0 引言
【研究意义】在肉品贮藏和加工中常常发生脂肪氧化,脂肪氧化产生的自由基能导致肌原纤维蛋白(MP)同时氧化,引起蛋白质凝胶特性发生改变,进而引起肉制品感官品质如硬度、弹性、黏性等发生改变[1,2]。研究氧化对MP凝胶特性的影响,揭示氧化对MP凝胶特性影响的根本原因,能为MP凝胶特性控制和鸡肉制品的质量控制提供理论依据。【前人研究进展】有报道认为氧化导致蛋白质凝胶强度和保水性显著下降。DECKER等[3]研究了离子催化氧化体系对火鸡肉白肌MP的凝胶质构和保水性的影响,发现蛋白质氧化过程中凝胶强度和保水性都显著下降。BERTRAM等[4]对氧化处理的猪肉MP研究发现,随着氧化程度的增加(H2O2浓度的增加),蛋白质的保水性降低。但也有报道认为温和氧化有利于提高其凝胶特性并导致保水性增加。SNIDER等[5]发现温和氧化有利于蛋清蛋白的聚集,提高其凝胶特性。SRINIVASAN等[6]冷冻储藏牛肉糜后发现冷冻促进了其蛋白和脂肪氧化,凝胶弹性也增大。XIONG等[7]也发现适度氧化的样品拥有更好的弹性和更均匀细腻的凝胶微观结构。KELLEHER等[8]研究发现在水溶性和脂溶性抗氧化剂混合的肉糜体系中,保水性随着氧化程度的升高而增加。MP凝胶作用力决定其凝胶特性,疏水作用力、静电作用力和氢键是影响此凝胶性质的主要作用力,二硫键的作用较小[9]。ZHANG等[10]和WANG等[11]用拉曼光谱法研究了MP凝胶的疏水作用力和氢键。【本研究切入点】虽然氧化对MP凝胶特性的影响已有一些报道,但结果却是矛盾的。其原因是采用的氧化体系不同,与FeCl3+H2O2氧化体系相比,采用亚油酸-脂肪氧化酶体系进行研究更接近氧化发生的实际情况。脂肪氧合酶是脂肪氧化的一种重要方式,氧化过程中会产生自由基和含羰基的化合物,后者进而氧化MP,但利用亚油酸-脂肪氧化酶体系研究氧化对MP凝胶特性影响原因却未见报道。【拟解决的关键问题】借助脂肪氧化酶-亚油酸氧化体系产生自由基使MP发生不同程度的氧化,研究蛋白质氧化对MP凝胶的质构特性和保水性的影响。通过研究氧化对MP凝胶特性、作用力(疏水相互作用、氢键、静电相互作用和二硫键)和分子组成的影响,从分子水平上揭示MP氧化对其凝胶特性影响的根本原因。1 材料与方法
试验于2017年6—11月在南京财经大学进行。1.1 主要材料与试剂
40日龄的活AA鸡(母鸡和公鸡各半),购于南京江宁西北村养鸡场,就地屠宰后取鸡胸肉,运输途中加冰保管,储存于-18℃下并在1个月内使用。脂肪氧化酶LOX 1000 000单位/mL和亚油酸色谱纯,含量≥99.0%均购于Sigma公司。
其他化学试剂均为分析纯。
1.2 主要仪器与设备
PHS-3C型pH计,上海精密科学仪器有限公司;Beckman Avanti J-26 XP落地式高速冷冻离心机,美国贝克曼库尔特公司;DS-1高速组织捣碎机,上海标本模型厂;TA. XT. Plus. 质构仪,英国Stable Micro System公司;TM 3000 扫描电子显微镜,日本日立公司;LABRAM 800型激光拉曼谱仪,法国Jobin Yvon公司;Zetasizer Nano ZS Zeta电位分析仪,英国马尔文公司;U-3900紫外分光光度计,日本日立公司;L-8900型全自动氨基酸分析仪,日本日立公司。1.3 试验方法
1.3.1 MP的提取 冷冻鸡胸肉在4℃下解冻20 min,剔除脂肪和结缔组织,切碎后参照XIONG等[12]的方法提取MP,提取的蛋白质在4℃下保存,3 d内用完。1.3.2 MP的氧化处理及凝胶的制备 取适量MP,用磷酸盐缓冲液(0.6 mol·L-1 KCl、0.01 mol·L-1 KH2PO4,pH 6.0)配制60、25、1 mg·mL-1的MP溶液,向其中分别加入0、0.2、1、2、4、10 mmol·L-1亚油酸溶液,分别加入5 000单位/mL脂肪氧化酶,在4℃下氧化24 h,用1 mmol·L-1 EDTA终止氧化,得到经过氧化处理的MP溶液[13]。
氧化处理后的MP样品水浴加热至70℃(1℃·min-1)制成凝胶,保温20 min,取出后自然冷却,4℃保存16 h后备用。25 mg·mL-1的MP凝胶用于质构特性、保水性和微观结构测定。1 mg·mL-1的MP凝胶用于静电相互作用和巯基含量的测定。60 mg·mL-1的MP凝胶用于拉曼光谱测定氢键和疏水相互作用。
1.3.3 MP羰基含量的测定 羰基含量的测定参照LEVINE等[14]的方法并略作修改,在离心管中加入0.5 mL蛋白溶液(25 mg·mL-1)和2 mL 2, 4-二硝基苯肼溶液(10 mmol·L-1),在室温下反应40 min,对照组只加入2 mL不含2, 4-二硝基苯肼的2 mol·L-1 HCl,不再添加之后步骤的试剂。向离心管中加入2.5 mL 20%的TCA(三氯乙酸),振荡后离心(11 000×g,5 min),弃上清,用2 mL乙醇-乙酸乙酯溶液(体积比1﹕1)清洗蛋白沉淀3次,除去未反应的试剂。加6 mol·L-1盐酸胍6 mL溶解蛋白沉淀。在37℃下放置30 min,空白组作为对照,测定370 nm处吸光值,使用摩尔吸光系数2.2×104 mol·L-1·cm-1计算羰基衍生物含量(μmol·g-1 protein)。
1.3.4 质构特性的测定 用质构仪按照杨玉玲等[15]的方法和条件测定MP热诱导凝胶的硬度和弹性。参数如下:P/6探头,测试前速度5 mm·s-1,测试中速度1 mm·s-1,测试后速度5 mm·s-1,探头探入距离为10 mm。每个样品重复3次。
1.3.5 保水性的测定 用高速冷冻离心机参照KOCHER等[16]的方法进行测定。将氧化后的MP凝胶和离心管称重(W1),于4℃下离心(10 000×g,15 min),除上清液后称量离心管和凝胶的重量(W2),空离心管的质量为W。根据下列公式计算凝胶保水性WHC(%)=(W2-W)/(W1-W)×100。每个处理3次重复。
1.3.6 微观结构的观察 将凝胶切块,用戊二醛固定3 h,清洗,利用乙醇(50%,70%,90%,95%和100%)梯度脱水,每个浓度处理30 min。再用叔丁醇置换,冷冻干燥,镀膜后用扫描电镜(SEM)观察微观结构,扫描电镜加速电压15 kV。
1.3.7 激光拉曼光谱法测试 按照ZHANG等[17]的方法和条件用拉曼光谱仪测定MP凝胶的疏水相互作用和氢键作用。激光波长:514.5 nm;激光出射功率:10 mW(照到样品上约4 mW);显微物镜:50倍长焦距;光栅:600;狭缝(Hole):200 μm;积分时间:60 s;重复3次累加得谱。用I760 cm-1处强度反映MP凝胶的疏水相互作用[18]。I850/I830值反映MP凝胶的氢键变化[19]。
1.3.8 Zeta电位法测试 MP凝胶的静电斥力用样品的Zeta电位表示。将1 mg·mL-1的MP凝胶样品注入Zeta电位皿后,盖上塞子,勿留气泡,进行Zeta电位测试。测试参数:散射角90°,平衡时间60 s,测试温度25℃。每个梯度重复3次。
1.3.9 MP巯基含量的测定 采用ELLMAN法[20]测定MP凝胶的总巯基和活性巯基含量。将0.5 mL MP样品(1 mg·mL-1)依次与4.5 mL缓冲液(由0.01 mol·L-1 EDTA,8 mol·L-1 尿素和0.1 mol·L-1 K2HPO4配制而成,pH 6.0)、100 μL Ellman试剂(由10 mmol·L-1 DTNB和0.01 mol·L-1 KH2PO4缓冲液配制而成,调pH为6.0)混合,使用旋涡振荡约1 min,使溶液混合充分,在室温、避光条件下静置25 min,用紫外分光光度计于412 nm下测定吸光值,摩尔消光系数设为1.36×10-4 mol·L-1·cm-1,巯基含量用nmol·mg-1 protein表示。每个样品测定3次。用不含尿素的缓冲液以同样的方法测定样品中活性巯基的含量。
1.3.10 MP氨基酸含量测定 氨基酸含量测定的方法参照PARK等[21]并做部分修改。称取一定量的MP凝胶于10 mL玻璃水解管中,加入6 mol·L-1的盐酸,在氮气环境下完全水解并浓缩,用0.02 mol·L-1的盐酸溶解后,以15 000 r/min离心5 min,得上清液备用。取适量上清液通过0.22 μm滤器过滤,采用氨基酸自动分析仪进行氨基酸组分分析。
1.4 统计分析
用SPSS17.0软件进行方差分析和主成分分析。2 结果
2.1 氧化对MP羰基含量的影响
图1反映了氧化条件下MP羰基含量的变化。在脂肪氧化酶-亚油酸-MP体系中,随着亚油酸浓度的增加,羰基含量不断增加,并最终达到2.96 μmol·g-1 protein,这表明MP氧化程度随亚油酸含量增加而逐渐升高。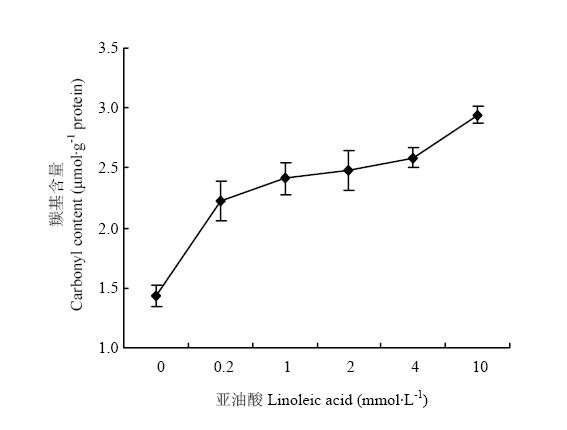 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1氧化对MP羰基含量的影响
-->Fig. 1Effect of oxidation on carbonyl content of MP
-->
蛋白质在氧化过程中,羰基基团的引入是蛋白质氧化过程中的明显变化,其含量可作为蛋白质氧化指标[22]。蛋白质分子自身结构中不含有天然存在的羰基,但是蛋白质氧化能够将羰基基团引入到蛋白质分子,在脂肪氧化酶-亚油酸氧化体系下,蛋白质分子与脂质氧化二级产物以及自由基结合,肽骨架的断裂,氨基酸侧链的直接氧化,这些都会导致羰基的产生。随着亚油酸浓度的增加,羰基含量逐渐升高,说明蛋白质氧化程度越来越高。
2.2 氧化对MP凝胶质构特性的影响
图2反映了不同氧化程度下MP凝胶硬度和弹性的变化。凝胶硬度随亚油酸浓度增加呈现先升高后降低的趋势,在2 mmol·L-1时达到最大值。亚油酸含量从0增加到2 mmol·L-1时,MP凝胶硬度从10.50 g增加到最大值12.95 g,与对照组相比,凝胶硬度增加了21.9%;随着亚油酸浓度继续增加,凝胶硬度迅速下降,在亚油酸10 mmol·L-1处下降到8.23 g,比对照组降低了21.6%。凝胶弹性在亚油酸含量为0.2 mmol·L-1时比对照组略有增加,之后随着氧化程度继续增加,弹性逐渐降低,在10 mmol·L-1处达到最低值,比对照组的凝胶弹性降低了64.3%。表明在脂质酶氧化体系下,低程度氧化改善了MP凝胶的硬度;高浓度氧化会降低MP凝胶的硬度和弹性,削弱MP凝胶的形成能力。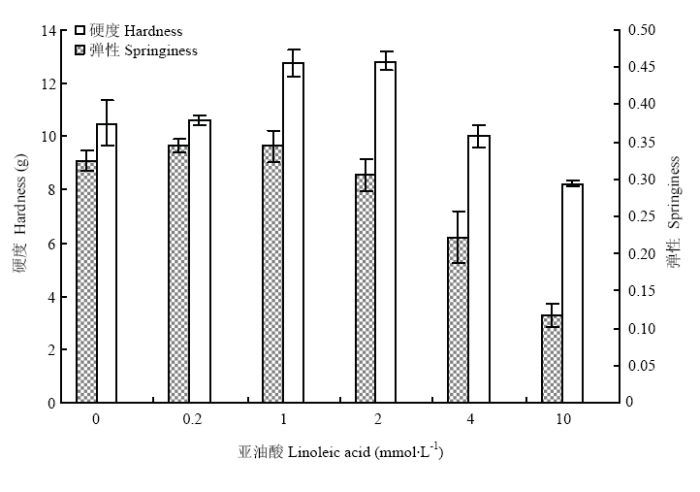 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2氧化对MP凝胶硬度和弹性的影响
-->Fig. 2Effects of oxidation on the hardness and springiness of MP gel
-->
2.3 氧化对MP凝胶保水性的影响
如图3所示,在0—0.2 mmol·L-1亚油酸浓度的低氧化程度下,MP凝胶的保水性变化不大;随着亚油酸浓度增加(0.2—10 mmol·L-1),氧化程度升高,保水性呈现出先增加后降低的趋势;当亚油酸浓度为2 mmol·L-1时,保水性到达最大值,然后再降低。表明适度氧化能改善凝胶的持水性。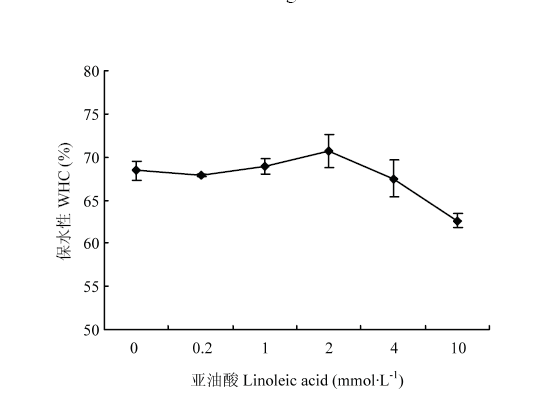 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图3氧化对MP凝胶保水性的影响
-->Fig. 3Effect of oxidation on water holding capacity of MP gel
-->
2.4 氧化对MP凝胶微观结构的影响
不同氧化程度的MP凝胶结构有明显区别(图4)。未经过氧化处理的MP凝胶孔隙比较均匀、网络结构也比较致密;随着氧化程度的增加(0.2—2 mmol·L-1),凝胶网络结构越来越致密,亚油酸浓度为2 mmol·L-1时形成的凝胶网络最为均匀细腻,孔径均一,呈现“蜂窝状”胶束。而随着氧化程度继续增大(2— 10 mmol·L-1),网络结构变少,凝胶孔径增大,胶束不均匀,形成大小不一的蛋白质胶束。亚油酸浓度为10 mmol·L-1时形成的凝胶网络均匀性最差,孔径变大,空隙增多。因此在亚油酸浓度为2 mmol·L-1处MP凝胶的网络结构最佳。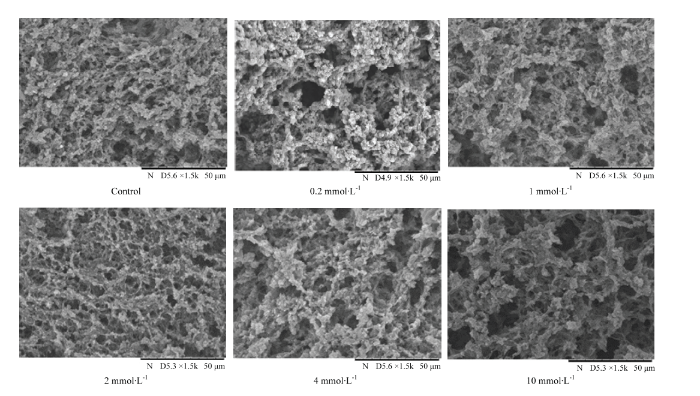 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图4氧化对MP凝胶微观结构的影响
-->Fig. 4Effect of oxidation on the microstructure of MP gel
-->
2.5 拉曼光谱测试结果
2.5.1 氧化对MP凝胶疏水作用力的影响 拉曼光谱760 cm-1处色氨酸环伸缩振动反映了MP凝胶的疏水相互作用[18]。如图5所示,随着氧化程度的升高,MP凝胶760 cm-1处归一化强度(I760)随着亚油酸浓度的增加先升高后降低,在2 mmol·L-1处达到最大值,表明疏水相互作用先增加(0—2 mmol·L-1),然后随着氧化程度进一步增强而降低,这说明随着氧化程度的增加,疏水作用力先增加后降低,在2 mmol·L-1处获得最大值。由此可知,低程度氧化可增大MP凝胶的疏水作用力,但高程度氧化减弱了MP凝胶的疏水作用力。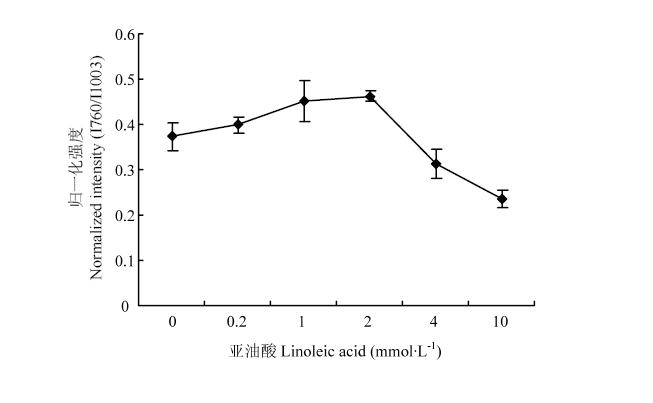 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图5归一化的760 cm-1处条带强度随亚油酸浓度的变化
-->Fig. 5Normalized intensity of the 760 cm-1 band in Raman spectroscopy as a function of linoleic acid concentration
-->
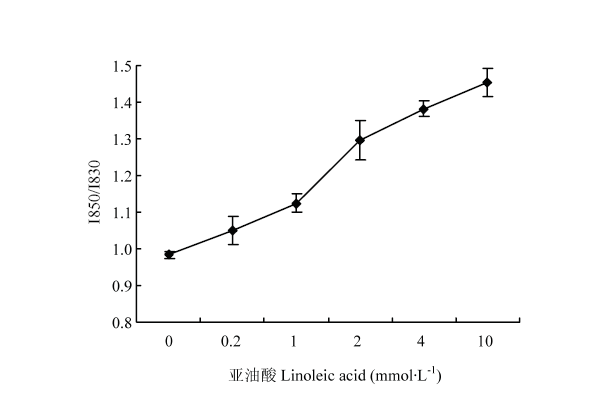
