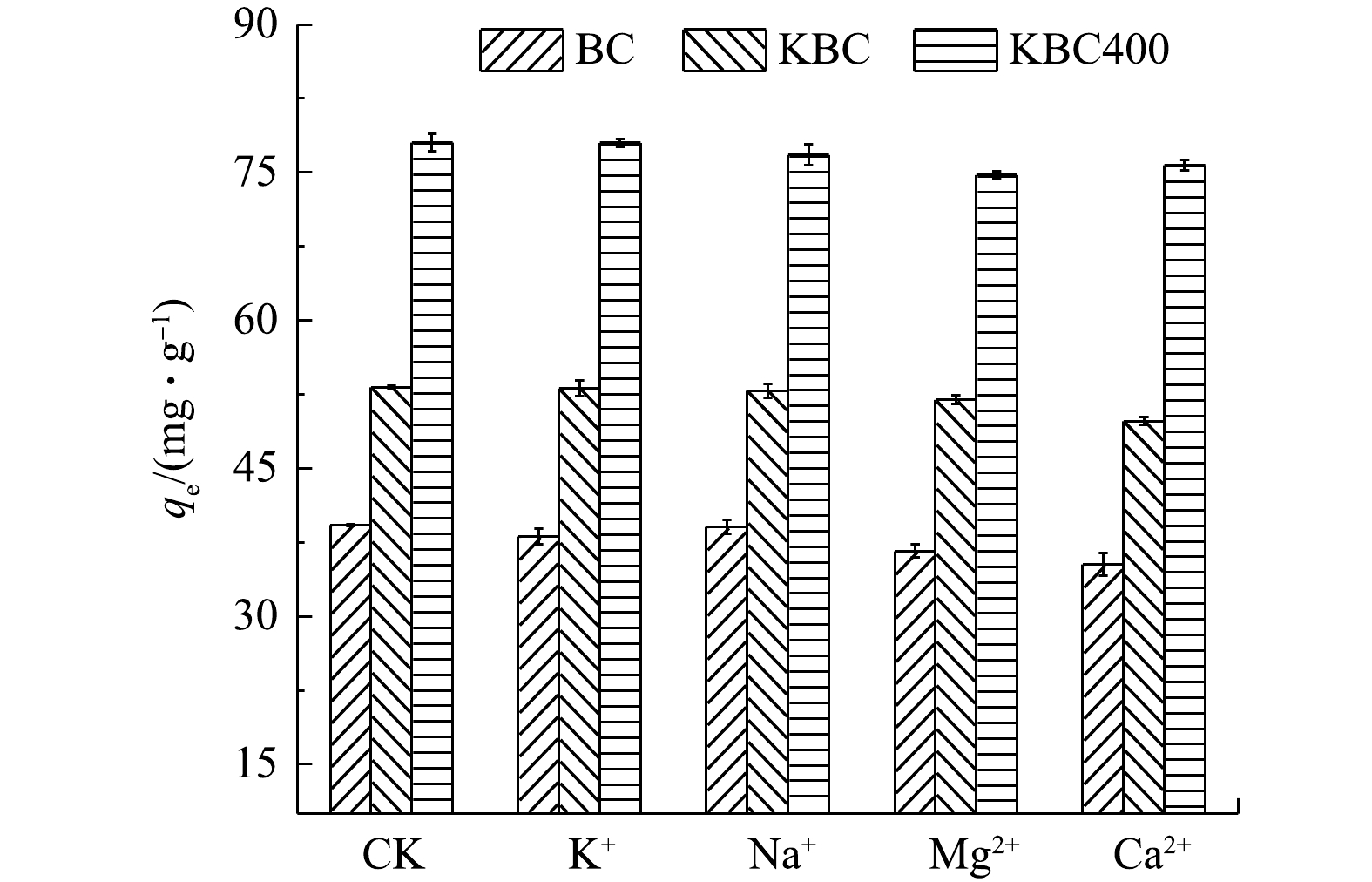
除了溶液pH外,吸附介质中的共存离子也会影响吸附剂的吸附效果,因此,本实验探讨了溶液中存在不同的碱金属离子(K+、Na+、Mg2+和Ca2+)对吸附效果的影响(图7)。其中,CK为未添加碱金属离子时BC、KBC和KBC400对Cd2+的吸附情况。对吸附影响较小的是Na+、K+;对吸附影响影响较大的是Mg2+,BC、KBC和KBC400对Cd2+的吸附量分别下降了25.16%、10.96%、3.08%。同时,Ca2+也会抑制BC、KBC和KBC400对Cd2+的吸附,吸附量分别下降了30.42%、12.29%和2.24%。出现这种现象可能是由于Mg2+、Ca2+较弱的共价性质,导致其水化程度较低,从而增强它们与吸附剂表面的相互作用[38-39]。以上结果表明,KBC400具有较好的抗离子干扰能力,其次是KBC。2.5. 吸附动力学
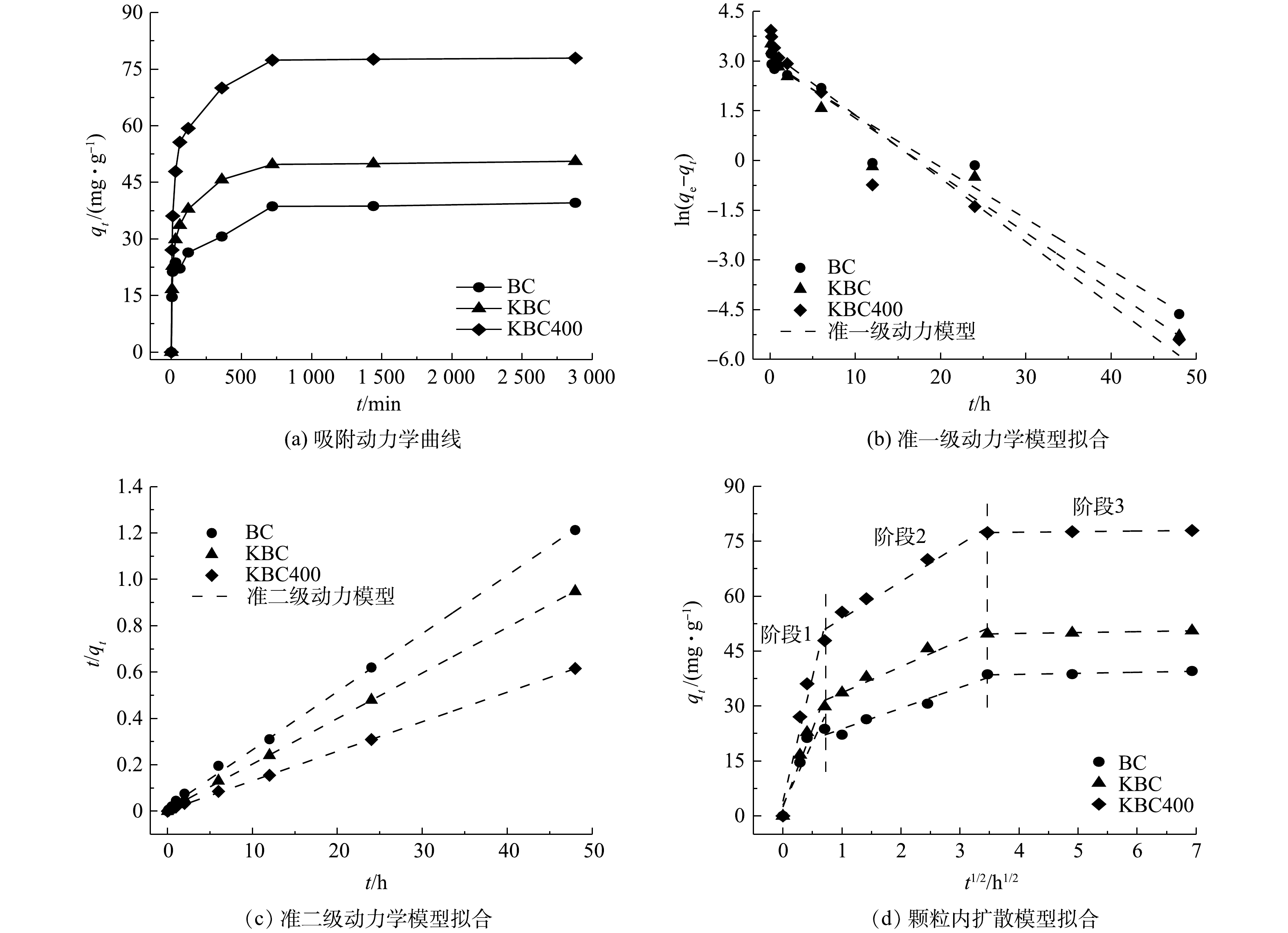
