 ,, 孙元, 王涛, 仇华吉
,, 孙元, 王涛, 仇华吉 ,中国农业科学院哈尔滨兽医研究所兽医生物技术国家重点实验室,哈尔滨 150069
,中国农业科学院哈尔滨兽医研究所兽医生物技术国家重点实验室,哈尔滨 150069African Swine Fever: A Major Threat to the Chinese Swine Industry
LUO YuZi ,, SUN Yuan, WANG Tao, QIU HuaJi
,, SUN Yuan, WANG Tao, QIU HuaJi ,Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin 150069
,Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin 150069通讯作者:
责任编辑: 林鉴非
收稿日期:2018-08-28接受日期:2018-09-17网络出版日期:2018-11-01
| 基金资助: |
Received:2018-08-28Accepted:2018-09-17Online:2018-11-01

摘要
关键词:
Abstract
Keywords:
PDF (1134KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
罗玉子, 孙元, 王涛, 仇华吉. 非洲猪瘟——我国养猪业的重大威胁[J]. 中国农业科学, 2018, 51(21): 4177-4187 doi:10.3864/j.issn.0578-1752.2018.21.016
LUO YuZi, SUN Yuan, WANG Tao, QIU HuaJi.
非洲猪瘟(African swine fever,ASF)是由非洲猪瘟病毒(African swine fever virus,ASFV)感染家猪和野猪引起的一种烈性传染病。不同日龄的猪均易感,临床表现为高热、皮肤发绀和各脏器出血,发病率和死亡率可高达100%,给疫区国家的养猪业造成了巨大的经济损失,并冲击生猪产业的国际贸易[1]。该病被世界动物卫生组织(OIE)列为法定报告的动物疫病,是我国重点防范的外来动物疫病之一,目前尚无有效疫苗和治疗药物。
目前ASF主要在撒哈拉以南的非洲地区、意大利撒丁岛、高加索地区以及俄罗斯和东欧部分国家流行[1,2,3,4,5]。2018年8月,该病首次传入我国[6],突如其来的ASF给我国养猪业带来空前的危机。我国生猪养殖规模大,很多猪场生物安全条件差,生猪跨区域调运频繁,因此该病在我国大范围扩散和流行的风险极高。同时,我国与非洲、欧洲多国以及俄罗斯的贸易日益频繁,加剧了ASF再次传入我国的风险。本文从ASF的病原学、流行病学、诊断和疫苗最新研究进展以及防控面临的挑战等方面进行概述,以期提高相关人员对该病的认识,增强相关部门的检疫防范意识,为我国ASF防控提供参考。
1 病原学
ASFV为有囊膜的双链DNA病毒,是非洲猪瘟相关病毒科(Asfarviridae)非洲猪瘟病毒属(Asfivirus)的唯一成员[7,8]。该病毒具有20面体对称结构,直径为175—215 nm,由内至外依次由病毒基因组、内核心壳、双层内膜、衣壳和囊膜5部分构成(图1)。基因组全长170—194 kb,编码150—200种蛋白[7,8]。图1
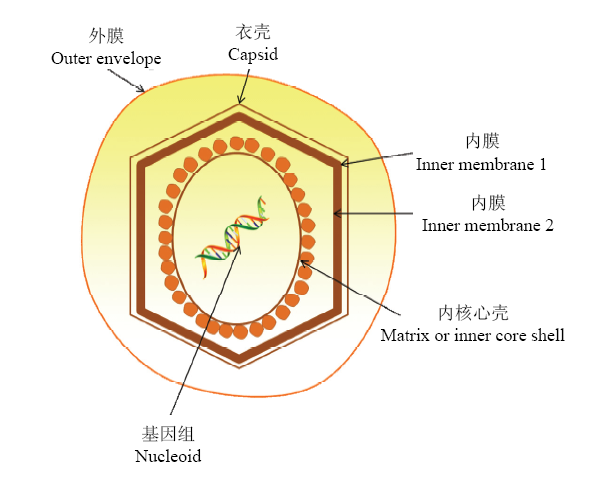 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1ASFV结构示意图
Fig. 1ASFV virion structure
ASFV结构蛋白较多,其中p72是主要的结构蛋白之一,该蛋白序列较保守,猪只感染ASFV后可诱导机体产生针对该蛋白的高滴度的抗体,常作为ASF血清学诊断的主要靶标。另外,ASFV基因组变异频繁,具有遗传多样性。根据p72基因末端一段478 bp的核酸序列,可将ASFV划分为24个基因型[9,10]。