2.5.2 氧化对MP凝胶氢键的影响 在图6中,未经氧化处理的对照样I850/I830比值为0.98;随着亚油酸含量增加,I850/I830比值快速增加;当亚油酸含量大于2 mmol·L-1时,I850/I830比值大于1.25。这表明,在对照组中,酪氨酸上-OH既与水形成氢键又与蛋白质上其他中性基团形成氢键。但随着氧化程度增加,蛋白质分子间氢键逐渐解体,到达2 mmol·L-1时,酪氨酸上的-OH完全暴露在水环境中,与溶剂水分子生成氢键,也就是说MP分子间的氢键作用随着氧化程度的升高而减少。
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图6归一化的I850/I830强度随亚油酸浓度的变化
-->Fig. 6Normalized ratio of I850/I830 doublet bands as a function of linoleic acid concentration
-->
2.6 氧化对MP凝胶静电相互作用的影响
Zeta电位与分散体系凝结的稳定性密切相关,可用于体现胶体粒子之间的静电相互作用[23,24]。通常蛋白质分子表面带净负电荷,静电相互作用表现为斥力,Zeta电位值为负值。当溶液中存在的胶粒携带了大量正或负电荷,即Zeta电位的绝对值较大时,胶粒间斥力占优势,不会发生絮凝。但当胶粒的Zeta电位绝对值较低时,粒子会相互接近并发生絮凝。在图7中,Zeta电位绝对值随着亚油酸浓度的增加而降低,表明静电相互作用随着氧化程度的增加而减弱。氧化引起蛋白质分子结构的变化,影响蛋白表面电荷,即导致Zeta电位的变化。MP分子中的某些基团会与水分子结合,如带电基团与水分子进行离子-偶极相互作用,Asn、Gln上的酰胺基团,Thr、Tys、Ser残基的-OH以及肽键进行偶极-偶极相互作用,非极性残基也能与水分子进行偶极-诱导-偶极相互作用。如带正电荷的赖氨酸与自由基和醛类物质反应生成中型的羰基衍生物[25]。Zeta电位的下降可能是由于随着氧化程度的增加,包埋的带电荷的氨基酸残基暴露出来,与氧化产物反应,生成电中性的其他物质,导致了Zeta电位的降低。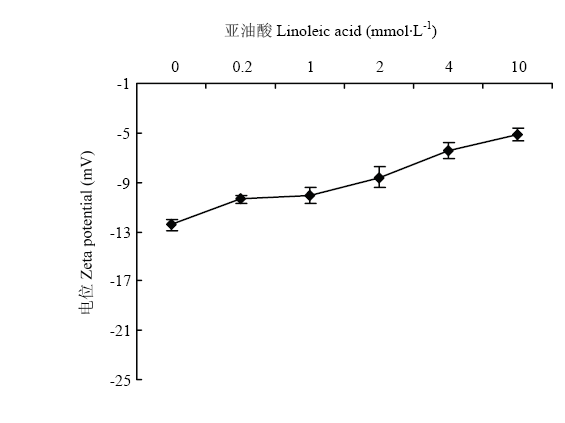 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图7Zeta电位随亚油酸浓度的变化
-->Fig. 7Zeta potential as a function of linoleic acid concentration
-->
2.7 氧化对巯基含量的影响
蛋白质中包含的巯基有两种形式,一种是暴露于蛋白质表面的,另一种是包埋在蛋白质内部的。总巯基包括以上二者,而活性巯基只包括前者[26]。可认为总巯基含量的减少代表二硫键的生成[27]。如图8所示,随着亚油酸浓度的升高,总巯基含量和活性巯基含量均呈现降低的趋势。在亚油酸浓度为10 mmol·L-1时,总巯基和活性巯基含量最低,表明氧化过程中二硫键不断生成。二硫键在MP凝胶形成以及维持凝胶功能特性和结构方面起着重要作用[28,29]。加热时MP分子展开,暴露出内部的巯基与疏水基团,巯基反应生成二硫键,疏水基团聚集,形成具有一定强度的三维网络结构,有助于稳定其中的脂质和水分,形成更好的凝胶网络结构,增加凝胶强度。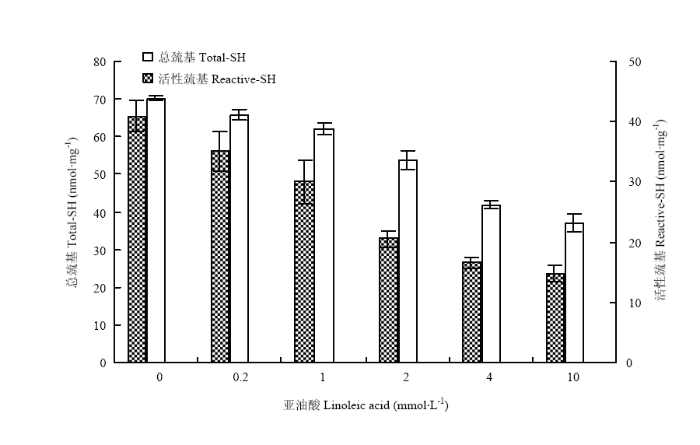 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图8氧化对总巯基和活性巯基含量的影响
-->Fig. 8Effects of oxidation on total sulfhydryl and reactive sulfhydryl content
-->
2.8 氧化对MP分子氨基酸含量的影响
表1中,Cys的含量随着氧化程度的升高而降低,Cys的巯基在凝胶形成过程中能够形成二硫键;侧链疏水性最强的3种氨基酸Leu,Ile和Phe的含量随氧化程度的变化不显著;但疏水性氨基酸(Ala,Met,Val,Leu,Ile和Phe)总量变化显著,并且最大值发生在亚油酸浓度为2 mmol·L-1处。Ser,Glu和Cys 3种氨基酸残基能够形成MP分子内氢键,这3种氨基酸的含量随着氧化程度的升高而降低。正电荷的氨基酸Lys,Arg和His随氧化程度变化不显著,但是解离后带负电荷的Glu含量随着氧化程度的升高而减少。Table 1
表1
表1不同氧化程度下MP的氨基酸含量
Table 1Amino acid content of MP under different degrees of oxidation (mg·g-1)
| 氨基酸 Amino acid | 亚油酸浓度Linoleic acid concentration | |||||
|---|---|---|---|---|---|---|
| 0 | 0.2 mmol·L-1 | 1 mmol·L-1 | 2 mmol·L-1 | 4 mmol·L-1 | 10 mmol·L-1 | |
| Ala | 43.85±0.58a | 41.47±0.34b | 41.08±0.17b | 41.64±0.94b | 39.39±0.52b | 39.83±0.61b |
| Arg | 51.97±0.54a | 49.81±0.84a | 48.14±0.65a | 51.23±0.28a | 48.35±0.68a | 49.05±0.94a |
| Asp | 66.03±0.57a | 68.67±0.34a | 65.96±0.68a | 67.76±0.84a | 65.99±0.21a | 66.63±0.52a |
| Cys | 7.23±0.15a | 6.97±0.11b | 6.82±0.17b | 6.68±0.21b | 6.52±0.14b | 6.27±0.35c |
| Glu | 142.85±0.51a | 140.38±0.27a | 136.95±0.34b | 132.62±0.84c | 132.99±0.35c | 133.78±0.68c |
| Gly | 27.19±0.57a | 23.12±0.36b | 22.33±0.57b | 23.58±0.95b | 22.35±0.28b | 18.64±0.58c |
| His | 13.53±0.35a | 13.21±0.68a | 12.87±0.47a | 13.71±0.33a | 12.92±0.95a | 13.25±0.62a |
| Ile | 35.94±0.35a | 35.27±0.94a | 35.31±0.65a | 36.21±0.34a | 34.42±0.38a | 34.92±0.52a |
| Leu | 61.75±0.28a | 62.64±0.97a | 62.68±1.21a | 64.44±0.57b | 60.82±0.68a | 61.89±0.21a |
| Lys | 9.19±0.65a | 8.69±0.52a | 8.57±0.68a | 8.96±0.62a | 8.54±0.47a | 8.74±0.95a |
| Met | 25.16±0.84a | 23.01±1.32b | 22.28±0.56b | 23.32±0.75b | 22.53±0.35b | 22.41±0.59b |
| Phe | 28.68±0.54a | 29.17±0.68a | 28.12±0.24a | 29.72±0.33a | 28.13±0.37a | 28.38±0.59a |
| Ser | 29.13±0.28a | 26.48±0.49b | 23.73±0.36c | 24.96±0.25c | 23.67±0.98c | 23.72±0.54c |
| Thr | 33.75±0.58a | 33.51±0.36a | 31.36±0.27a | 32.96±0.95a | 31.37±0.84a | 31.53±0.68a |
| Tyr | 22.85±0.63a | 20.47±0.21a | 19.77±0.37a | 20.96±0.57a | 20.43±0.64a | 20.25±0.84a |
| Val | 34.23±0.97a | 35.19±0.27a | 35.81±0.86a | 35.12±0.54a | 33.44±0.67a | 33.62±0.35a |
新窗口打开
2.9 主成分分析
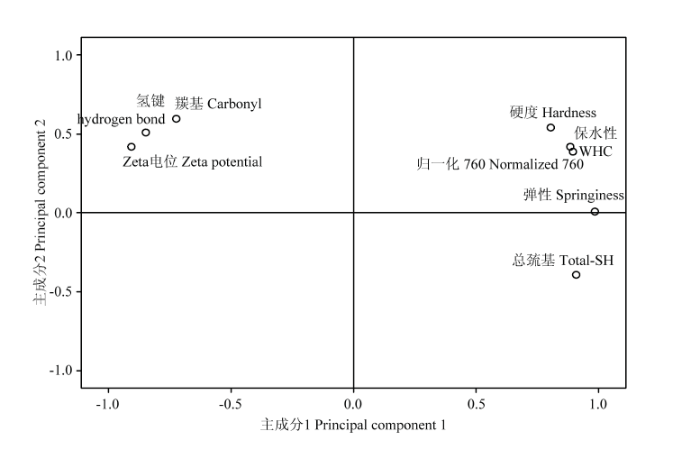
用SPSS软件对凝胶特性和作用力进行主成分分析(表2)。从表2可见,第一个主成分能解释总体方差变异的72.124%,两个主成分的累积解释方差已经达到95.096%,且这两个主成分的特征值均超过1,基本可以解释原来所有指标包含的信息,故选取这两个主成分作为评价不同氧化程度下MP凝胶特性和化学作用力关系的综合指标进行评价(图9)。Table 2
表2
表2不同氧化程度处理下MP凝胶的主成分分析
Table 2Principal component analysis of MP gel at different degrees of oxidation
| 主成分PC | 特征值Eigenvalue | 解释方差Total variance (%) | 累积特征值Cumulative eigenvalue | 累积解释方差Cumulative (%) |
|---|---|---|---|---|
| 1 | 6.491 | 72.124 | 6.491 | 72.124 |
| 2 | 2.068 | 22.972 | 8.559 | 95.096 |
新窗口打开
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图9不同氧化程度下主成分评分
-->Fig. 9Principal component scores of different degrees of oxidation
-->
从图9可见,第一主成分与硬度、弹性、保水性、总巯基和I760均呈现正相关性,与羰基含量、氢键和Zeta电位呈现负相关性。第二主成分与羰基含量正相关性比较高,与总巯基呈现负相关性。硬度、弹性、保水性与I760都处于第一象限并且比较接近,这表明疏水相互作用对脂质酶氧化体系下MP凝胶形成起着重要甚至是决定性作用。
3 讨论
3.1 不同氧化体系下的MP凝胶质构特性比较
在脂肪氧化酶-亚油酸-MP体系中,亚油酸是油脂氧化反应产生自由基的底物,本试验中随着亚油酸含量增加,MP中羰基含量不断增加,这表明MP氧化程度随亚油酸含量增加而逐渐升高。因此,可以用亚油酸含量高低代表氧化程度大小。DEAN等[22]也获得了相同的结论。本试验中,亚油酸含量从0增加到2 mmol·L-1时,MP凝胶硬度逐渐增加到最大值,随着亚油酸浓度继续增加,凝胶硬度迅速下降。原因可能是氧化改变了凝胶的疏水作用力或二硫键[30]。凝胶弹性在亚油酸含量为0.2 mmol·L-1时比对照组略有增加,之后随着氧化程度继续增加,弹性逐渐降低。胡忠良[31]研究了羟自由基氧化体系(H2O2+FeCl3)对鸡胸肉MP凝胶硬度和弹性的影响,发现随着氧化程度的升高,凝胶的硬度、弹性均先增加后降低,与本试验结果类似。而李银等[32]发现在羟自由基氧化体系下猪肉MP凝胶硬度随着H2O2浓度增加逐渐降低,造成这种差别的原因可能是MP的来源不同,本试验中的MP来自鸡胸肉,而李银等[32]的MP则来自猪长肌;也可能是试验设计的问题,如果氧化体系反应物浓度间隔太大,有可能漏掉其中的某些变化。3.2 本氧化体系下的MP凝胶特性与微观结构
MP凝胶的保水性与凝胶硬度一样,随着氧化程度升高逐渐增加,当亚油酸浓度为2 mmol·L-1时达到最大值,而后逐渐降低。在MP形成凝胶的过程中,亚油酸-脂肪氧化酶氧化体系会生成自由基中间物以及氢过氧化物如α, β-不饱和醛(丙烯醛和4-羟基-2-壬烯醛)和丙二醛(MDA)[33,34],可能会促进蛋白质分子之间以及蛋白质分子-脂质交联的形成,从而提高了MP凝胶的保水性和硬度。XIONG等[35]的观点是氧化使蛋白质的溶解度和巯基含量减少,导致蛋白凝胶保水性的下降。但也有研究发现,氧化并不一定降低蛋白质的加工特性,KELLEHER等[36]对鲭鱼肉糜研究发现随着氧化程度增加,肉糜保水性也持续增加。良好的凝胶网状结构能更好地束缚水分,增强凝胶抗压能力,使得凝胶的硬度和保水性增加。使用扫描电镜对凝胶的微观结构进行观察,发现在亚油酸浓度为2 mmol·L-1时,MP形成的凝胶网络最为均匀细腻,孔径均一,呈现“蜂窝状”胶束;而亚油酸浓度为10 mmol·L-1时,形成的凝胶网络均匀性最差,孔径变大。这可能是由于亚油酸浓度在0—2 mmol·L-1范围内,轻度氧化能促进蛋白质分子变性和展开,但变性速度随亚油酸浓度增加仅缓慢增加,致使较多变性的MP分子能从容重排,形成致密细腻的凝胶结构;而在高浓度氧化剂下,剧烈的氧化使过多的蛋白质分子快速变性并聚集,来不及有序重排,因此形成的凝胶网络均匀性最差,孔径变大。胡忠良等[31]利用羟基自由基体系研究氧化对MP凝胶微观结构的影响时,也发现过度氧化导致凝胶微观结构的变差。3.3 氧化反应与作用力变化
