 ,北京市农林科学院畜牧兽医研究所,北京100097
,北京市农林科学院畜牧兽医研究所,北京100097Virulence, E Gene Sequence and Antigenic Difference of 4 Duck Tembusu Virus Isolations
YANG ZhiYuan, DUAN HuiJuan, WANG XiaoLei, LIU LiXin, ZHAO JiCheng, PAN Jie, LIU YueHuan, LIN Jian ,Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agricultural and Forestry Sciences, Beijing 100097
,Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agricultural and Forestry Sciences, Beijing 100097通讯作者:
责任编辑: 林鉴非
收稿日期:2019-04-23接受日期:2019-06-28网络出版日期:2019-12-01
| 基金资助: |
Received:2019-04-23Accepted:2019-06-28Online:2019-12-01
作者简介 About authors
杨志远,E-mail:yangzy88@126.com

摘要
关键词:
Abstract
Keywords:
PDF (899KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
杨志远, 段会娟, 王小蕾, 刘立新, 赵际成, 潘洁, 刘月焕, 林健. 4株鸭坦布苏病毒的毒力、E基因序列和抗原差异性[J]. 中国农业科学, 2019, 52(23): 4406-4414 doi:10.3864/j.issn.0578-1752.2019.23.021
YANG ZhiYuan, DUAN HuiJuan, WANG XiaoLei, LIU LiXin, ZHAO JiCheng, PAN Jie, LIU YueHuan, LIN Jian.
0 引言
【研究意义】鸭坦布苏病毒(duck Tembusu virus, DTMUV)属于黄病毒科黄病毒属,2010年春季开始在浙江、江苏、山东、河北和北京等地区暴发,以鸭产蛋量下降为主要临床特征[1,2,3]。疫病暴发初期,研究人员根据临床症状和主要病理变化,曾将该病命名为鸭出血性卵巢炎[4]、类减蛋综合征[5]和鸭病毒性脑炎[6],2011年首届“水禽疫病防控研讨会”将该病统一命名为“鸭坦布苏病毒病”[7]。北京鸭、樱桃谷鸭、金定鸭、麻鸭、康贝尔鸭和鹅等禽类均可发病[8],目前已成为养鸭生产中的一种地方流行性疫病,几乎每年都会给养鸭业造成明显的经济损失。开展鸭坦布苏病毒的流行情况调查和遗传变异分析,可为该病的有效防控提供科学依据。【前人研究进展】Tembusu病毒首次于1968年在马来西亚Sarawak地区的蚊子体内分离到[9],随后分别于1982和1992年在泰国北部日本脑炎流行地区的蚊子体内发现[10,11]。2000年,有研究报道一种与Tembusu病毒核酸的同源性为92%的Sitiawan病毒,感染鸡后能够引起鸡生长发育受阻[12]。引起我国鸭群发病的坦布苏病毒与Bagaza病毒的核酸高度同源[13]。万春和等在鸭坦布苏病毒病灭活疫苗、活疫苗和活载体疫苗研制方面做了大量的研究工作,试验结果均表明疫苗有效[14,15,16,17,18,19,20,21]。在使用疫苗的选择压力下,鸭坦布苏病毒的遗传变异情况如何,目前已有多名研究人员对流行的鸭坦布苏病毒株主要进行了E基因的序列分析[22,23,24,25,26],因为囊膜蛋白(E蛋白)是黄病毒最大的结构蛋白和主要的包膜蛋白,是宿主抗感染免疫的重要保护性抗原[27]。结果表明,各分离株之间没有显著性差异,但没有直接数据表明抗原性有无差异。【本研究切入点】自2010年鸭坦布苏病毒病暴发以来,虽然曾一度呈现零星散发,但从2012年起该病在我国主要养鸭地区再次大面积流行[28]。该病流行期间,病毒的生物学特性、基因序列和抗原性是否发生变异,尚未见有系统的研究报道。为了回答这些问题,我们利用2010至2016年间分离自山东、河北、广西、安徽、河南等不同地区的DTMUV开展了毒力、E蛋白基因和抗原差异性的比较研究,为DTMUV分子流行病学的研究提供参考信息,为该病的防控和疫苗的研制奠定理论基础。【拟解决的关键问题】鸭坦布苏病毒的毒力是否发生了变化,抗原性是否改变。1 材料与方法
本试验于2015年2月至2017年8月在北京市农林科学院畜牧兽医研究所完成。1.1 试验材料
1.1.1 毒株 DTMUV-SD株(2010)、DTMUV-HB株(2011)、DTMUV-GX1株(2012)、DTMUV-BJ株(2013)、DTMUV-AH株(2014)、DTMUV-GX2株(2015)、DTMUV-HN株(2015)、DTMUV-AX株(2016)由北京市农林科学院畜牧兽医研究所动物疫病研究室分离鉴定、保存。1.1.2 实验动物 180日龄健康DTMUV抗体阴性北京鸭和10日龄易感鸭胚,购自北京南口北京鸭育种中心,试验鸭及种鸭群未经DTMUV疫苗免疫且无DTMUV疫病流行史;SPF鸡胚购自北京梅里亚维通实验动物有限公司。
1.1.3 细胞 C6/36细胞,123代,购自中国典型培养物保藏中心(武汉大学),北京市农林科学院畜牧兽医研究所动物疫病研究室增殖扩繁。
1.1.4 试剂 0.5%乳汉液、0.33%鹅红细胞悬液等,均由北京市农林科学院畜牧兽医研究所动物疫病研究室配制;MEM培养基,购自Hyclone公司;胎牛血清(FBS),购自Life Technology公司;TaKaRa MiniBEST Viral RNA/DNA Extraction Kit Ver.5.0、TaKaRa RNA PCR Kit (AMV) Ver.3.0、均购自TaKaRa公司。
1.2 试验方法
1.2.1 病毒的增殖及鸡胚半数致死量(ELD50)的测定 将DTMUV-HB、DTMUV-AH、DTMUV-GX1、DTMUV-GX2 4株病毒用无菌0.5%乳汉液1﹕200倍稀释。将稀释的毒种经尿囊腔途径接种10日龄易感鸭胚,每胚0.1 mL,置36—37℃孵育,收获尿囊液。-70℃保存备用。4株病毒尿囊液分别用无菌0.5%乳汉液作10倍系列稀释至10-6,每个稀释度经卵黄囊接种6日龄SPF鸡胚5枚,每胚0.1 mL,置36—37℃孵育,观察和记录接种后24—168 h死亡鸡胚,采用K?rber法计算ELD50。1.2.2 人工感染鸭试验及病毒阳性血清的制备 共40只180日龄健康北京鸭,经检测DTMUV抗体呈阴性,随机分成5组,每组8只,1组健康对照,肌肉注射PBS 0.5 mL/只,其余4组各用1株试验用毒株进行人工感染,胸部肌肉接种病毒液0.5 mL/只(含100个ELD50),观察临床症状;攻毒后2 d采血分离血清,按照文献[29]进行病毒分离;攻毒后8 d每组随机取5只进行剖检,观察病理变化。剩余试验鸭在攻毒后14 d,使用相同剂量和相同毒株再次接种加强免疫,接种后15 d采血,分离血清,分别制备成相应毒株的阳性血清,-30℃保存备用。