吸附时间对蚕沙基生物炭吸附Cd2+的影响如图8所示。在不同时间下,比较生物炭的吸附量大小为KBC400>KBC>BC。在12 h内,生物炭对Cd2+的吸附容量迅速增大。这是因为生物炭表面的活性位点丰富,能与Cd2+迅速结合。之后生物炭表面的活性位点渐渐饱和(即达到吸附平衡),即使吸附时间增加,吸附容量也变化不大。其中,浸渍-热解活化方式能够更快速的吸附Cd2+。因为KOH热解后提高了生物炭的比表面积,导致与Cd2+的接触面积有所增加。Weber-Morris颗粒内扩散模型拟合结果如图8(d)所示。该模型假设吸附主要可分为3个阶段:首先,吸附质通过水膜层从外部流体介质中向吸附剂的外表面扩散,即外扩散过程;然后,吸附质由材料外表面进一步向孔隙中活性位点扩散,即内扩散过程;最后是吸附质被活性位点吸附的过程,该阶段可能涉及化学反应,此模型能直观地反映吸附剂在吸附的各个阶段的吸附速率大小,且一般主要由前2个阶段控制[38]。Cd2+在KBC、KBC400上的快速吸附阶段发生在6 h内,而初始快速去除阶段则发生在0.5 h内。