测定蛋白质疏水作用力的方法主要有两种,一种是ANS探针法,另一种为拉曼光谱法,前一种方法必须在极稀的溶液中进行测定,后者更适合于凝胶蛋白质样品的测定,因此本试验用拉曼光谱的I760强度表达MP凝胶的疏水作用力[37]。结果发现,随着氧化程度的增加,疏水作用力先增加后降低,在2 mmol·L-1处获得最大值。在MP凝胶的形成过程中,随着氧化程度的升高(0—2 mmol·L-1),MP分子部分展开,使得疏水性基团暴露,增强了疏水相互作用,此外脂质酶氧化体系的二级产物、氢过氧化物和过氧自由基会与氨基酸基团反应,如赖氨酸、半胱氨酸和组氨酸能与α, β-不饱和醛反应,从而引入一条疏水性侧链,这使得疏水相互作用在低氧化程度下增强。但是,当亚油酸浓度继续增加(2—10 mmol·L-1),疏水相互作用降低,可能是因为疏水性基团被氧化为亲水性基团,比如蛋氨酸被氧化为蛋氨酸亚砜。LI等[38]用ANS探针法研究了经FeSO4-抗坏血酸氧化体系处理的肌球蛋白的疏水性变化趋势,发现表面疏水性随着氧化程度的增加而增加;WU等[39]报道了大豆蛋白氧化会导致表面疏水性的降低。造成各种结果不一致的原因很多,包括测定方法,蛋白质原料、氧化体系等试验设计和条件等。拉曼光谱I850与I830共轭双峰比值变化能反映出MP中氢键的变化趋势图[19]。当I850/I830≥1.25时,代表酪氨酸上的-OH与溶剂水分子生成氢键;I850/I830≤0.5时,表明酪氨酸残基包埋在疏水环境中,与蛋白质分子上其他极性基团生成氢键。本试验中未经氧化处理的对照组比值为0.98,表明酪氨酸残基形成的氢键既包括暴露的氢键,也包括埋藏的氢键;随氧化程度增加,I850/I830比值增加,表明MP酪氨酸残基苯环上的-OH与MP分子其他残基(如-COOH)生成的氢键逐渐转变为与水分子生成的氢键,即MP分子间的氢键作用随着氧化程度的升高而减少。在蛋白质经过高度氧化后,MP分子展开,而且MP分子疏水性降低,这使络氨酸残基处于亲水性微环境,埋藏的MP分子内部的氢键转变为MP分子与水分子之间的氢键。XU等[40]研究加热过程中(20℃—70℃)猪肉MP I850/I830的变化,发现共轭双峰的比值从1.13升高到2.56,他们认为加热使酪氨酸残基暴露在水溶液环境中。SUN等[41]对猪肉MP研究也证实埋藏的络氨酸残基会导致I850/I830共轭双峰值的降低,与本试验结果一致。本研究中MP凝胶的静电作用力随着氧化程度的升高而减弱,这可能是由于带电荷的氨基酸含量发生变化导致。一般情况下,总巯基的减少表示二硫键的生成[27]。MP分子中含有的大量巯基,在氧化体系中能被不同程度的氧化,并且产生相关共价化合物[42,43]。巯基(-SH)能被氧化成为二硫键(-S-S-)、次磺酸(RSOH)、亚磺酸(-SO2H)和磺酸(-SO3H)等形式[44]。其中巯基(-SH)被氧化成为可逆的氧化形式:二硫键(-S-S-)和次磺酸(RSOH),导致二硫键含量增加;但是也可能会被氧化成为不可逆的氧化形式:亚磺酸(-SO2H)和磺酸(-SO3H),这会导致MP分子中总巯基含量以及二硫键含量的降低。本研究中巯基含量随氧化程度的升高而持续降低,说明氧化一定程度上导致二硫键增加。随着亚油酸浓度的升高,氧化程度不断增强,MP分子的半胱氨酸残基的-SH被氧化成二硫键或其他含硫氧化物[45],其总巯基和活性巯基含量降低是必然的。
3.4 氧化体系下MP组成、作用力与凝胶特性的内在关系
本研究中疏水性氨基酸Ala、Leu、Ile和Phe的含量随氧化程度的变化不显著,Val和Met含量随氧化程度升高而降低,但疏水性氨基酸的总量(Ala、Met、Val、Leu、Ile和Phe)随氧化程度升高而显著变化,在亚油酸为2 mmol·L-1处达到最大值。在氧化过程中,疏水性氨基酸暴露于表面,并通过疏水相互作用相互联接,因此导致测定的MP凝胶疏水相互作用也在亚油酸2 mmol·L-1时达到了最大。通过对MP凝胶特性和作用力指标进行主成分分析发现,疏水相互作用对氧化体系下MP凝胶特性形成起决定性作用,这解释了MP凝胶的硬度在2 mmol·L-1处最大的原因,同时也说明了氧化通过改变MP分子中疏水氨基酸残基含量和分布,改变凝胶的疏水相互作用,进而控制凝胶的质构特性。Ser,Glu和Cys 3种氨基酸残基可形成分子内氢键,蛋白质氧化破坏了这3种氨基酸,使这3种氨基酸数量下降,从而导致了分子内氢键降低。MP凝胶中的氢键可能与凝胶的弹性相关。PARK等[21]对经过氧化的MP进行氨基酸分析时同样发现,Cys和Met的含量随着亚油酸浓度的升高而降低,与本研究一致。本试验中带正电荷的氨基酸基团(Lys,Arg,和His)和带负电的氨基酸基团(Asp)随亚油酸浓度变化不显著,但是带负电荷的Glu被氧化修饰,与脂质氧化二级产物以及自由基结合,生成羰基,这导致了蛋白质分子电位降低,凝胶中静电斥力降低。良好的凝胶是分子间引力和斥力相平衡的结果,斥力是防止凝胶絮凝的关键性作用力。这解释了高氧化程度时凝胶微观结构出现高孔径变大,胶束不均匀的现象。因此氧化通过降低MP分子中Glu含量改变凝胶的静电斥力,进而改变凝胶的超微结构和弹性。4 结论
氧化作用改变了MP的组成,使其作用力发生变化,致使MP的超微结构、质构特性和保水性发生变化。Ser、Glu和Cys 3种氨基酸残基能够形成分子内氢键,随着亚油酸浓度的增加,蛋白质氧化程度越来越高,蛋白质氧化破坏了3种氨基酸,使这3种氨基酸数量下降,从而减弱了分子内氢键作用;Cys的巯基在凝胶形成过程中能够形成二硫键,Cys的含量随着氧化程度的升高而降低,导致二硫键增加。氧化降低了MP分子中解离后带负电荷的Glu含量,从而减弱凝胶的静电相互作用,进而影响凝胶的超微结构和凝胶的弹性。疏水性氨基酸的总量随氧化程度升高而增加,在亚油酸为2 mmol·L-1处达到最大值,导致MP凝胶的疏水相互作用在亚油酸2 mmol·L-1时达到了最大,疏水相互作用对脂质酶氧化体系下MP凝胶形成起着决定性作用,所以MP凝胶的硬度和保水性在2 mmol·L-1处最大,凝胶的超微结构最佳。The authors have declared that no competing interests exist.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | . The development of protein oxidation as assessed by the total carbonyl content and its influence on color and texture deterioration during the refrigerated storage (+4 C/60 d) of frankfurters, were studied. The effect of the addition of a rosemary essential oil at different levels (150, 300, and 600 ppm) on the protein oxidative stability of the frankfurters was also evaluated. Frankfurters with no added essential oil were used as controls. The amount of carbonyls from protein oxidation significantly increased during refrigerated storage, and this increase was significantly higher in control frankfurters than in those treated with 300 and 600 ppm. Rosemary essential oil at levels of 300 and 600 ppm successfully protected the heme molecule from degradation and significantly inhibited the increase of nonheme iron (NHI) in refrigerated stored frankfurters. Color changes were directly related to oxidation processes because frankfurters with added antioxidants (300 and 600 ppm) suffered less color modifications than the controls. The addition of rosemary essential oil enhanced texture characteristics of frankfurters by reducing hardness, adhesiveness, gumminess, chewiness, and controlling the lost of elasticity during refrigeration. Statistically significant correlations were calculated between protein oxidation and instrumentally measured parameters, suggesting that the alteration of protein functionality caused by oxidation likely affected color and texture characteristics of frankfurters. |
| [2] | . The effect of modified atmosphere packaging (70% O 2/30% CO 2) and skin packaging (no oxygen) on protein oxidation and texture of longissimus dorsi was investigated during storage for 14 days at 4 °C. High oxygen atmosphere resulted in reduced tenderness and juiciness and SDS–PAGE revealed cross-linking of myosin heavy chain through disulfide bonding, and the content of protein thiols was reduced indicating protein oxidation. Myofibril fragmentation was reduced in meat stored in high oxygen atmosphere indicating less proteolysis and/or cross-linking of proteins. Protein carbonyl content was not affected by the packaging atmospheres. This study shows that packaging in modified atmosphere containing a high level of oxygen can result in protein cross-linking and reduced tenderness and juiciness of the meat. |
| [3] | . ABSTRACT Oxidative damage to turkey white muscle myofibrillar proteins (MP) was investigated by measuring changes in chemical, physical, and functional properties after exposure to iron or copper (25 ) and ascorbate. Both iron- and copper-oxidized MP exhibited increased absorbance at 265 nm and carbony! content compared to controls. Increasing the ascorbate concentration from 0 to 25 mM increased the absorbance 6.8- and 4.0-fold and the carbonyl content 2.6- and 1.9-fold for MP in the presence of iron and copper, respectively. The iron- and copper-oxidized MP showed lower solubility, gel strength, and gel water-holding capacity than controls. SDS-polyacryiamide gel electrophoresis demonstrated that both iron- and copper-catalyzed oxidation resulted in a major loss of myosin and actin with concomitant formation of protein polymers. These data suggest that the decreased functionality of proteins in muscle foods exposed to oxidative environments could be due to chemical and physical changes resulting from oxidative reactions. |