1.2.3 分离毒株E基因分析 利用TaKaRa MiniBEST Viral RNA/DNA Extraction Kit Ver.5.0提取7株分离病毒株尿囊液病毒RNA,参照GenBank已发布DTMUV的E基因序列设计一对引物,引物序列为DTMUV-EF:5′-ttcagctgtctggggatgc-3′,DTMUV-ER:5′-cggcattgacatttactgcc-3′,由生工生物工程(上海)股份有限公司合成。通过TaKaRa RNA PCR Kit (AMV) Ver.3.0进行RT-PCR扩增,目的片段大小为1 506 bp,PCR产物送测序后应用DNAstar ( Version 5. 07) 分析软件比较不同时间不同地区的鸭坦布苏病毒E蛋白的核苷酸和氨基酸序列相似性,并绘制系统发育进化树。
1.2.4 分离毒株交叉血凝抑制试验 参照文献[30],将DTMUV-HB、DTMUV-AH、DTMUV-GX1、DTMUV- GX2 4株病毒接种C6/36细胞后,获得了对0.33%鹅红细胞的血凝特性,利用实验室建立的鸭坦布苏病毒血凝抑制试验操作方法,分别测定了4株病毒阳性血清对4株病毒的HI效价。
1.2.5 分离毒株细胞交叉中和试验 将C6/36细胞接种96孔微量细胞培养板,利用C6/36细胞进行DTMUV-HB、DTMUV-AH、DTMUV-GX1、DTMUV- GX2 4株病毒株的TCID50测定,将毒种用MEM培养基作10倍系列稀释,取10-1、10-2、10-3、10-4、10-5 稀释度,每个稀释度分别接种5孔,每孔100 μL,置28.5℃作用2 h,然后弃去上清液,加入含2%胎牛血清的MEM培养液100 μL,28.5℃培养120 h。每孔取25 μL上清液测定对0.33%鹅红细胞的血凝性,以75%以上的凝集判为HA阳性。记录接种孔中HA阳性的孔数,采用Reed-Muench法计算。根据4株病毒的病毒含量,采用固定病毒稀释血清法,将4种免疫阳性血清56℃灭活30 min,作2-1—2-6稀释,分别与等量的含200个TCID50的4株病毒液进行交叉混合,37℃作用1 h后,接种于96孔C6/36单层细胞培养板,每孔100 μL,每个稀释度接种5孔,28℃作用2 h,弃去,加入含2%胎牛血清的维持液100 μL。同时设置含100个TCID50、1个TCID50和0.1个TCID50的病毒对照,空白细胞对照,血清对照,继续培养120 h,测定每孔对0.33%鹅红细胞的血凝性,以75%以上的凝集判为HA阳性,按照Reed-Muench公式计算血清中和效价。
1.2.6 抗原相关性判定标准 根据公式R=$\sqrt {r1·r2}$计算抗原间的相关性[31],其中,R:2毒株间抗原性差异;r1:血清A对病毒B的中和效价/血清A对病毒A的中和效价;r2 :血清B对病毒A的中和效价/血清B对病毒B的中和效价。
如果R=1,表明2株间抗原相同;如果0.67≤R≤1.5,表明2株间抗原性无明显差异;如果0.5≤R<0.67,表明2毒株间抗原性有小的差异;如果R<0.5,表明2毒株间抗原性有明显差异;R值越小,抗原性差异越大。
2 结果
2.1 病毒增殖与ELD50的测定
DTMUV-HB、DTMUV-AH、DTMUV-GX1、DTMUV-GX2 4株病毒株接种鸭胚后均在48—72 h内死亡,死亡鸭胚发育矮小,收获的尿囊液透明清亮。4株分离毒株测定的病毒含量分别为104.9、104.7、104.9和105.3 ELD50/0.1mL。2.2 DTMUV分离株对北京鸭的毒力
DTMUV-HB、DTMUV-AH、DTMUV-GX1、DTMUV-GX2 4株病毒以相同攻毒剂量(含100个ELD50)人工感染试验鸭3 d后,攻毒鸭临床上均出现采食量下降、一过性精神沉郁、体重减轻、拉绿色稀便等症状,健康对照鸭精神状态良好,未见明显异常。攻毒后2 d,4株病毒攻毒鸭的病毒分离阳性率均在85%以上,健康对照鸭未分离到病毒,结果见表1。攻毒后8 d,剖检攻毒鸭均出现明显的卵巢或输卵管萎缩、出血、变形等病变,健康对照鸭未见明显异常。除剖检鸭外,剩余试验鸭均存活。结果表明,4株试验用DTMUV对北京鸭的毒力没有明显差异。Table 1
表1
表1DTMUV人工感染北京鸭试验
Table 1
| 动物数量(只) Animal | 攻毒用毒株 Virus | 攻毒途径及剂量 Routes and dose | 病毒分离阳性率 Positive rate of virus isolation |
|---|---|---|---|
| 8 | DTMUV-HB | 肌肉注射 Intramuscular injection 0.5mL (100ELD50) | 100%(8A/8B) |
| 8 | DTMUV-AH | 87.5%(7/8) | |
| 8 | DTMUV-GX1 | 87.5%(7/8) | |
| 8 | DTMUV-GX2 | 100%(8/8) | |
| 8 | / | / | 0%(0/8) |
新窗口打开|下载CSV
2.3 DTMUV分离株E基因分析
提取DTMUV-SD、DTMUV-HB、DTMUV-GX1、DTMUV-BJ、DTMUV-AH、DTMUV-GX2、DTMUV- HN、DTMUV-AX等8株病毒核酸,并进行RT-PCR扩增,PCR产物经凝胶电泳均看到约1 500 bp大小的目的片段,结果如图1。送TaKaRa测序与国内其他DTMUV比较后发现,核苷酸序列相似性为95.7%—100%,推导氨基酸序列相似性在98.2%以上。遗传进化分析表明,7株DTMUV与近几年登录在GenBank的鸭坦布苏病毒在系统进化上共同构成了一个进化分支,图2表明目前在我国鸭群中流行的DTMUV的E基因未出现明显的遗传变异。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图18株DTMUV的E基因RT-PCR扩增
M: DL2000 Marker; 1: DTMUV-SD; 2: DTMUV-HB; 3: DTMUV-GX1; 4: DTMUV-BJ; 5: DTMUV-AH; 6: DTMUV-GX2; 7: DTMUV-HN; 8: DTMUV-AX; 9: Negative control
Fig. 1RT- PCR amplification of E genes of 8 DTMUV isolates
图2
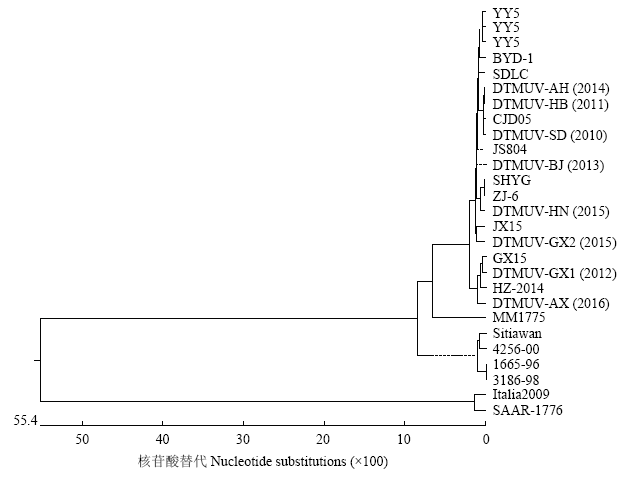 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2DTMUV分离株E基因进化树
Fig. 2Phylogenetic analysis of E gene of DTMUV isolates
2.4 DTMUV分离毒株交叉血凝抑制试验
分别测定4种病毒株阳性血清对4株DTMUV抗原的交叉HI效价,每种病毒阳性血清有3份,取几何平均值,结果见表2。按照抗原相关系数R值的计算方法计算4毒株间的抗原性差异,详见表3。4毒株间的HI抗原性差异R值在0.79—1.12之间,表明没有明显的抗原性差异。Table 2
表2
表24株DTMUV的HI交叉试验
Table 2
| 毒株 Virus | 4株DTMUV阳性血清HI效价 Positive sera HI titer of 4 DTMUV isolates | |||
|---|---|---|---|---|