ASFV基因组结构比较特殊,其末端是由37个核苷酸(nt)组成的共价闭合环(loop)结构,紧邻末端的是串联重复序列和多基因家族,中间是一段比较保守的基因序列(图2)。ASFV的复制机制与痘病毒相似[7],其复制的主要靶细胞是单核细胞、巨噬细胞。病毒可通过巨胞饮或者网格蛋白介导的内吞作用侵入宿主细胞,脱去内膜后主要在胞质中进行转录和翻译,在病毒“工厂”进行组装,然后通过出芽方式释放到细胞外,进入下一轮感染周期[11,12]。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2ASFV基因组结构示意图
Fig. 2Genomic organization of ASFV
ASFV耐低温,56℃ 70 min或60℃ 20 min可灭活病毒,对乙醚和氯仿敏感。2%氢氧化钠、2%—3%次氯酸钠、0.3%福尔马林、3%邻苯基酚或碘化合物作用30 min,均可灭活该病毒。ASFV在感染猪的污染物中可存活1个月,在腐败的血液或冷鲜肉中可存活近4个月,在冷藏的猪血液中可存活18个月,在冰冻猪肉或肉制品中可以存活数年至数十年,在未熟的肉品、腌肉、泔水中可长时间存活。
2 流行病学
ASFV可感染家猪和野猪引起高热、广泛性出血和高死亡率,而对于非洲野猪,如疣猪(warthogs)和非洲丛林野猪(bushpigs)则呈隐性感染[13]。非洲钝缘蜱属(Ornithodoros)的软蜱是ASFV的自然宿主和传播媒介。ASF的潜伏期一般为3—19 d,急性型一般3—4 d,OIE法典规定的潜伏期为15 d[4]。根据毒力和临床表现差异,可将ASF分为最急性型(强毒株)、急性型(强毒株)、亚急性型(中等毒力毒株)和慢性型(弱毒株)。最急性型常无临床症状,突然死亡,死亡率高达100%;急性型发病率和死亡率可达100%,表现为高热(可达42℃),沉郁、厌食,耳、四肢、腹部等处的皮肤发绀,内脏广泛性出血,其中脾脏肿大出血是ASF的重要鉴别特征;亚急性型和慢性型较急性型病情轻,病死率低,病程可持续数周至数月,可见血清学转阳。
1921年肯尼亚首次报道ASF疫情[14],随后该病流行于撒哈拉以南的非洲地区。据报道ASFV早已在东非和南非的疣猪和非洲钝缘软蜱中存在了多个世纪[15]。经历了上世纪50至80年代从非洲大陆到欧洲和美国的几段长距离传播之后,近30年以来,除了意大利的撒丁岛,ASF局限于非洲地区。直到2007年,ASF从非洲老疫区传播至东欧的格鲁吉亚,随后迅速蔓延至整个高加索地区和俄罗斯联邦等地。该病于2012年传入乌克兰,2013年传入白俄罗斯,2014年传入波兰、立陶宛、拉脱维亚、爱沙尼亚,2016年传入摩尔多瓦,2017年传入捷克和罗马尼亚,2018年8月传入中国和比利时[1,2,3,4,5,6]。这是比利时时隔 33年再次爆发ASF疫情。截止到目前,全球近60个国家发生过ASF疫情。ASF自2018年8月首次传入我国辽宁以来,不到3个月时间迅速蔓延至河南、江苏、浙江、安徽、黑龙江、内蒙古和吉林、天津、山西、云南、湖南、贵州等13个省(直辖市、自治区)[4,6],累计爆发疫情50多起,扑杀猪只超过20万头,疫情呈现区域流行态势,进一步扩散和蔓延的风险极高,防控形势异常严峻。同时,近年来全球ASF疫情明显抬头,在俄罗斯和东欧国家不断蔓延,并呈持续扩散态势。俄罗斯ASF疫情异常严峻,仅2018年已爆发疫情上百起,其境内野猪的潜在感染使得该病在俄罗斯快速根除的可能性很小。我国作为猪产品最大的进口国,与非洲、欧洲以及俄罗斯等周边国家的贸易不断增多,使ASF再次传入我国的风险极高。
2.1 传染源