| [4] | . Water-binding properties of myofibrils extracted from porcine muscle, and added hemoglobin with and without exposure to H2O2, were characterized using low-field proton NMR T2 relaxometry. The effects of pH and ionic strength in the samples were investigated as pH was adjusted to 5.4, 6.2, and 7.0 and ionic strength was adjusted to 0.29, 0.46, and 0.71 M, respectively. The formation of dityrosine as a measure of oxidative protein cross-linking revealed a significant increase in dityrosine concentrations upon H2O2 activation. The formation of dityrosine was strongly pH-dependent and increased with decreasing pH. In addition, increased levels of thiobarbituric acid reactive substances were observed upon addition of H2O2, implying that lipid oxidation was enhanced, however, with a different oxidation pattern as compared to the myofibrillar proteins. Low-field NMR relaxation measurements revealed reduced T2 relaxation times upon H2O2 activation, which corresponds to reduced water-holding capacity upon oxidation. However, a direct relationship between degree of oxidation and T2 relaxation time was not observed with various pH values and ionic strengths, and further studies are needed for a complete understanding of the effect of oxidation on myofibrillar functionality. |
| [5] | . First page of article |
| [6] | . Oxidation of proteins (carbonyls) and lipids (TBARS) in beef heart surimi-like materials during preparation and storage (2°C) was inhibited by propyl gallate (0.02%) or -tocopherol (0.2%). Inhibition of oxidation did not affect surimi gel property (storage modulus, G'). Storage promoted oxidation of proteins in 0.2% ascorbate-washed mince, leading to increases in peak (65 55°C) and final (70°C) G' of thermally induced surimi gel. Protein carbonyls in stored surimi and its sol (salted), as well as TBARS of the sol, strongly correlated with both peak and final G of gels. Incorporation of tripolyphosphate into washed mince promoted gelation whether the surimi-like material was oxidized or not. |
| [7] | . ABSTRACT: The objective of the study was to examine how oxidatively induced protein cross-linking would influence the gelation properties of myofibrillar protein (MP) under meat processing conditions. MP suspensions in 0.6 M NaCl at pH 6 were treated with an iron-catalyzed oxidizing system (IOS: 10 μM FeCl3, 0.1 mM ascorbic acid, 0.05 to 5 mM H2O2) or a H2O2-activated metmyoglobin oxidizing system (MOS: 0.01 to 0.1 mM metmyoglobin/H2O2) that produced hydroxyl radical and ferryl species, respectively. Both oxidizing systems promoted MP thermal gelation, which was evidenced by rapid protein–protein interaction and the enhancement in storage modulus (elasticity) of the gel network as revealed by dynamic rheological testing in the 20 to 74 °C temperature range. This gelation-enhancing effect was attributed to the shift of myosin aggregation in the early stage of heating from predominantly head–head association (nonoxidized control samples) to prevalently tail–tail cross-linking through disulfide bonds. However, both hardness and water-holding capacity of chilled gels tended to decline when MP was exposed to ≥1 mM H2O2 in IOS and to all concentrations of metmyoglobin in MOS. Microscopic examination confirmed a more porous structure in oxidized gels when compared with nonoxidized protein gels. The results demonstrated that mild oxidation altered the mode of myosin aggregation in favor of an elastic gel network formation, but it did not improve or had a negative effect on water-binding properties of MP gels.Practical Application: Mild oxidation promotes protein network formation and enhances gelation of myofibrillar protein under normal salt and pH conditions used in meat processing. This oxidative effect, which involves disulfide linkages, is somewhat similar to that in bakery product processing where oxidants are used to improve dough performance through gluten protein interaction. |
| [8] | . |
| [9] | . |
| [10] | . The effects of high pressure (100–500MPa) on chemical forces and water holding capacity of heat-induced myofibrillar protein (MP) gel were investigated. As pressure increased, total sulfhydryl (SH) group content decreased and absolute value of zeta potential increased, which suggested the formation of disulfide bonds and increased the strength of electrostatic repulsion. Surface hydrophobicity and normalized intensity of the 760cm611 band showed a maximum value at 200MPa, indicating that 200MPa was the optimum pressure for hydrophobic interactions. Hydrogen bonding of MP gel was strengthened at pressures of 300MPa and above. Bound water (T2b) had lower water mobility and was more closely associated with proteins. Free water (T22) had higher water mobility. More free water was attracted by proteins or trapped in gel structure, and transferred to bound or immobilized water as pressure increased. A value of 200MPa was the optimum pressure for the water holding capacity of MP gel. |
| [11] | . |
| [12] | . <P>Physical changes in chicken gastrocnemius myofibrils incubated in 0.1 to 1.0 M NaCl solutions with or without 10 mM ortho-(P), pyro-(PP), tripoly-(TPP) or hexameta- (HMP) phosphate at pH 6.0 were examined by phase-contrast microscopy, electrophoresis, and solubility. PP and TPP performed similarly in promoting protein extraction, P had no apparent effect, and HMP exhibited an intermediate effect. PP, TPP, and HMP treatments markedly improved protein solubility in 0.3 and 0.4 M NaCl through the release of myosin, but the phosphate effect diminished in ≥ 0.6 M NaCl. Overall, phosphates influenced the ultrastructure of myofibrils and extraction of their constituents in the order: PP 65 TPP > HMP > P 65 nonphosphate control.</P> |
| [13] | . |
| [14] | . |