| DTMUV-HB | DTMUV-AH | DTMUV-GX1 | DTMUV-GX2 | |
| DTMUV-HB | 1:806.3 | 1:806.3 | 1:806.3 | 1:640.0 |
| DTMUV-AH | 1:160.0 | 1:201.6 | 1:201.6 | 1:100.8 |
| DTMUV-GX1 | 1:127.0 | 1:127.0 | 1:100.8 | 1:100.8 |
| DTMUV-GX2 | 1:201.6 | 1:254.0 | 1:254.0 | 1:201.6 |
新窗口打开|下载CSV
Table 3
表3
表34株DTMUV的抗原差异性R值
Table 3
| R值 R value | DTMUV-HB | DTMUV-AH | DTMUV-GX-1 | DTMUV-GX-2 |
|---|---|---|---|---|
| DTMUV-HB | ** | 0.79 | 0.91 | 1.20 |
| DTMUV-AH | 0.89 | ** | 0.82 | 0.91 |
| DTMUV-GX-1 | 1.12 | 1.12 | ** | 0.93 |
| DTMUV-GX-2 | 0.89 | 0.79 | 1.12 | ** |
新窗口打开|下载CSV
2.5 DTMUV分离毒株细胞交叉中和试验
2.5.1 TCID50测定结果 DTMUV-HB、DTMUV-AH、DTMUV-GX1、DTMUV-GX2 4株病毒的TCID50测定结果分别为10-3.8/0.1mL、10-3.8/0.1mL、10-3.8/0.1mL、10-4.5/0.1mL。2.5.2 细胞交叉中和试验 不同毒株与阳性血清作用1 h后,接种细胞,培养168 h,观察记录细胞病变(通过测定HA效价进行最终判定),计算阳性血清对不同毒株的中和效价,结果见表4和图3。每种病毒阳性血清有3份,取几何平均值,按照抗原相关系数R值的计算方法计算4毒株间的抗原性差异,详见表3。结果表明,R值在0.79—1.20之间,4毒株间的没有明显的抗原差异性,与血凝抑制试验结果一致。
Table 4
表4
表44株DTMUV的细胞交叉中和试验
Table 4
| 毒株 Virus | 4株DTMUV阳性血清中和效价 Positive sera neutralization titer of 4 DTMUV isolates | |||
|---|---|---|---|---|
| DTMUV-HB | DTMUV-AH | DTMUV-GX1 | DTMUV-GX2 | |
| DTMUV-HB | 1:54.6 | 1:29.3 | 1:34.9 | 1:55.4 |
| DTMUV-AH | 1:44.3 | 1:38.3 | 1:34.9 | 1:48.9 |
| DTMUV-GX1 | 1:59.3 | 1:33.4 | 1:45.3 | 1:45.4 |
| DTMUV-GX2 | 1:62.0 | 1:28.4 | 1:37.7 | 1:44.0 |
新窗口打开|下载CSV
图3
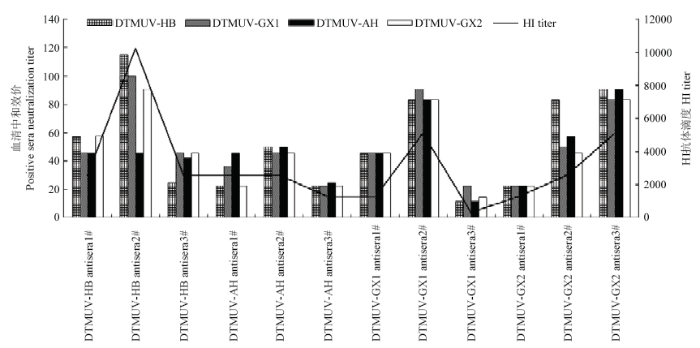 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图34株DTMUV阳性血清中和效价
Fig. 3Positive sera neutralization titer of 4 DTMUV isolates
3 讨论
鸭坦布苏病毒病2010年在我国江浙一带开始流行,随后迅速扩散到我国主要的鸭养殖地区,目前已发展为地方流行性常在疫病。该病流行期间,DTMUV是否发生了变异?毒力有无增强?抗原性是否发生变化?现有的疫苗是否能对目前的“流行毒株”产生保护?这些是国内各养鸭企业、养鸭场包括疫苗生产企业最为关心的问题。我们利用2010—2016年间不同地区分离到的DTMUV毒株从病毒毒力、E蛋白基因序列和抗原差异性上进行了系统的比较和研究,为初步回答上述问题提供了数据支撑和理论依据。本研究选用的毒株均为从发病鸭场分离得到,时间跨度从2010—2016年,分布地点有河北、河南、安徽和广西等地。从ELD50和对鸭的人工感染试验来看,病毒含量范围在104.7—105.3 ELD50/0.1mL,人工感染鸭的病毒分离阳性率均在85%以上,4株DTMUV对易感动物的毒力没有明显差异,与课题组前期的研究结果一致[29, 32-33]。多名研究人员也进行了动物回归试验,所得的试验结果与本文基本一致。于可响[34]对2010年到2012年分离的6株病毒进行了ELD50的测定,范围在105.0—105.5 ELD50/0.2mL。2015年范萍萍[35]利用安徽铜陵分离株进行了动物回归试验,同样也是在攻毒后第3天采食量、产蛋量开始下降,剖检鸭卵巢萎缩、脾脏肿胀、胰脏出血等病变。
目前多篇关于鸭坦布苏病毒株基因序列分析的研究表明,不同地区、不同时间的分离株基因组并无显著性差异,尚未发生较大变异。万春和等[36]发现不同来源(鸡源、鸭源、鹅源、鸽源和蚊源)坦布苏病毒E基因同源性均较高,未发现该病毒在不同家禽之间有明显宿主特异性。张帅[37]2010—2012年通过对包括其实验室分离的ZJ-407、YY5、ZJ-06在内的35株DTMUV基因序列进行比对分析,发现不同来源的各株病毒之间核酸和氨基酸的同源性分别为97.0%—100%和97.4%— 100%,不同来源的鸭坦布苏病毒之间E蛋白基因未发生明显改变。这与本文对于实验室分离毒株E基因的分析结果一致。
R值判定是用于两种抗原物质相似程度的有效方法,可用于不同物种蛋白抗原相似性的分析。本文通过不同抗原与各自的抗体进行交叉反应,采用R值分析方法,经数学处理扣除试验误差,定量给出试验用毒株抗原的相似程度。从交叉血凝抑制试验、细胞交叉中和试验2个方面对分离的4株DTMUV抗原性作比较分析,其R值范围分别为0.79—1.12和0.79—1.20,抗原比值均大于0.67,根据抗原相关性判定标准可初步判断试验用DTMUV没有明显的抗原性差异。该结论与本研究进行的对试验动物毒力、E基因序列比对及同源性分析结论相符。YU[28]等利用2010—2012年分离的病毒株进行了血清交叉中和试验,结果表明分离毒株为同一血清型。与本文的研究结果一致。
关于鸭坦布苏病毒抗体的检测方法有ELISA[38,39]、乳胶凝集试验[40]、中和试验等,尚未见有HI抗体测定方法的报道。本研究利用实验室建立的HI试验方法对血清中的HI抗体和中和抗体的相关性同时做了比较,从试验结果来看具有良好的相关性,相比ELISA方法和中和试验法,HI抗体检测法具有可靠、简单、用时短和不需要特殊设备等优点,可用于该病的诊断和疫苗效力检验的替代检验方法。
4 结论
目前,DTMUV还没有发现其他血清型,本文试验研究所用的4株DTMUV在毒力、E基因序列和抗原性上没有明显的差异,提示我们目前国内流行的毒株还未产生较大的变异。应坚持进行流行病学调查和疫情监控,监测该病毒的变异趋势。但目前来说,不论是利用发病早期分离的毒株制备的疫苗,还是经过弱化的弱毒苗,或是基因工程疫苗,都可以有效控制鸭坦布苏病毒病的发生和流行,是预防和控制本病最经济有效的办法。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.3201/eid1710.101890URL [本文引用: 1]