感染ASFV的家猪、野猪、软蜱,猪肉及其制品、受污染的饲料、运输车辆、人员、设施等均为重要的传染源。家猪对ASFV高度易感,一旦感染可出现高热、出血和高死亡率,是疫情扩散的主要传染源[16]。非洲疣猪分布广泛,与家猪和生活在洞穴中的钝缘蜱接触机会较多,因此是ASFV在非洲最重要的感染源。软蜱通过叮咬带毒疣猪而被感染,再通过叮咬易感猪而传播病毒。非洲丛林猪感染ASFV后其病毒血症可持续91 d,但不表现病症[17]。亚临床感染、慢性感染或耐过猪是重要的传染源。这些猪在长达数周内仍具有感染性,可通过软蜱叮咬或直接及间接接触将疫病传染给其他易感猪[18]。非洲巨型森林猪很少受ASFV感染[19],在疫病传播中的作用较小。2.2 传播方式
ASF的宿主和传播媒介涉及家猪、各种野猪和部分软蜱,在其间保持着复杂的循环。在非洲,软蜱感染ASFV后通过叮咬传染给野猪,未感染ASFV的软蜱通过叮咬感染的野猪获得病毒,通过叮咬再感染其他野猪,形成“野猪-软蜱-野猪”循环(森林循环),另外还存在家猪-家猪和野猪-野猪循环(图3)。图3
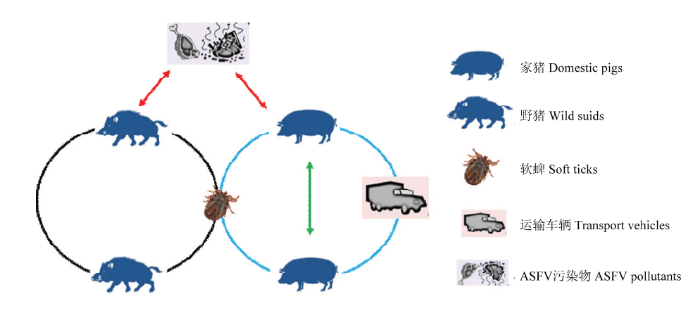 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3ASFV的传播循环
Fig. 3The transmission cycles of ASFV
从野生宿主到家猪的传播机制还不完全清楚[19]。南非野猪和家猪之间杂交、家猪误食带毒的野猪肉、家猪与野猪共存区域通过蜱传播给家猪都可能为ASFV的传播提供机会。ASFV一旦传入家猪,感染猪的排泄物、分泌物、血液、组织等均含有病毒,成为危险的传染源。ASFV可通过直接接触感染猪尸体、污染物、肉制品在地区甚至国际范围内传播[20,21]。
2.3 分子流行病学
分子流行病学研究对调查ASF的流行病学模式以及病源的追溯具有重要作用。最初通过ASFV基因组限制性酶切图谱分析及测序技术,根据基因组长度的差异来分析病毒流行情况。目前主要先基于p72(B646L)基因分型,再根据B602L、E183L或CP204L基因进一步区分亚型[22]。基于B646L基因已鉴定出24个基因型[8,9],其中20个基因型仅存在于东非和南非[23]。基因I型或称ESAC-WA基因型,由欧洲、南美洲、加勒比海和西非等地区的分离株组成[24]。最近流行于高加索和俄罗斯的ASFV为基因II型[2]。基因II型ASFV最初很可能是从莫桑比克传入马达加斯加。在非洲东部和南部,一些基因型(如VIII和XIX)高度同源,这些毒株可能仅限于猪之间传播,或在猪与寄生于家猪的蜱间传播。另外一些基因型(如V、X、XI、XII、XIII和XIV)毒株同时存在于猪-蜱循环和猪-猪循环[25,26]。遗传多样性也可能受到不同分离株协同感染、重排和病毒进化的影响[27]。我国爆发的ASF疫情是由ASFV基因II型毒株引起[6]。不同ASFV基因型地理分布不同(图4),这说明了ASF流行病学的复杂性。图4
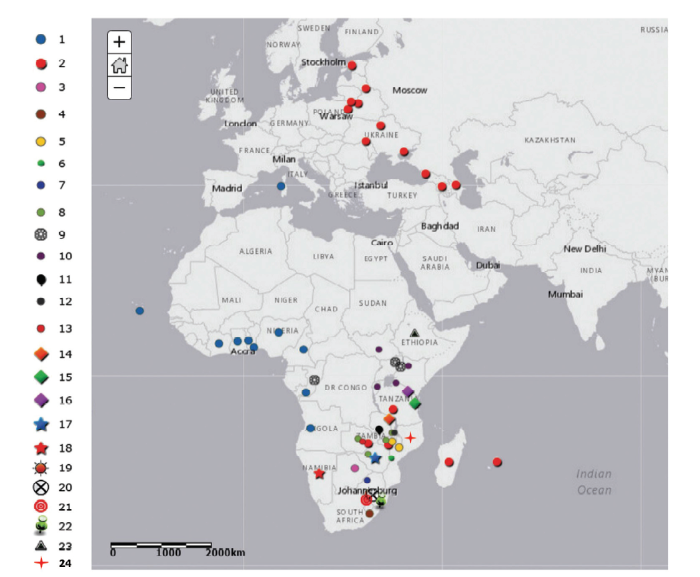 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4ASFV不同基因型及地理分布(根据INIA-CISA, 2016整理)
Fig. 4Genotypes and geographical distribution of ASFV (adapted from INIA-CISA, 2016)
3 非洲猪瘟的诊断及疫苗研究
3.1 诊断
3.1.1 病原学检测方法 ASFV结构复杂,在感染细胞内可检测到100多种病毒蛋白,其中具有免疫原性和诊断意义的主要有p72、p54、p30等蛋白。PCR方法因其具有很高的灵敏度和特异性,是国际贸易中OIE指定的ASFV检测方法。PCR技术同时适用于检测不适合进行病毒分离的腐败组织或血液样品。(1)普通PCR 普通PCR主要为基于相对保守的ASFV p72基因设计引物建立的诊断方法[28,29],可用于ASF的监测和诊断。最近的一份研究报告显示,OIE推荐的PCR方法敏感性和特异性降低,推测可能是由于引物和病毒靶基因的核苷酸不匹配所致[30]。因此,本团队建立了改进的PCR方法,可以检测目前流行的ASFV毒株 [28]。