| [15] | . 【目的】研究加热温度对肌原纤维蛋白二级结构和凝胶特性的影响,并探讨肌原纤维蛋白二级结构与凝胶特性之间的内在关系。【方法】将活AA鸡20只(40日龄)屠宰,取鸡胸肉在-18℃下储存,用于提取鸡胸肉肌原纤维蛋白。用圆二色谱(CD)研究加热过程中肌原纤维蛋白二级结构(α-螺旋,β-折叠,β-转角和无规则卷曲)的变化;使用流变仪测定加热温度对肌原纤维蛋白的流变性质参数储能模量G’和相位角正切值 (Tanδ)的影响;将肌原纤维蛋白在不同温度下制备成凝胶,运用质构仪研究成胶温度对凝胶硬度和弹性的影响;用低场核磁共振仪(NMR)测定不同加热温度下成胶的肌原纤维蛋白凝胶的弛豫时间T2,以此研究不同温度下制得凝胶的水分布特性。利用SPSS17.0对所得的数据进行相关性分析等处理,以便阐明加热温度与肌原纤维蛋白二级结构及其凝胶特性的关系。【结果】加热温度显著影响肌原纤维蛋白的二级结构。随着加热温度升高,肌原纤维蛋白二级结构中α-螺旋含量逐渐降低。在30℃时α-螺旋含量为95.77%,加热温度在30—40℃以及70—80℃之间时α-螺旋含量变化很小,在40—70℃之间显著下降(P<0.05),到80℃时下降到45.05%。 β-折叠含量在30—45℃之间随温度上升缓慢增加,在40—70℃之间显著增加(P<0.05),超过70℃后含量仅略有增加;在30—80℃加热范围内,β-折叠含量从0.20%增加到12.65%。α-螺旋含量降低代表蛋白质分子展开程度增加,而β-折叠含量增加代表蛋白质分子间聚集程度增加。加热温度影响肌原纤维蛋白的流变性、质构特性和水分布特性。G’开始增加时的温度为42℃,表明肌原纤维蛋白在此温度下开始胶凝。在42—50℃之间,G’迅速增加到峰值177 Pa,之后G’迅速下降 (50—55℃),在55—75℃范围内G’再次快速增加;肌原纤维蛋白凝胶硬度在40—75℃内随温度上升而显著增大,在75℃时硬度达到最大值51.4 g。凝胶弹性在55℃达到弹性最大值0.754;在NMR图谱中T2有 3个峰,其中T22表示不可移动水,肌原纤维蛋白成胶温度在40—60℃内的凝胶T22值随加热温度上升从403.7 ms降到265.6 ms,即T22向快弛豫方向移动,表明随着温度的升高,水分子移动性降低。经相关性分析发现,加热温度、β-折叠含量与凝胶G’和凝胶硬度呈极显著正相关(P<0.01),相关系数均高于0.849,说明加热引起了蛋白质分子展开、聚集、并导致蛋白质分子胶凝、凝胶的G’及硬度显著变化。α-螺旋、β-折叠含量与凝胶的弹性和T22之间相关性不显著(P>0.05)。综合分析加热温度对α-螺旋含量、β-折叠含量和G’的影响,发现加热温度超过40℃时,加热同时导致肌原纤维分子展开、分子间聚集和胶凝;并发现展开后的肌原纤维蛋白分子部分重排成β-折叠结构是导致凝胶G’增加的关键因素;分析加热温度对β-折叠含量和凝胶硬度的影响,发现β-折叠结构含量增加也是导致凝胶硬度增加的关键因素。【结论】肌原纤维蛋白从30℃加热到80℃时,其α-螺旋含量显著下降,β-折叠含量显著提高,加热引起蛋白质二级结构发生重大变化,肌原纤维蛋白在42℃开始胶凝。凝胶硬度在75℃时达最大值51.4 g。加热温度、β-折叠含量与凝胶的G’和硬度呈极显著正相关,加热过程中β-折叠含量增加是导致凝胶G’和硬度增加的关键。 . 【目的】研究加热温度对肌原纤维蛋白二级结构和凝胶特性的影响,并探讨肌原纤维蛋白二级结构与凝胶特性之间的内在关系。【方法】将活AA鸡20只(40日龄)屠宰,取鸡胸肉在-18℃下储存,用于提取鸡胸肉肌原纤维蛋白。用圆二色谱(CD)研究加热过程中肌原纤维蛋白二级结构(α-螺旋,β-折叠,β-转角和无规则卷曲)的变化;使用流变仪测定加热温度对肌原纤维蛋白的流变性质参数储能模量G’和相位角正切值 (Tanδ)的影响;将肌原纤维蛋白在不同温度下制备成凝胶,运用质构仪研究成胶温度对凝胶硬度和弹性的影响;用低场核磁共振仪(NMR)测定不同加热温度下成胶的肌原纤维蛋白凝胶的弛豫时间T2,以此研究不同温度下制得凝胶的水分布特性。利用SPSS17.0对所得的数据进行相关性分析等处理,以便阐明加热温度与肌原纤维蛋白二级结构及其凝胶特性的关系。【结果】加热温度显著影响肌原纤维蛋白的二级结构。随着加热温度升高,肌原纤维蛋白二级结构中α-螺旋含量逐渐降低。在30℃时α-螺旋含量为95.77%,加热温度在30—40℃以及70—80℃之间时α-螺旋含量变化很小,在40—70℃之间显著下降(P<0.05),到80℃时下降到45.05%。 β-折叠含量在30—45℃之间随温度上升缓慢增加,在40—70℃之间显著增加(P<0.05),超过70℃后含量仅略有增加;在30—80℃加热范围内,β-折叠含量从0.20%增加到12.65%。α-螺旋含量降低代表蛋白质分子展开程度增加,而β-折叠含量增加代表蛋白质分子间聚集程度增加。加热温度影响肌原纤维蛋白的流变性、质构特性和水分布特性。G’开始增加时的温度为42℃,表明肌原纤维蛋白在此温度下开始胶凝。在42—50℃之间,G’迅速增加到峰值177 Pa,之后G’迅速下降 (50—55℃),在55—75℃范围内G’再次快速增加;肌原纤维蛋白凝胶硬度在40—75℃内随温度上升而显著增大,在75℃时硬度达到最大值51.4 g。凝胶弹性在55℃达到弹性最大值0.754;在NMR图谱中T2有 3个峰,其中T22表示不可移动水,肌原纤维蛋白成胶温度在40—60℃内的凝胶T22值随加热温度上升从403.7 ms降到265.6 ms,即T22向快弛豫方向移动,表明随着温度的升高,水分子移动性降低。经相关性分析发现,加热温度、β-折叠含量与凝胶G’和凝胶硬度呈极显著正相关(P<0.01),相关系数均高于0.849,说明加热引起了蛋白质分子展开、聚集、并导致蛋白质分子胶凝、凝胶的G’及硬度显著变化。α-螺旋、β-折叠含量与凝胶的弹性和T22之间相关性不显著(P>0.05)。综合分析加热温度对α-螺旋含量、β-折叠含量和G’的影响,发现加热温度超过40℃时,加热同时导致肌原纤维分子展开、分子间聚集和胶凝;并发现展开后的肌原纤维蛋白分子部分重排成β-折叠结构是导致凝胶G’增加的关键因素;分析加热温度对β-折叠含量和凝胶硬度的影响,发现β-折叠结构含量增加也是导致凝胶硬度增加的关键因素。【结论】肌原纤维蛋白从30℃加热到80℃时,其α-螺旋含量显著下降,β-折叠含量显著提高,加热引起蛋白质二级结构发生重大变化,肌原纤维蛋白在42℃开始胶凝。凝胶硬度在75℃时达最大值51.4 g。加热温度、β-折叠含量与凝胶的G’和硬度呈极显著正相关,加热过程中β-折叠含量增加是导致凝胶G’和硬度增加的关键。 |
| [16] | . A method developed to measure water-holding properties of protein gels provides high precision and expanded analysis. A cylindrical gel sample was placed in an inner cell (which at its base contained a filter membrane) of a commercial microfiltration unit. The unit was then spun in a horizontal rotor microcentrifuge. Rates of water loss and degree of compression were determined. The rate of water loss could be modeled as the summation of two exponential decay functions. Degree of compression correlated with held-water. Gel rehydration ability and microstructure were used to study effects of centrifugal compression on gel structure. |
| [17] | . |
| [18] | . ABSTRACT Alpha-lactalbumin and beta-lactoglobulin solutions [15 % (w/v) in D2O, pD 6.8, 20 mM NaCl] were heated at 50, 70, or 90-degrees-C for 30, 60, or 90 min. Only the samples heated at 90-degrees-C formed transparent gels. The amide I' and amide III' bands of the Raman spectra implicated an increase of beta-sheet structure with a simultaneous decrease of helical structure in heated alpha-lactalbumin, while an increase of beta-sheet with a simultaneous decrease of turn structure was suggested in heated beta-lactoglobulin. Changes in other regions of the Raman spectra could be interpreted as changes in disulfide conformation as well as in microenvironment around several amino acid residues, i.e., Trp, Tyr, and His. These changes were observed after heating at 70 and 90-degrees-C but were more intense in the gelled (90-degrees-C) than in the ungelled samples and in beta-lactoglobulin than in alpha-lactalbumin. These observations are in accord with the fact that beta-lactoglobulin forms gels much more easily than alpha-lactalbumin. |
| [19] | . Raman spectroscopy can be a useful tool to probe protein structure in solid and liquid food systems. Bands in the Raman spectrum arising from amide I, amide III and skeletal stretching modes of peptides and proteins are useful for characterizing backbone conformation, including the estimation of secondary structure fractions. Bands attributed to various stretching or bending vibrational modes of functional groups of amino-acid residues can be used to monitor the environment around these side-chains. In particular, valuable information may be obtained on SS or SH groups of cystinyl or cysteinyl residues, CH groups of aliphatic residues, and aromatic rings of tryptophanyl, tyrosinyl and phenylalanyl residues. One important parameter distinguishing Raman spectroscopy from many other spectroscopic methods is its applicability to systems containing high concentrations of proteins, which is critical for the investigation of structural changes during processes such as coagulum or gel formation. Thus, changes in both intramolecular and intermolecular interactions can be studied. In this chapter, examples are presented on the application of Raman spectroscopy to investigate protein structure as a function of processing, such as heating, drying, salt addition or homogenization with lipids, which may be important to correlate with protein functionality in food systems. |
| [20] | . |
| [21] | . Susceptibility of amino acids in myofibrillar protein isolate (MPI) exposed to three oxidizing matrixes commonly encountered in muscle foods was compared. MPI suspensions (20 mg protein/mL) in 15 mM piperazine- N,N bis(2-ethane sulphonic acid) buffer (pH 6.0) were oxidized with an iron-catalyzed oxidizing system (IOS, 0.01 mM FeCl 3, 0.1 mM ascorbic acid, 0.0–10.0 mM H 2O 2), a lipid-oxidizing system (LOS, 0.0–10.0 mM linoleic acid, 3750 units of lipoxidase/mL), or a metmyoglobin (MetMb) oxidizing system (MOS, 0.0–0.5 mM H 2O 2/MetMb) for 24 h at 4 °C. Changes were quantitatively analyzed by determining amino acids on a reverse-phase liquid chromatographic (LC) system. In IOS, the amount of cysteine, methionine and tyrosine decreased ( P < 0.05) with increasing [H 2O 2]. In LOS, only cysteine and methionine were lowered at increasing linoleic acid concentrations. In MOS, the quantity of alanine, cysteine, glycine, histidine, leucine and lysine, as well as the total amount of amino acids were significantly reduced at high concentrations of MetMb/H 2O 2. The results suggest that under typical meat processing conditions, iron- and metmyoglobin-catalyzed reactions play a major role in the oxidation of amino acids in muscle proteins. |