In China in 2010, a disease outbreak in egg-laying ducks was associated with a flavivirus. The virus was isolated and partially sequenced. The isolate exhibited 87%-91% identity with strains of Tembusu virus, a mosquito-borne flavivirus of the Ntaya virus group. These findings demonstrate emergence of Tembusu virus in ducks.
DOI:10.1371/journal.pone.0018106URLPMID:21455312 [本文引用: 1]

Since April 2010, a severe outbreak of duck viral infection, with egg drop, feed uptake decline and ovary-oviduct disease, has spread around the major duck-producing regions in China. A new virus, named BYD virus, was isolated in different areas, and a similar disease was reproduced in healthy egg-producing ducks, infecting with the isolated virus. The virus was re-isolated from the affected ducks and replicated well in primary duck embryo fibroblasts and Vero cells, causing the cytopathic effect. The virus was identified as an enveloped positive-stranded RNA virus with a size of approximately 55 nm in diameter. Genomic sequencing of the isolated virus revealed that it is closely related to Tembusu virus (a mosquito-borne Ntaya group flavivirus), with 87-91% nucleotide identity of the partial E (envelope) proteins to that of Tembusu virus and 72% of the entire genome coding sequence with Bagaza virus, the most closely related flavivirus with an entirely sequenced genome. Collectively our systematic studies fulfill Koch's postulates, and therefore, the causative agent of the duck egg drop syndrome occurring in China is a new flavivirus. Flavivirus is an emerging and re-emerging zoonotic pathogen and BYD virus that causes severe egg-drop, could be disastrous for the duck industry. More importantly its public health concerns should also be evaluated, and its epidemiology should be closely watched due to the zoonotic nature of flaviviruses.
DOI:10.1016/j.virol.2011.06.003URL [本文引用: 1]

During investigations into an outbreak of egg production decline, retarded growth, and even death among ducks in Southeast China, a novel Tembusu virus strain named Tembusu virus Fengxian 2010 (FX2010) was isolated. This virus replicated in embryonated chicken eggs and caused embryo death. In cross-neutralization tests, antiserum to the partial E protein of Tembusu virus Mm1775 strain neutralized FX2010, whereas antiserum to Japanese encephalitis virus did not. FX2010 is an enveloped RNA virus of approximately 4550 nm in diameter. Sequence analysis of its E and NS5 genes showed that both genes share up to 99.6% nucleotide sequence identity with Baiyangdian virus, and up to 88% nucleotide sequence identity with their counterparts in Tembusu virus. FX2010 was transmitted without mosquito, and caused systemic infection and lesions in experimentally infected ducks. These results indicate that FX2010 and BYD virus are newly emerged Tembusu virus strains that cause an infectious disease in ducks. (C) 2011 Elsevier Inc.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]

从以产蛋下降为主的樱桃谷种鸭以及出现神经症状的雏鸭各分离出1株病毒,分别命名为BZ株和LC株。该2株病毒对SPF鸡胚和健康鸭胚均能产生相同的病变,分离病毒不能凝集鸡、鸭、鹅、鸽等的红细胞,在鸭胚成纤维细胞(DEF)能够产生典型的细胞病变(CPE),电镜下观察到约50 nm的病毒粒子。病理组织学研究表明,二者在临床上均可导致脑组织危害,表现为脑膜水肿、血管充血和皮质层神经胶质细胞增生等。血清学检测表明,分离病毒与禽流感病毒(AIV)、鸭瘟病毒(DEV)、新城疫病毒(NDV)等病原无交叉。生物学特性鉴定该病原为有囊膜单股RNA病毒。利用不同禽病的特异性引物分别进行PCR或RTPCR,均未扩增出特异条带。设计随机引物进行RTPCR,扩增出基因片段,利用GenBank进行Blast同源比较,结果发现,分离病毒与以色列火鸡脑膜脑炎病毒(Israel turkey meningoencephalitis virus,TMEV)和在马来西亚发现的Tembumu 病毒至少在2段基因上具有较高的同源性,属于黄病毒属。测序表明,分离病毒与Tembumu 病毒的非结构蛋白(NS5基因)和囊膜蛋白(E基因)的核苷酸同源性为86.7%~90.2% 和87.0%~91.8%,与TMEV的NS5基因和E基因的同源性为72.4%~73.2%和72.7%~72.8%。2分离株之间E基因和NS5基因的核苷酸同源性均为99.5%。血清中和试验表明,BZ株阳性血清可以中和LC病毒,因此证实二者可能是同一种病毒。综合以上研究,建议将该病命名为“鸭病毒性脑炎”(Duck viral encephalitis disease)。
URL [本文引用: 1]

从以产蛋下降为主的樱桃谷种鸭以及出现神经症状的雏鸭各分离出1株病毒,分别命名为BZ株和LC株。该2株病毒对SPF鸡胚和健康鸭胚均能产生相同的病变,分离病毒不能凝集鸡、鸭、鹅、鸽等的红细胞,在鸭胚成纤维细胞(DEF)能够产生典型的细胞病变(CPE),电镜下观察到约50 nm的病毒粒子。病理组织学研究表明,二者在临床上均可导致脑组织危害,表现为脑膜水肿、血管充血和皮质层神经胶质细胞增生等。血清学检测表明,分离病毒与禽流感病毒(AIV)、鸭瘟病毒(DEV)、新城疫病毒(NDV)等病原无交叉。生物学特性鉴定该病原为有囊膜单股RNA病毒。利用不同禽病的特异性引物分别进行PCR或RTPCR,均未扩增出特异条带。设计随机引物进行RTPCR,扩增出基因片段,利用GenBank进行Blast同源比较,结果发现,分离病毒与以色列火鸡脑膜脑炎病毒(Israel turkey meningoencephalitis virus,TMEV)和在马来西亚发现的Tembumu 病毒至少在2段基因上具有较高的同源性,属于黄病毒属。测序表明,分离病毒与Tembumu 病毒的非结构蛋白(NS5基因)和囊膜蛋白(E基因)的核苷酸同源性为86.7%~90.2% 和87.0%~91.8%,与TMEV的NS5基因和E基因的同源性为72.4%~73.2%和72.7%~72.8%。2分离株之间E基因和NS5基因的核苷酸同源性均为99.5%。血清中和试验表明,BZ株阳性血清可以中和LC病毒,因此证实二者可能是同一种病毒。综合以上研究,建议将该病命名为“鸭病毒性脑炎”(Duck viral encephalitis disease)。
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1080/00034983.1975.11686984URLPMID:235907 [本文引用: 1]

Thirty isolations of Tembusu virus and four of Sindbis virus were obtained from approximately 280 000 mosquitoes collected between October 1968 and February 1970 in Sarawak, particularly from K. Tijirak, a Land Dyak village 19 miles South of Kuching. Twenty-two isolations of Tembusu virus and two of Sindbis virus were from Culex tritaeniorhynchus; two of Tembusu virus and two of Sindbis virus came from Culex gelidus. Tembusu virus was active throughout the year at K. Tijirak, the highest infection rates in C. tritaeniorhynchus being in January-March and May-August, when the C. tritaeniorhynchus population was declining and ageing. These results confirm that C. tritaeniorhynchus is the principal arthopod host of Tembusu virus in Sarawak. Antibody studies suggest that birds, particularly domestic fowl, are probably vertebrate maintenance hosts of Tembusu and Sindbis viruses in Sarawak.
DOI:10.1016/0035-9203(86)90397-4URLPMID:2885948 [本文引用: 1]

From 16 June to 15 August, 1982 CDC light traps were used to collect mosquitoes in the province of Kamphaengphet, N. Thailand. 353,042 mosquitoes comprising 59 species were collected and identified, and 345,173 were placed in pools for attempted virus isolation by inoculation of C6/36 Aedes albopictus mosquito cell cultures. Viruses were isolated from 63 mosquito pools. These comprised 56 flaviviruses, identified as 35 isolates of Japanese encephalitis (JE) virus strains, 18 strains of Tembusu (TEM) virus and three untyped flaviviruses (FLA); three alphaviruses, identified as the first isolates of Getah (GET) virus to have been made in Thailand; and four viruses which are still unidentified. Most virus isolates were from Culex tritaeniorhynchus mosquitoes collected in carbon dioxide baited light traps. JE virus was isolated only over a ten-day period and the last isolate was obtained one week before the peak of admission of human encephalitis cases at Kamphaengphet Provincial Hospital. Rapid screening of isolates grown on Ae. pseudoscutellaris (LSTM-AP-61) mosquito cells by indirect immunofluorescence using flavivirus group-specific and JE-specific monoclonal antibodies showed a high degree of correlation with plaque reduction neutralization tests. An antigen capture enzyme immunoassay (EIA) test successfully identified about 50% of the JE virus positive pools, but the method saved considerable processing time.
URLPMID:10695806 [本文引用: 1]