此外,与BC相比,KBC和KBC400具有良好的吸附性能,0.5 h吸附容量分别达29.88 mg·g?1、47.89 mg·g?1,占其总吸附量的59.06%和61.43%。而BC在1 h内吸附量才达到22.17 mg·g?1,占其总吸附量的56.01%。初始快速吸附可归因于溶液扩散、表面静电吸引和离子交换等过程。对比Weber-Morris模型的斜率可知,浸渍-热解(KBC400)活化方式能够更快速的吸附镉离子。
由表2中的准一级、二级动力学模型的拟合结果可知,BC、KBC和KBC400的吸附过程都更加符合准二级动力学模型(R2≥0.99),说明发生在生物炭表面的Cd2+的吸附行为主要以化学过程为主,即吸附过程可能存在吸附质与吸附剂之间的电子共用或化学成键作用[40]。此外,KBC和KBC400的准一级和准二级动力学模型的R2皆大于0.9,说明经过KOH活化后的生物炭的吸附可能同时存在物理和化学吸附过程[35, 41]。
2.6. 吸附等温线
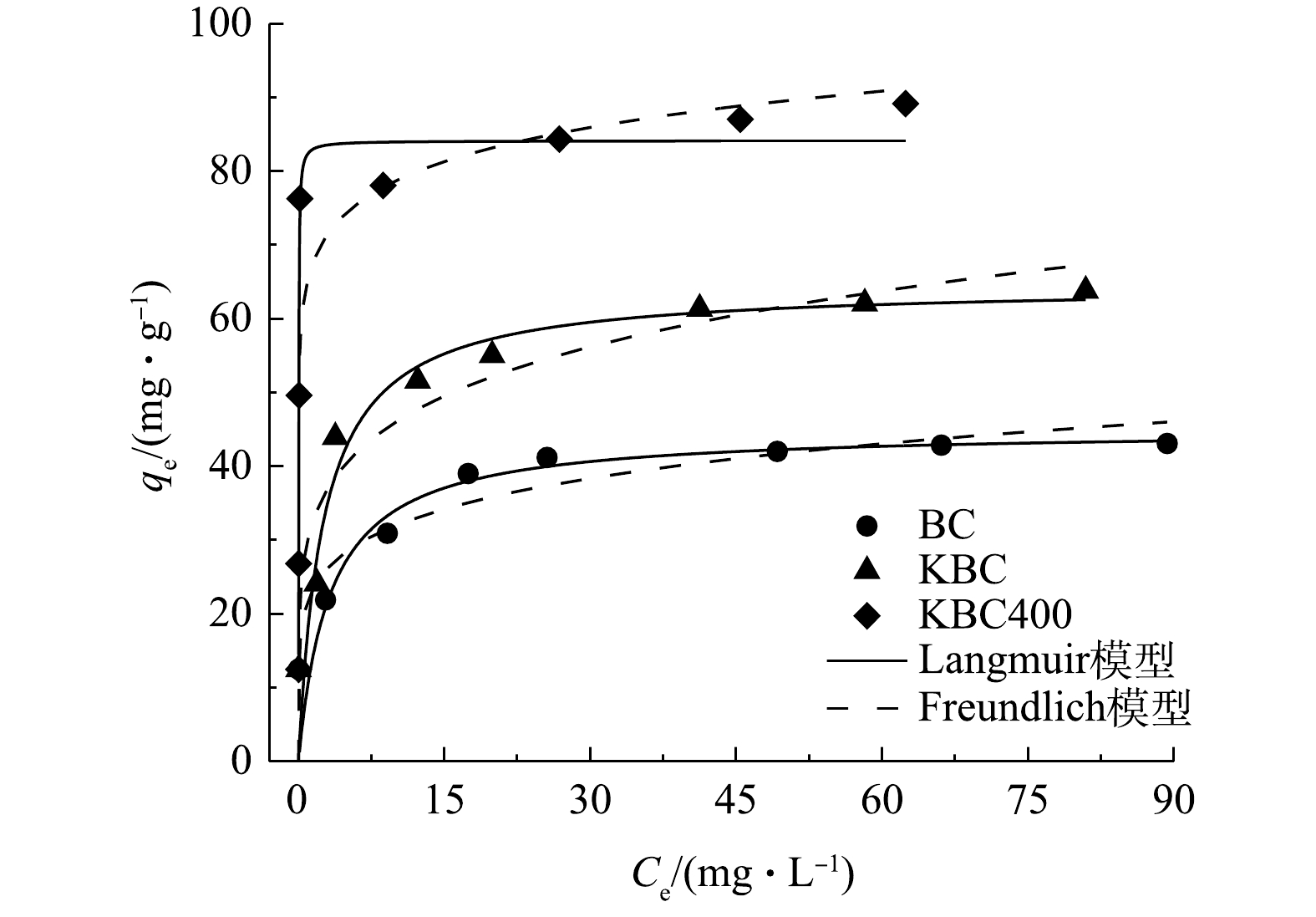