| [22] | . |
| [23] | |
| [24] | . A detailed mathematical model for flocculation of colloidal suspensions in presence of salts and polymers is described and validated. In former case, the classical DLVO theory, which accounts for relevant variables such as pH and salt concentration, is incorporated into a geometrically sectioned discrete population balance model. For processes involving polymers, flocculation via simple charge neutralization is modeled using a modified DLVO theory in which the effect of adsorbed polymer layers on van der Waals attraction is included. The fractal dimension of aggregates is obtained by dynamic scaling of experimental data for time evolution of mean aggregate size. The particle surface potential is assumed to be approximately equal to the zeta potential. The model predictions are in close agreement with experimental results for flocculation of colloidal hematite suspensions in the presence of KCl and polyacrylic acid at different concentrations. In particular, given values of model parameters, e.g., Hamaker constant, fractal dimension, surface potential, and thickness of adsorbed polymer layer, the model can realistically describe the kinetics of flocculation by a simple charge neutralization mechanism and track the evolution of floc size distribution. Representative examples of sensitivity of the flocculation model to perturbations in surface potential and fractal dimension and to modification in the DLVO theory for polymer-coated particles are included. |
| [25] | . The aim of this lecture is to impart some insight into the mechanisms involved in gel formation. These mechanisms are determined by the balance between forces underlying chain-chain and chain-solvent interactions. Mechanisms and conformations favored by either of these interactions are listed in Table I. |
| [26] | . |
| [27] | . The effects of high intensity ultrasound (HIU) modification (power: 200, 400, 600, 800 and 1000W, corresponding to intensities of: 88, 117, 150, 173 and 193Wcm612) on the physicochemical properties of myofibrillar protein (MP) and water in MP gel were studied. As the HIU power increased, the solubility, surface hydrophobicity and absolute value of the zeta potential increased, while turbidity and total sulfhydryl (SH) group content decreased. The G′ and G″ of MP during thermal gelation decreased as ultrasound intensity increased due to greater protein denaturation. Low field NMR data showed that bound water (T2b) had lower water mobility and was more closely associated with proteins in HIU treated MP gel, while the proportions of immobilized water (PT21) and free water (PT22) had their maximum and minimum value, respectively, at 600W. Moderate HIU (≦600W) treatments had denser and uniform gel microstructure, which improved water holding capacity (WHC) of the gels, while stronger HIU (>600W) treatments had larger and irregular gel microstructures, accompanied by decreased WHC. |
| [28] | . ABSTRACT Ground fillets of frozen Alaska pollock (Theragra chalcogramma), a low-fat fish, and Pacific mackerel (Pneumataphorus japanicus), a high-fat fish, heated for 20 min at temperature intervals ranging from 40 to 115 °C showed a linear decrease in the content of -SH (sulfhydryl) groups and a concomitant increase in the content of S-S (disulfide) bonds from 50 to 115 °C. At 95 °C, the reaction was rapid and had reached an equilibrium after 20 min. These experiments indicate that temperatures higher than 50 °C are required for oxidative transformation of -SH groups to S-S bonds. Heating at 115 °C caused a loss in cystine plus cysteine. Heating to 95 °C and drum-drying caused the formation of S-S bonds from -SH groups and reduced protein and amino acid digestibility when fed to rainbow trout (Salmo gairdneri) as compared with the raw fish protein. Freeze-drying did not affect digestibility and no formation of S-S bonds was found. It is postulated that heat-induced S-S cross-linking from -SH oxidation causes a reduction in protein and amino acid digestibility in drum-dried samples. The effect of heating on digestibility was greater in the low-fat pollock than in the high-fat mackerel. |
| [29] | . In this short review we discuss the role of cysteine residues and cystine bridges for the functional aggregation of food proteins. We evaluate how formation and cleavage of disulphide bonds proceeds at a molecular level, and how inter- and intramolecular disulfide bonds can be detected and modified. The differences between heat-, high-pressure-, and denaturant-induced unfolding and aggregation are discussed. The effect of disulphide bonding between aggregates of proteins and protein mixtures on the functional macroscopic properties of space filling networks in protein gels is briefly presented. |
| [30] | . ABSTRACT Myofibrils isolated under different antioxidative conditions from three groups of chicken muscle [breast (Bctrl), leg (L), and breast with its iron and fat content adjusted to the level of leg muscle (Badj)] exhibited complex viscoelastic characteristics during thermal gelation. Without antioxidants in myofibril isolation buffer, Badj myofibrils had decreased storage moduli (G‘) and loss moduli (G‘‘) compared with Bctrl myofibrils, but these values were much higher than those of L myofibrils. Lipid oxidation (TBA value) was inhibited in myofibril samples prepared using antioxidant buffers. Antioxidant treatments increased G‘ and G‘‘ of Badj myofibrils to a level comparable to G‘ and G‘‘ of the Bctrl counterparts. Different distributions of prooxidants in chicken white and red myofibrillar proteins were not an apparent major factor causing functional discrepancies between the two types of proteins. The discrepancies could be ascribed largely to fiber type-dependent myosin isoforms and polymorphism. Keywords: Chicken muscle; fiber type; oxidation; protein gelation; rheology |