A virus isolate, ThCAr105/92, from a pool of mosquitos, Culex tritaeniorhynchus, collected in Chiang Mai, Thailand in 1992, appeared to be a member of the genus Flavivirus of the family Flaviviridae, based on the reverse transcription polymerase chain reaction (RT-PCR) using flavivirus cross-reacting primer pairs, electron microscopic examination, and serological tests. However, RT-PCR using Japanese encephalitis (JE) virus-specific primers showed that the isolate was different from JE virus. Sucrose density gradient sedimentation of the virus replicated in C6/36 cells indicated that the virus is relatively unstable in the infected culture fluids at 37 degrees C. Antibody prepared against this virus and a virus seed for the isolate were tested by cross neutralization against a panel of flaviviruses and the results showed that the new isolate was a distinct subtype of Tembusu virus.
DOI:10.4269/ajtmh.2000.63.94URLPMID:11358004 [本文引用: 1]

A new virus named Sitiawan virus (SV) was isolated from sick broiler chicks in chicken embryos. The virus replicated well with cytopathogenic effect (CPE) in the chicken B-lymphocyte cell line LSCC-BK3. The virus was an enveloped RNA virus of approximately 41 nm in size with hemagglutinating activity (HA) to goose erythrocytes. It was cross-reactive with Japanese encephalitis virus (JEV), a member of flaviviruses by HA inhibition tests but not by cross-virus neutralization tests. The cDNA fragment of NS5 gene was amplified with primers corresponding to NS5 gene of flaviviruses. The nucleotide sequences were 92% homologous to Tembusu virus, a member of the mosquito-borne virus cluster of the genus Flavivirus. In cross-neutralization tests with Tembusu virus, antiserum to SV did not neutralize Tembusu virus, and antiserum to Tembusu virus neutralized more weakly to SV than against homologous virus. These results indicate that SV is a new virus which can be differentiated serologically from Tembusu virus but is otherwise similar with respect to nucleotide sequence. The virus causes encephalitis, growth retardation, and increased blood glucose levels in inoculated chicks.
DOI:10.1128/JVI.07132-11URL [本文引用: 1]

Duck tembusu virus (DTMUV) is an emerging agent that causes a severe disease in ducks. We report herein the first complete genome sequences of duck tembusu virus strains YY5, ZJ-407, and GH-2, isolated from Shaoxing ducks, breeder ducks, and geese, respectively, in China. The genomes of YY5, ZJ-407, and GH-2 are all 10,990 nucleotides (nt) in length and encode a putative polyprotein of 3,426 amino acids. It is flanked by a 5' and a 3' noncoding region (NCR) of 94 and 618 nt, respectively. Knowledge of the whole sequence of DTMUV will be useful for further studies of the mechanisms of virus replication and pathogenesis.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.vaccine.2014.07.082URL [本文引用: 1]

A newly emerged tembusu virus that causes egg-drop has been affecting ducks in China since 2010. Currently, no vaccine is available for this disease. A live attenuated duck enteritis virus (DEV; a herpesvirus) vaccine has been used routinely to control lethal DEV in ducks since the 1960s. Here, we constructed two recombinant DEVs by transfecting overlapping fosmid DNAs. One virus, rDEV-TE, expresses the truncated form of the envelope glycoprotein (TE) of duck tembusu virus (DTMUV), and the other virus, rDEV-PrM/TE, expresses both the TE and pre-membrane proteins (PrM). Animal study demonstrated that both recombinant viruses induced measurable anti-DTMUV neutralizing antibodies in ducks. After two doses of recombinant virus, rDEV-PrM/TE completely protected ducks from DTMUV challenge, whereas rDEV-TE only conferred partial protection. These results demonstrate that recombinant DEV expressing the TE and pre-membrane proteins is protective and can serve as a potential candidate vaccine to prevent DTMUV infection in ducks. (C) 2014 Elsevier Ltd.
DOI:10.1016/j.vetmic.2019.03.022URLPMID:30955813 [本文引用: 1]

Infection with duck Tembusu virus (DTMUV) can cause large economic losses to the duck-rearing industry in China. In this study, we isolated a virulent strain of DTMUV (SDS) from sparrows near a duck farm and attenuated it via serially passaging (alternately for 100 passages) in specific pathogen-free chicken and duck embryos. We attenuated the virus after the 60th passage (SDS-60), based on the production of embryos that were free of visible lesions and still alive. The 70th adapted strain (SDS-70), obtained with a virus titer of 10-2.46 EID50 was chosen to be the live attenuated vaccine. After immunizing ducklings with the SDS-70 strain, they obtained 100% protection against infection by the SDS-10 virulent strain. Our data demonstrate that the vaccine can protect ducks from becoming infected with TMUV. Our study also shows that this newly developed attenuated vaccine candidate provides excellent immunogenicity, is safe, and has the potential to control DTMUV infections in ducks.
DOI:10.1055/a-1059-9739URLPMID:31841290 [本文引用: 1]

Popliteal aneurysms have the highest incidence of all peripheral aneurysms. The clinical symptoms are dominated by chronic embolism, resulting in irreversible ischemia with the associated risk of amputation and mortality, but rupture is less important. Acute aneurysm thrombosis bears a high risk of amputation and mortality. Endovascular exclusion with covered stents instead of open surgery has gained widespread acceptance and is based on reliable data. The principle of flow diversion for aneurysm treatment is well known for the cerebral vasculature, and is now emerging as a potential alternative with promising results and is challenging the concept of complete endovascular aneurysm exclusion or surgical bypassing. Since 2011, thirty-four out of 142 electively treated popliteal aneurysms and 8 thrombosed aneurysms were treated with a bare metal woven Nitinol stent. In this single centre series with continuous mid- to long-term follow-up, as described below, this option showed reliable results in terms of clinical outcome, material fatigue and preservation of outflow vasculature with the option for conversion. The limitation of the technique is determined by the available maximum outer stent diameter of 7.5?mm.
DOI:10.3390/v6062428URLPMID:24956180 [本文引用: 1]

Duck Tembusu virus (DTMUV) is a recently emerging pathogenic flavivirus that has resulted in a huge economic loss in the duck industry. However, no vaccine is currently available to control this pathogen. Consequently, a practical strategy to construct a vaccine against this pathogen should be determined. In this study, duck enteritis virus (DEV) was examined as a candidate vaccine vector to deliver the envelope (E) of DTMUV. A modified mini-F vector was inserted into the SORF3 and US2 gene junctions of the attenuated DEV vaccine strain C-KCE genome to generate an infectious bacterial artificial chromosome (BAC) of C-KCE (vBAC-C-KCE). The envelope (E) gene of DTMUV was inserted into the C-KCE genome through the mating-assisted genetically integrated cloning (MAGIC) strategy, resulting in the recombinant vector, pBAC-C-KCE-E. A bivalent vaccine C-KCE-E was generated by eliminating the BAC backbone. Immunofluorescence and western blot analysis results indicated that the E proteins were vigorously expressed in C-KCE-E-infected chicken embryo fibroblasts (CEFs). Duck experiments demonstrated that the insertion of the E gene did not alter the protective efficacy of C-KCE. Moreover, C-KCE-E-immunized ducks induced neutralization antibodies against DTMUV. These results demonstrated, for the first time, that recombinant C-KCE-E can serve as a potential bivalent vaccine against DEV and DTMUV.
DOI:10.1637/10960-101514-RegURLPMID:26473674 [本文引用: 1]