不同Cd2+浓度溶液对蚕沙基生物炭吸附的影响以及等温模型拟合曲线如图9所示。在不同浓度下,对比吸附量可得出对应关系为KBC400>KBC>BC。在Cd2+质量浓度低于30 mg·L?1时,生物炭的吸附容量快速增加。这是因为污染物浓度较低,生物炭表面结合位点充足,可迅速与之结合。之后结合位点渐渐饱和,虽然污染物浓度增加但吸附量趋于平缓。BC、KBC和KBC400实际测量最大吸附量分别为43.09、63.80和89.15 mg·g?1。本研究采用Langmuir和Freundlich 2种等温模型对数据进行拟合(表3),Langmuir等温模型代表均质的单层吸附,BC、KBC和KBC400的拟合结果显示R2值为0.874~0.971,说明Langmuir模型能够更好的描述生物炭材料在不同Cd2+浓度下的吸附行为。
通过Langmuir等温线模型拟合可知,BC、KBC和KBC400的理论最大吸附量分别为44.99、64.54和84.12 mg·g?1,与实际吸附量相近。通过KOH活化能提高蚕沙基生物炭的吸附容量,相比于BC分别提高了43%、99%。同时,在本研究条件下,浸渍-热解活化方式制备的生物炭对Cd2+的吸附量提升最多,表明KBC400在去除Cd2+污染具有一定应用潜力。
2) 2种KOH活化法均可增加生物炭的吸附量,但KBC400的吸附能力高于KBC。在投加量为0.4 g·L?1和pH为5.0的条件下,KBC和KBC400最大吸附量分别为63.80 mg·g?1和89.15 mg·g?1。两者的吸附过程更符合准二级动力学模型和Langmuir等温线吸附模型,说明该吸附过程主要由化学反应控制,属于单层均质吸附。
3)蚕沙基生物炭主要通过静电引力和Cd2+-π结合作用吸附去除水体中Cd2+。2步KOH(热解-浸渍)活化法制备蚕沙基生物炭,第1步(浸渍)和第2步(热解)对吸附Cd2+的相对贡献率分别为28.69%和71.31%。
参考文献

 下载:
下载: 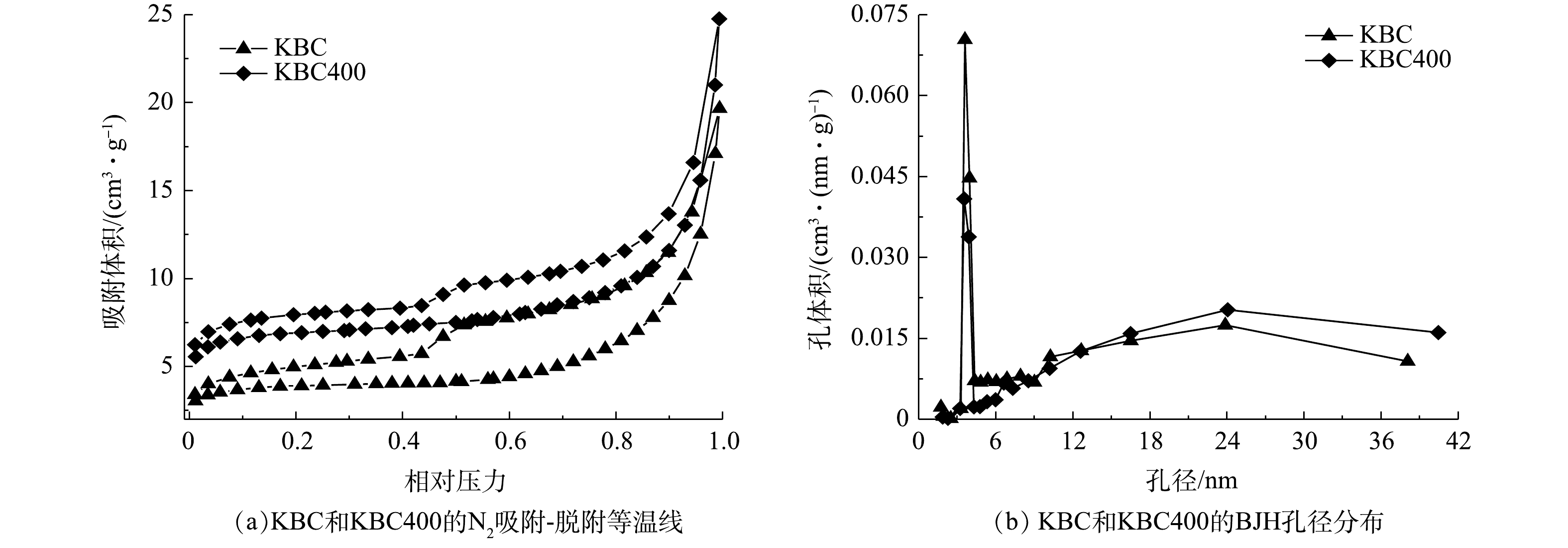
 点击查看大图
点击查看大图