| [31] | . . |
| [32] | . 为研究羟自由基(·OH)氧化体系中肌原纤维蛋白(myofibrillar protein,MP)氧化及其凝胶特性的变化,试验分析了羟自由基氧化体系中不同H2O2浓度对蛋白氧化程度及MP凝胶白度、持水力、质构特性(texture profiles analysis,TPA)与弹性模量等特征指标的影响。结果表明:随H2O2浓度的增加,MP中羰基值上升,蛋白氧化程度加剧,凝胶白度、保水性、硬度、咀嚼性及弹性模量则与H2O2浓度呈显著负相关。与对照组相比,当H2O2浓度增加至20 mmol/L时,羰基含量增加至2.82 nmol/mg蛋白(p<0.05),凝胶白度、持水性及硬度则分别下降了2.83%、14.65%及52.77%(p<0.05)。扫描电镜(scanning electron micrograph,SEM)观察表明,MP氧化导致凝胶微观结构破坏,形成空隙较大且分布不均的网络;低场核磁共振分析(nuclear magnetic resonance,NMR)结果显示,随H2O2浓度的增加,MP凝胶中的一部分不易流动水"态变"为自由水,凝胶持水力降低。综上所述,·OH氧化体系中肌原纤维蛋白氧化会影响其凝胶形成,破坏蛋白凝胶结构,降低凝胶功能,这为肉类生产加工过程中蛋白氧化控制提供理论参考。 . 为研究羟自由基(·OH)氧化体系中肌原纤维蛋白(myofibrillar protein,MP)氧化及其凝胶特性的变化,试验分析了羟自由基氧化体系中不同H2O2浓度对蛋白氧化程度及MP凝胶白度、持水力、质构特性(texture profiles analysis,TPA)与弹性模量等特征指标的影响。结果表明:随H2O2浓度的增加,MP中羰基值上升,蛋白氧化程度加剧,凝胶白度、保水性、硬度、咀嚼性及弹性模量则与H2O2浓度呈显著负相关。与对照组相比,当H2O2浓度增加至20 mmol/L时,羰基含量增加至2.82 nmol/mg蛋白(p<0.05),凝胶白度、持水性及硬度则分别下降了2.83%、14.65%及52.77%(p<0.05)。扫描电镜(scanning electron micrograph,SEM)观察表明,MP氧化导致凝胶微观结构破坏,形成空隙较大且分布不均的网络;低场核磁共振分析(nuclear magnetic resonance,NMR)结果显示,随H2O2浓度的增加,MP凝胶中的一部分不易流动水"态变"为自由水,凝胶持水力降低。综上所述,·OH氧化体系中肌原纤维蛋白氧化会影响其凝胶形成,破坏蛋白凝胶结构,降低凝胶功能,这为肉类生产加工过程中蛋白氧化控制提供理论参考。 |
| [33] | . Acrolein (CH2[Note: See the image of page 4882 for this formatted text] ==CH--CHO) is known as a ubiquitous pollutant in the environment. Here we show that this notorious aldehyde is not just a pollutant, but also a lipid peroxidation product that could be ubiquitously generated in biological systems. Upon incubation with BSA, acrolein was rapidly incorporated into the protein and generated the protein-linked carbonyl derivative, a putative marker of oxidatively modified proteins under oxidative stress. To verify the presence of protein-bound acrolein in vivo, the mAb (mAb5F6) against the acrolein-modified keyhole limpet hemocyanin was raised. It was found that the acrolein-lysine adduct, N -(3-formyl-3,4-dehydropiperidino)lysine, constitutes an epitope of the antibody. Immunohistochemical analysis of atherosclerotic lesions from a human aorta demonstrated that antigenic materials recognized by mAb5F6 indeed constituted the lesions, in which intense positivity was associated primarily with macrophage-derived foam cells and the thickening neointima of arterial walls. The observations that (i) oxidative modification of low-density lipoprotein with Cu2+generated the acrolein-low-density lipoprotein adducts and (ii) the iron-catalyzed oxidation of arachidonate in the presence of protein resulted in the formation of antigenic materials suggested that polyunsaturated fatty acids are sources of acrolein that cause the production of protein-bound acrolein. These data suggest that the protein-bound acrolein represents potential markers of oxidative stress and long-term damage to protein in aging, atherosclerosis, and diabetes. |
| [34] | . Malondialdehyde (MDA), a naturally occurring dialdehyde produced in the membrane lipid peroxidation, is known to react with lysine residues of proteins, but the MDA-lysine adducts generated in the proteins have not been characterized adequately. In the present study, we provide evidence that the enaminal-type MDA-lysine adduct, N(epsilon)-(2-propenal)lysine, is formed in human low-density lipoprotein (LDL) upon reaction with MDA or Cu2+. We found that the incubation of N(alpha)-acetyllysine with MDA generated N(alpha)-acetyl-N(epsilon)-(2-propenal)lysine as the predominant product. In addition, a polyclonal antiserum raised against the MDA-modified protein was found to contain antibody populations that could be purified by affinity gel prepared by covalent attachment of N(alpha)-acetyl-N(epsilon)-(2-propenal)lysine. It was concluded that the affinity-purified anti-N(epsilon)-(2-propenal) lysine antibody was highly specific to the enaminal derivative of both lysine residues and phosphatidylethanolamine, based on the observations that (i) MDA was the only aldehyde which generated immunoreactive materials in proteins; (ii) among structurally defined MDA-lysine adducts tested, the antibody recognized the enaminal adduct only; and (iii) immunoreactivity to N-(2-propenal)serine was still significant but much weaker than its reactivity to N-(2-propenal)ethanolamine. Furthermore, analysis of antibody recognition sites with a variety of N-(2-propenal)alkylamines revealed that the mono-specific antibody recognized the N-2-propenal-N-ethyl moiety [-(CH2)2-NH-CH=CH-CHO] of enaminal adducts. Determination by a competitive enzyme-linked immunosorbent assay demonstrated that N(epsilon)-(2-propenal)lysine accounted for 33.7 and 3.1% of the lysine residues that disappeared during in vitro incubation of LDL with MDA and Cu2+, respectively. These results suggest that N(epsilon)-(2-propenal)lysine represents a major form of MDA covalently attached to proteins. |
| [35] | . A review of published reports on the mechanism of protein oxidation is presented. The oxidative modifications of amino acid residues and changes in the polypeptide backbone, which lead to alterations in the physicochemical properties of proteins, are discussed with emphasis on changes in structural attributes of proteins and solubility. The nutritional quality of oxidized proteins is assessed. ... |
| [36] | . |
| [37] | [D]. [D]. |
| [38] | . Hand-deboned samples of dark ground meat from 6-week-old broilers were treated with varying combinations of FeSO4, ascorbic acid, and α-tocopherol. Mixtures were incubated at 37 °C for 0, 50, 100, and 200 min. Lipid oxidation was determined by measuring thiobarbituric acid reactive substances. Myosin was isolated from incubated samples, and denaturation was determined by measuring intrinsic tryptophan fluorescence, 1-anilino-8-naphthalenesulfonate binding, and Ca2+-ATPase activity. Myosin denaturation during heating was positively correlated with lipid oxidation. Exogeneously added α-tocopherol reduced lipid oxidation and decreased myosin denaturation during heating. Keywords: α-Tocopherol; myosin; lipid oxidation; chicken meat |