To evaluate the potential use of an inactivated virus-based vaccine for the control and prevention of the newly emerged duck Tembusu virus infection in China, a duck Tembusu virus isolate, Tembusu-HB, was propagated in 12-day-old duck embryos and inactivated by treatment with formaldehyde. The inactivated viral antigen was emulsified with mineral oil, and five batches of the vaccine were manufactured. The immunogenicity and protection efficacy of the vaccine were evaluated in Beijing ducks and Beijing white geese. Results showed that more than 80% of immunized ducks were protected against virulent virus challenge after two intramuscular or subcutaneous injections of the inactivated vaccine, as evidenced by the negative virus isolation results. The protection is also correlated with a positive virus-specific antibody response as detected by ELISA. In contrast, none of the control ducks and geese had any detectable antibody response. Virus was isolated from all control ducks and geese after virulent virus challenge. Interestingly, a variable level of protection (20%-80%) was observed in Beijing white geese immunized twice with the same batches of vaccine, suggesting a species-specific effect of the vaccine. Overall, the results clearly suggest that the inactivated duck Tembusu virus vaccine is immunogenic and provides protection against virulent virus challenge.
DOI:10.1016/j.vaccine.2016.03.030URLPMID:27016654 [本文引用: 1]

To obtain an effective vaccine candidate against duck Tembusu viral (DTMUV) disease which causes egg-drop and great economical loss in the Chinese duck industry, liposome vaccines containing recombinant E protein were prepared and assessed in this study. The recombinant plasmid (PET28a-E) was constructed and transformed into BL21 (DE3) cells to produce E proteins. The recombinant E proteins were purified and entrapped by liposomes through reverse-phase evaporation. Eighty-four cherry valley ducks were randomly divided into seven groups and inoculated intramuscularly at one- or seven-day-old with liposomes-E protein or Freund's adjuvant-E protein vaccine. Blood samples were collected from the first week to the tenth week for serum antibody, plasma for viremia, as well as oropharyngeal and cloacal swabs for virus shedding analyses after being challenged with a 10(2.4) 50% tissue culture infective dose (TCID50) of duck Tembusu virus. Results showed that serum antibody level of the liposomes vaccine was higher than the Freund's adjuvant vaccine, and inoculating twice was superior to once; furthermore, the viremia and virus shedding tests also proved that the liposomes vaccine can provide complete protection against DTMUV challenge. These results demonstrated that the liposomes-E protein vaccine could be used as a potential candidate vaccine to prevent DTMUV infection in ducks.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/978-3-7091-0572-6_6URLPMID:15119763 [本文引用: 1]

There are two major groups of encephalitic flaviviruses, those that infect and are transmitted by ticks, particularly Ixodes spp. and those that infect and are transmitted by mosquitoes, particularly Culex spp. The tick-borne encephalitic flaviviruses exhibit evolutionary characteristics that are largely determined by the protracted life cycle of the tick, its habitat and the prevailing climatic conditions. These viruses appear to have evolved gradually from non-encephalitic viruses that radiated eastwards and north eastwards out of Africa into Asia and the southern islands, then northwards to far east Asia and finally westwards across Eurasia to western Europe, during the past two to four thousand years. Only one of these recognized species has found its way to North America viz. Powassan virus. In contrast, the evolution of the recognized mosquito-borne encephalitic flaviviruses reflects the wide range of mosquito species that they infect. They emerged out of Africa relatively recently and at roughly the same time, i.e., probably during the past few centuries. Although many of these mosquito-borne viruses are geographically widely dispersed, with the exception of West Nile virus, they are found either in the Old World or the New World, never in both, and we are now beginning to understand the reasons. Phylogenetic trees will be used here to describe the evolution, epidemiology and dispersal characteristics of these viruses, taking into account the importance of virus persistence and recombination.
DOI:10.1371/journal.pone.0071319URLPMID:23990944 [本文引用: 2]

Since the first reported cases of ducks infected with a previously unknown flavivirus in eastern China in April 2010, the virus, provisionally designated Duck Tembusu Virus (DTMUV), has spread widely in domestic ducks in China and caused significant economic losses to poultry industry. In this study, we examined in detail structural, antigenic, and evolutionary properties of envelope (E) proteins of six DTMUV isolates spanning 2010-2012, each being isolated from individual farms with different geographical locations where disease outbreaks were documented. Structural analysis showed that E proteins of DTMUV and its closely related flavivirus (Japanese Encephalitis Virus) shared a conserved array of predicted functional domains and motifs. Among the six DTMUV strains, mutations were observed only at thirteen amino acid positions across three separate domains of the E protein. Interestingly, these genetic polymorphisms resulted in no detectable change in viral neutralization properties as demonstrated in a serum neutralization assay. Furthermore, phylogenetic analysis of the nucleotide sequences of the E proteins showed that viruses evolved into two distinct genotypes, termed as DTMUV.I and DTMUV.II, with II emerging as the dominant genotype. New findings described here shall give insights into the antigenicity and evolution of this new pathogen and provide guidance for further functional studies of the E protein for which no effective vaccine has yet been developed.
DOI:10.3864/j.issn.0578-1752.2014.23.021URL [本文引用: 2]

【Objective】 The objective of the study is to illustrate the viremia of ducks challenged with duck hemorrhagic ovaritis virus (DHOV-HB Strain) , to gain insight into the pathogenesis of DHOV and provide data for diagnosis and vaccine development.【Method】A group of twenty-four 250-day-old Peking ducks with implanted chip were infected with 100-fold diluted DHOV-HB (3×104ELD50) orally, 15 ducks as negative control were kept in isolation unit under the same condition, clinical signs were observed daily. On 1-10 day post inoculation (DPI), 10 serum samples were collected from each group via wing vein daily for virus-isolation and antibody determination (select the same duck as possible). The serum samples of 1-10 DPI were inoculated into 6-day-old SPF chicken embryos via yolk-sac route. Five SPF chicken embryos were inoculated for each sample at the inoculum of 0.1 mL per embryo. Then they were hatched at 37℃ further. The chicken embryos were observed regularly twice a day. Reap and count the death chicken embryos during 24-168 h in time. Then the death chicken embryos were cut into small pieces, and then centrifuged the grinding homogenate. The viral nucleic acid was detected by RT-PCR in the supernatant. If there are more than one (including one) death chicken embryos, and the nucleic acid testing was positive, then it was concluded that virus isolation was positive. Using the classical neutralization test method to detect the serum antibody of 4-10 DPI, 5 SPF chicken embryos were inoculated for each serum dilution. The inoculation method and criteria of embryos’ specific death were performed as previously described. The determination standards are as follows: results with serum protect more than 80% (4/5) chicken embryos, the serum antibody was recognized as positive, 20%-60%(1/5-3/5) were as doubtful, and 0%(0/5) as negative.【Result】The viremia and antibody responses were closely related after infection with DHOV. The viremia could be detected as early as 1 DPI, peaked at 1-3 DPI. By this time, the positive isolation rate of serum were all 100% (10/10). On 4-6 DPI, the positive isolation rate of serum began dropping which were 90% (9/10), 70% (7/10), and 30% (3/10), respectively, and no virus was recovered anymore on 7-10 DPI. The results of the neutralization test indicated that there were low titer antibodies in the serum while the virus isolation positive rate showed a decreasing trend on 4 DPI, showed up as the death time of the embryos postponed while the antibodies of the inoculated ducks were all negative temporarily. It was 80%(8/10) negative, 20%(2/10) doubtful on 6 DPI , 70% (7/10) positive, 10% (1/10) negative, 20% (2/10) doubtful on 7 DPI, 90% (9/10) positive 10% (1/10) doubtful on 9 DPI, and the antibodies of the inoculated ducks were all positive on 10 DPI. Virus isolation (1-10d) and antibody test (4-10d) results were all negative in the negative control ducks. 【Conclusion】The duration of viremia stage of ducks infected with DHOV-HB was short, the virus was recovered in 100% (10/10) on 1-3 DPI, decreased on 4 DPI and disappeared on 7 DPI. The antibody-positive rate was 70% (7/10) on 7 DPI and reached 100% (10/10) on 10 DPI.Further analysis showed that the quickly absence of viremia was closely related to specific antibody response. The antibody-positive rate was 70% (7/10) on 7 DPI and reached 100% (10/10) on 10 DPI.
DOI:10.3864/j.issn.0578-1752.2014.23.021URL [本文引用: 2]

【Objective】 The objective of the study is to illustrate the viremia of ducks challenged with duck hemorrhagic ovaritis virus (DHOV-HB Strain) , to gain insight into the pathogenesis of DHOV and provide data for diagnosis and vaccine development.【Method】A group of twenty-four 250-day-old Peking ducks with implanted chip were infected with 100-fold diluted DHOV-HB (3×104ELD50) orally, 15 ducks as negative control were kept in isolation unit under the same condition, clinical signs were observed daily. On 1-10 day post inoculation (DPI), 10 serum samples were collected from each group via wing vein daily for virus-isolation and antibody determination (select the same duck as possible). The serum samples of 1-10 DPI were inoculated into 6-day-old SPF chicken embryos via yolk-sac route. Five SPF chicken embryos were inoculated for each sample at the inoculum of 0.1 mL per embryo. Then they were hatched at 37℃ further. The chicken embryos were observed regularly twice a day. Reap and count the death chicken embryos during 24-168 h in time. Then the death chicken embryos were cut into small pieces, and then centrifuged the grinding homogenate. The viral nucleic acid was detected by RT-PCR in the supernatant. If there are more than one (including one) death chicken embryos, and the nucleic acid testing was positive, then it was concluded that virus isolation was positive. Using the classical neutralization test method to detect the serum antibody of 4-10 DPI, 5 SPF chicken embryos were inoculated for each serum dilution. The inoculation method and criteria of embryos’ specific death were performed as previously described. The determination standards are as follows: results with serum protect more than 80% (4/5) chicken embryos, the serum antibody was recognized as positive, 20%-60%(1/5-3/5) were as doubtful, and 0%(0/5) as negative.【Result】The viremia and antibody responses were closely related after infection with DHOV. The viremia could be detected as early as 1 DPI, peaked at 1-3 DPI. By this time, the positive isolation rate of serum were all 100% (10/10). On 4-6 DPI, the positive isolation rate of serum began dropping which were 90% (9/10), 70% (7/10), and 30% (3/10), respectively, and no virus was recovered anymore on 7-10 DPI. The results of the neutralization test indicated that there were low titer antibodies in the serum while the virus isolation positive rate showed a decreasing trend on 4 DPI, showed up as the death time of the embryos postponed while the antibodies of the inoculated ducks were all negative temporarily. It was 80%(8/10) negative, 20%(2/10) doubtful on 6 DPI , 70% (7/10) positive, 10% (1/10) negative, 20% (2/10) doubtful on 7 DPI, 90% (9/10) positive 10% (1/10) doubtful on 9 DPI, and the antibodies of the inoculated ducks were all positive on 10 DPI. Virus isolation (1-10d) and antibody test (4-10d) results were all negative in the negative control ducks. 【Conclusion】The duration of viremia stage of ducks infected with DHOV-HB was short, the virus was recovered in 100% (10/10) on 1-3 DPI, decreased on 4 DPI and disappeared on 7 DPI. The antibody-positive rate was 70% (7/10) on 7 DPI and reached 100% (10/10) on 10 DPI.Further analysis showed that the quickly absence of viremia was closely related to specific antibody response. The antibody-positive rate was 70% (7/10) on 7 DPI and reached 100% (10/10) on 10 DPI.
DOI:10.4269/ajtmh.1958.7.561URLPMID:13571577 [本文引用: 1]
DOI:10.1084/jem.92.5.441URLPMID:14778924 [本文引用: 1]

Antigenic variants of influenza A virus strains emerge on serial passage in ovo in the presence of immune serum against different but related strains. An old laboratory strain (PR8) which had been through hundreds of animal passages was as readily modified by this procedure as recently recovered strains. Such variants apparently can be obtained at will and show antigenic patterns which are reproducible and appear to be predictable in terms of the immune serum used for their selection. Variant strains retain their new antigenic patterns on serial passage in ovo in the absence of immune serum. Limited serial passage in ovo of strains in the absence of immune serum did not result in the emergence of antigenic variants. Similarly, serial passages of strains in ovo in the presence of immune serum against widely different strains, which failed to show significant cross-neutralization, did not lead to the appearance of antigenic variants.
DOI:10.3864/j.issn.0578-1752.2016.14.017URL [本文引用: 1]

【Objective】The objective of this study is to understand the process and regularities of ovarian lesions on ducks infected with duck Tembusu virus, and to provide data for the efficiency evaluation of vaccine using laying ducks ovary pathological inspection.【Method】Seventy 260-day-old laying cherry ducks were inoculated intramuscularly with duck Tembusu virus (HB strain) at dosage of 0.5ml(100 DID50)/duck. Clinical symptoms were observed, and the egg production and feed intake were recorded daily. Serum samples were collected from all ducks for virus isolation via wing vein on 2 days post inoculation (dpi). Each serum sample was inoculated into five 6-day-old SPF chicken embryos via yolk-sac route at the inoculum of 0.1 ml per embryo. Then they were hatched at 37℃ further. The chicken embryos died in 24h were abandoned. The viral nucleic acid was detected by RT-PCR in the death chicken embryos during 24-72 h. If there were more than one (including one) death chicken embryos, and the nucleic acid testing was positive, then it was concluded that virus isolation was positive. On 4-10 dpi, 10 ducks were necropsied and the gross lesions of the reproductive system were observed every day. The pathologic rate was calculated, and the gross lesions of the reproductive system were conducted including whether there were eggs in the fallopian tube, whether the follicle was deformed, hemorrhaged or ruptured or not. According to the statistical pathologic rate, the time, the content of the examination and the criterions for determining lesion ovary were confirmed. 【Result】 (1) Feed intake and egg production decreased significantly on 3-6 dpi. The mental state of the duck on 7 dpi improved, and feed intake began to rise on 8 dpi. (2) Virus isolation of 70 ducks were all positive except one duck. The virus positive isolation rate was 98.6% (69/70) on 2 dpi. (3) On 4-10 dpi, a total of 64 laying duck reproductive organs can be determined. (4) On 4-10 dpi, the ovarian lesions rate were 66.7% (6/9), 100% (10/10), 100% (10/10), 100% (9/9), 100% (9/9), 100% (9/9) and 100% (8/8) respectively. (5) Eggs were found in fallopian tube of 2 ducks among 10 ducks that were necropsied on 4 dpi, and there was no egg found in fallopian tube of the remaining 62 ducks. The egg negative rate was 96.9(62/64). (6) On 4-10 dpi, the proportion of deformed follicular duck and hemorrhaged follicular duck were both 96.9% (62/64), and the proportion of deformed and hemorrhaged follicular duck was 95.3%(61/64), while the proportion of ruptured follicular duck was 34.4% (22/64).【Conclusion】 (1) The time for the examination of the pathological changes of ovary was determined as 7 to 8 dpi. (2) The criterion of abnormal follicle is that one of the lesions of deformation and hemorrhage or both were found. The criterion of lesion ovary is that three abnormal follicles or more appeared and no egg in the fallopian tube.
DOI:10.3864/j.issn.0578-1752.2016.14.017URL [本文引用: 1]