| [39] | . BACKGROUND: Protein oxidation results in covalent modification of structure and deterioration of functional properties of target protein. Oxidation extent of soy protein was affected by the content and type of lipid peroxidation (LPO) products in defatted soybean flours during storage and processing. Malondialdehyde (MDA) was selected as a secondary byproduct of LPO to investigate the effects of oxidative modification of LPO-derived reactive aldehyde on soy protein structure. RESULTS: MDA reacted with -amino and sulfhydryl groups of soy protein, and resulted in an increase in protein carbonyl groups but a decrease in sulfhydryl/disulfide, free amines and lysine. The decrease in solubility, surface hydrophobicity and intrinsic fluorescence indirectly indicated that MDA induced soy protein aggregation, and results of high-performance size-exclusion chromatography directly showed that gradual aggregation of soy protein was induced by increasing concentration of MDA. Results of electrophoresis indicated that MDA caused soy protein aggregation, and non-disulfide covalent bonds were involved in aggregate formation. CONCLUSION: The results showed that sensitivity of soy protein was related to MDA concentration. Soy protein gradually aggregated with increase of MDA concentration; -conglycinin was more sensitive to MDA modification than glycinin. Copyright 漏 2009 Society of Chemical Industry |
| [40] | . |
| [41] | . Abstract Structural changes of myofibrillar proteins from raw pork muscle and Cantonese sausage at different processing periods were elucidated using Raman spectroscopy. Fourier deconvolution combined with iterative curve fitting were used to analyze the amide I Raman band. Results from amide I, amide III, and C-C stretching vibrations in 890-1060 cm(-1) showed that 02±-helix decreased accompanied by an increase in 0205-sheet structure during the first 18 h, and a rebuilding process of secondary structures was observed at the rest stage due to proteolysis. The hierarchical cluster analysis results of amide I and amide III confirmed this rebuilding process. Changes in a doublet near 850 and 830 cm(-1) suggested that some tyrosine residues became buried in a more hydrophobic environment due to intermolecular interactions. Raman spectra in the 2855-2940 cm(-1) region suggested that the environment of aliphatic side chains might have been changed during the final stage and further confirmed above rebuilding process. |
| [42] | . Free radicals formed during the reaction of H 2 O 2 and metmyoglobin in the presence of bovine serum albumin (BSA) were investigated using freeze quench and spin-trap ESR spectroscopy. Increasing concentrations of BSA (0–300 μ M) resulted in drastic changes in the characteristic freeze quench ESR signal of H 2 O 2 -activated metmyoglobin (perferryl protein radical) under physiological conditions (pH = 7.4; I = 0.16). The radical species formed during reaction of 100 μ M H 2 O 2 , 100 μ M metmyoglobin, and 200 μ M BSA have half-lives of approximately 13 min at 25°C, in contrast to the perferryl protein radical that has a half-life of approximately 28 s at 25°C. The radical species formed in the presence of BSA were reactive towards ascorbate, glutathione, cysteine, and tyrosine. Substitution of BSA with defatted BSA, γ -globulin or β -lactoglobulin also resulted in formation of long-lived free radical species (half-lives: 13–18 min); however, the ability to form these was dependent of the specific protein and decreased in the following order: BSA > defatted BSA > γ -globulin > β -lactoglobulin. The spin-trap α -phenyl- tert -butylnitrone (PBN) showed the presence of transient protein radical species formed in the reaction between MMb, H 2 O 2 , and BSA. Transient radical species that could be proposed as intermediates in the formation of the long-lived protein radicals detected by freeze-quench ESR. Dityrosine was formed in the reaction between MMb, H 2 O 2 , and BSA, showing the involvement of tyrosine residues in the present reaction. The described chemical interaction between H 2 O 2 -activated myoglobin and other proteins have major consequences on future interpretations of the significance of the perferryl protein radical in biological systems where proteins are abundant. |
| [43] | . An overview of myoglobin-initiated lipid oxidation in simple model systems, muscle, and muscle-based foods is presented. The potential role of myoglobin spin and redox states in initiating lipid oxidation is reviewed. Proposed mechanisms for myoglobin-initiated lipid oxidation in muscle tissue (pH 7.4) and meat (pH 5.5) are evaluated with the purpose of putting forward general mechanisms explaining present observations regarding the catalytic events. |
| [44] | . Oxidant species are known to contribute to disease and dysfunction in biological systems. However, evidence has been progressively accumulating that demonstrates a more fundamental role for many oxidant species in the regulation of everyday function of healthy cells. Redox dependent signaling events involving the post-translational, oxidative modification of proteins has now been accepted as an important regulatory process, although the full extent of such mechanisms is yet to be determined. Some protein cysteinyl thiols are known to be susceptible to a number of redox-dependent modifications, including an interchange between the reduced thiol and several different oxidized disulfide states. Here, the role of oxidants as regulatory entities is reviewed, as are the many different ways protein disulfide formation can be analysed in complex protein mixtures. This includes an overview of many of the Proteomic strategies that can be used to identify proteins that form disulfides when pro-oxidizing conditions arise in cells, as well as related methods for studying intermediates that may precede disulfide formation. |
| [45] | . No Abstract available for this article. |