【Objective】The objective of this study is to understand the process and regularities of ovarian lesions on ducks infected with duck Tembusu virus, and to provide data for the efficiency evaluation of vaccine using laying ducks ovary pathological inspection.【Method】Seventy 260-day-old laying cherry ducks were inoculated intramuscularly with duck Tembusu virus (HB strain) at dosage of 0.5ml(100 DID50)/duck. Clinical symptoms were observed, and the egg production and feed intake were recorded daily. Serum samples were collected from all ducks for virus isolation via wing vein on 2 days post inoculation (dpi). Each serum sample was inoculated into five 6-day-old SPF chicken embryos via yolk-sac route at the inoculum of 0.1 ml per embryo. Then they were hatched at 37℃ further. The chicken embryos died in 24h were abandoned. The viral nucleic acid was detected by RT-PCR in the death chicken embryos during 24-72 h. If there were more than one (including one) death chicken embryos, and the nucleic acid testing was positive, then it was concluded that virus isolation was positive. On 4-10 dpi, 10 ducks were necropsied and the gross lesions of the reproductive system were observed every day. The pathologic rate was calculated, and the gross lesions of the reproductive system were conducted including whether there were eggs in the fallopian tube, whether the follicle was deformed, hemorrhaged or ruptured or not. According to the statistical pathologic rate, the time, the content of the examination and the criterions for determining lesion ovary were confirmed. 【Result】 (1) Feed intake and egg production decreased significantly on 3-6 dpi. The mental state of the duck on 7 dpi improved, and feed intake began to rise on 8 dpi. (2) Virus isolation of 70 ducks were all positive except one duck. The virus positive isolation rate was 98.6% (69/70) on 2 dpi. (3) On 4-10 dpi, a total of 64 laying duck reproductive organs can be determined. (4) On 4-10 dpi, the ovarian lesions rate were 66.7% (6/9), 100% (10/10), 100% (10/10), 100% (9/9), 100% (9/9), 100% (9/9) and 100% (8/8) respectively. (5) Eggs were found in fallopian tube of 2 ducks among 10 ducks that were necropsied on 4 dpi, and there was no egg found in fallopian tube of the remaining 62 ducks. The egg negative rate was 96.9(62/64). (6) On 4-10 dpi, the proportion of deformed follicular duck and hemorrhaged follicular duck were both 96.9% (62/64), and the proportion of deformed and hemorrhaged follicular duck was 95.3%(61/64), while the proportion of ruptured follicular duck was 34.4% (22/64).【Conclusion】 (1) The time for the examination of the pathological changes of ovary was determined as 7 to 8 dpi. (2) The criterion of abnormal follicle is that one of the lesions of deformation and hemorrhage or both were found. The criterion of lesion ovary is that three abnormal follicles or more appeared and no egg in the fallopian tube.
DOI:10.3864/j.issn.0578-1752.2011.10.022URL [本文引用: 1]

【Objective】 The objective of the study is to create an experimental infection model of Duck Hemorrhagic Ovaritis, study the disease characterization and provide an available model for developing vaccine and evaluating antiviral medicine.【Method】 The infected 196-day-old ducks were inoculated with 104 ELD50 of a DHOV isolate, DHOV-HB-F4, via a combination of intranasal, intraoral and intraocular inoculation method. Clinical signs were observed and recorded daily. At 5, 7, 9, 21, 28 and 34 day after inoculation, serum samples were taken, randomly selected and sacrificed for necropsy. Gross lesions were recorded and various tissues were collected for histological examination. Various tissues including ovaries, liver, brain, and spleen were collected for re-isolating virus. The method of fixed virus and diluted sera was used to detect the neutralizing antibody.【Result】The food consumption of infected ducks showed significant decrease (decreased by 80%) at 3, 4, 5 day post inoculation (DPI), recovered to 50% of normal intake at 6 DPI and to normal level at 11DPI. The egg production of infected ducks was significantly decreased at 3, 4, 5 DPI and zero at 6, 7, 8 DPI. The laying rate of infected ducks recovered to 60% of normal at 36 DPI and 80% at 39 DPI. DHO virus could be successfully re-isolated from three ducks’ ovaries and liver on 5 DPI (3/3), two ducks’ ovaries (2/2) and one duck’s spleen and brain(1/2) on 7 DPI, one duck’s ovaries(1/2) on 9 DPI. At 21 and 28 DPI, DHO virus could not be re-isolated from the infected ducks’ tissues. The infected ducks showed ovary degeneration and deformation, follicle membrane hyperaemia and haemorrhagia; ovary, liver, brain and spleen characterized by the reticular cells hyperplasia which showed acute Hemorrhagic Ovaritis, interstitial hepatitis, nonpurulent meningoencephalitis, necrotizing splenitis, interstitial nephritis and mild myocarditis. The infected ducks only produced lower-titer neutralizing antibody on week 3 post inoculation.【Conclusion】The experimental ducks showed acute Hemorrhagic Ovaritis after infected by Duck Hemorrhagic Ovaritis. The experimental infection model of Duck Hemorrhagic Ovaritis was developed in Peking duck which could be a model for screening and evaluating the antiviral medicine and vaccine.
DOI:10.3864/j.issn.0578-1752.2011.10.022URL [本文引用: 1]

【Objective】 The objective of the study is to create an experimental infection model of Duck Hemorrhagic Ovaritis, study the disease characterization and provide an available model for developing vaccine and evaluating antiviral medicine.【Method】 The infected 196-day-old ducks were inoculated with 104 ELD50 of a DHOV isolate, DHOV-HB-F4, via a combination of intranasal, intraoral and intraocular inoculation method. Clinical signs were observed and recorded daily. At 5, 7, 9, 21, 28 and 34 day after inoculation, serum samples were taken, randomly selected and sacrificed for necropsy. Gross lesions were recorded and various tissues were collected for histological examination. Various tissues including ovaries, liver, brain, and spleen were collected for re-isolating virus. The method of fixed virus and diluted sera was used to detect the neutralizing antibody.【Result】The food consumption of infected ducks showed significant decrease (decreased by 80%) at 3, 4, 5 day post inoculation (DPI), recovered to 50% of normal intake at 6 DPI and to normal level at 11DPI. The egg production of infected ducks was significantly decreased at 3, 4, 5 DPI and zero at 6, 7, 8 DPI. The laying rate of infected ducks recovered to 60% of normal at 36 DPI and 80% at 39 DPI. DHO virus could be successfully re-isolated from three ducks’ ovaries and liver on 5 DPI (3/3), two ducks’ ovaries (2/2) and one duck’s spleen and brain(1/2) on 7 DPI, one duck’s ovaries(1/2) on 9 DPI. At 21 and 28 DPI, DHO virus could not be re-isolated from the infected ducks’ tissues. The infected ducks showed ovary degeneration and deformation, follicle membrane hyperaemia and haemorrhagia; ovary, liver, brain and spleen characterized by the reticular cells hyperplasia which showed acute Hemorrhagic Ovaritis, interstitial hepatitis, nonpurulent meningoencephalitis, necrotizing splenitis, interstitial nephritis and mild myocarditis. The infected ducks only produced lower-titer neutralizing antibody on week 3 post inoculation.【Conclusion】The experimental ducks showed acute Hemorrhagic Ovaritis after infected by Duck Hemorrhagic Ovaritis. The experimental infection model of Duck Hemorrhagic Ovaritis was developed in Peking duck which could be a model for screening and evaluating the antiviral medicine and vaccine.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0053026URLPMID:23300851 [本文引用: 1]

Since April 2010, domesticated ducks in China have been suffering from an emerging infectious disease characterized by retarded growth, high fever, loss of appetite, decline in egg production, and death. The causative agent was identified as a duck Tembusu virus (DTMUV), a member of the Ntaya virus (NTAV) group within the genus Flavivirus, family Flaviviridae. DTMUV is highly contagious and spreads rapidly in many species of ducks. More than 10 million shelducks have been infected and approximately 1 million died in 2010. The disease remains a constant threat to the duck industry; however, it is not known whether DTMUV can infect humans or other mammalians, despite the fact that the virus has spread widely in southeast China, one of the most densely populated areas in the world. The lack of reliable methods to detect the serum antibodies against DTMUV has limited our ability to conduct epidemiological investigations in various natural hosts and to evaluate the efficiency of vaccines to DTMUV.
URL [本文引用: 1]

An outbreak of egg-drop syndrome occurred on a Sheldrake duck farm in Longhai in Fujian Province, China, in 2012. The main clinical symptoms were sharply reduced egg production, crooked necks, and death. We isolated the virus from the sick ducks, identified it, and observed the histopathologic changes after viral infection. We detected viral RNA in the blood and feces of the infected ducks and developed a latex-agglutination diagnostic method to detect anti-Tembusu-virus antibodies. Our results show that the pathogenic virus is a Tembusu virus. The histopathologic changes included follicular cell degeneration and necrosis, follicular cavity filled with blood cells, massive necrosis in the brain, and degeneration and necrosis of the nerve and glial cells. When the transmission of the virus in the infected ducks was studied, the duck blood was positive for viral nucleic acid for up to 29 days, and the feces were positive for viral nucleic acid for up to 13 days. We successfully established a simple, rapid, and easy-to-use latex-agglutination diagnostic method for the detection of antibodies against duck Tembusu virus.