(2)荧光定量PCR(quantitative Real-time PCR,qPCR) qPCR利用特异性寡核苷酸探针的荧光信号检测目标序列的扩增,具有快速、灵敏、交叉污染低、并可对结果定量等优点。2003年,KING等建立了针对ASFV p72基因的qPCR,其引物和探针获得OIE认证[31]。2007年,MCKLLEN等建立了分子信号实时PCR,该方法灵敏度较高,可与ASFV症状相似的猪瘟进行鉴别诊断[32]。
(3)多重PCR(multiplex PCR) 随着集约化养猪业的发展,混合感染比较普遍[33]。此外现地存在与ASF临床症状非常相似的疾病,如猪瘟,仅根据临床症状和病理变化,难以鉴别[34]。多重PCR方法可有效鉴别诊断的同时,兼具普通PCR快速、灵敏的特点。HU等建立的多重PCR检测可以同时检测并鉴别ASFV、猪瘟病毒(classical swine fever virus,CSFV)、高致病性猪繁殖与呼吸综合征病毒(highly pathogenic porcine reproductive and respiratory syndrome virus,HP-PRRSV)和伪狂犬病病毒(pseudorabies virus,PRV),并可用于这些疫病的流行病学监测[35]。
(4)等温扩增技术 Invader分析是美国三波技术公司开发的一种等温非“PCR”的DNA和RNA定性和定量检测方法[36]。基于Invader技术设计的ASFV特异性信号探针和Invader寡核苷酸可特异地检测ASFV,并且与CSFV没有交叉反应。JAMES等利用环介导等温扩增(loop-mediated isothermal amplification,LAMP)技术靶向特异性拓扑异构酶II基因检测ASFV,而与CSFV无交叉反应[37]。GAO等建立了检测ASFV的交叉引物扩增(cross-priming amplification,CPA)方法,并与试纸条联用,达到快速诊断ASF的目的[38]。同时该方法不需要昂贵的热循环仪器,在廉价的水浴设备中即可完成,适合现地快速检测。
(5)红细胞吸附试验(hemadsorption test,HAD) HAD试验是利用猪红细胞能够吸附在感染ASFV的猪单核细胞或巨噬细胞表面,形成特征性花环的特性,并且大多数ASFV毒株均可以产生这种吸附现象。由于该方法耗时长、操作繁琐,不能用于非血细胞吸附毒株的诊断,只能作为ELISA、PCR等阳性结果确认的一个参考试验。
(6)荧光抗体试验(the fluorescent antibody test,FAT) FAT通过使用异硫氰酸荧光素结合的特异性抗体检测细胞内抗原。该方法可以用来检测疑似猪组织中的ASFV抗原。FAT可用于检测无HAD现象的ASFV毒株,从而识别非血细胞吸附病毒株。另外,该方法还可根据荧光强弱来估测抗原含量,初步进行病毒定量。BOTIJAL等通过FAT快捷地检测出ASFV,并且该方法具有很高的敏感性,但是荧光素的非特异性反应会造成假阳性结果[39]。尽管FAT是急性ASF的一种高度敏感的检测方法,但是对亚急性和慢性ASF的检测灵敏度较低。
3.1.2 血清学检测方法 用于诊断ASFV感染的主要血清学方法包括ELISA抗体检测、间接免疫荧光抗体试验(indirect fluorescent antibody assay,IFA)等。ELISA抗体检测是国际贸易中OIE指定的ASF诊断方法[4]。基于全病毒抗原或表达抗原(如p72、p54等)的间接ELISA或利用针对某一蛋白(如p72)的单克隆抗体建立的阻断ELISA方法可以检测血清中的ASFV抗体,主要适用于亚急型和慢性ASF的诊断。当ELISA检测结果不确定或制备抗原困难或复杂时,可选用IFA方法。
3.2 疫苗研究进展
3.2.1 灭活疫苗 ASF灭活疫苗免疫猪后可检测到ASFV特异性抗体,但是没有保护作用,未来研发前景不大[40]。3.2.2 核酸疫苗 细胞免疫和体液免疫应答均在抵抗病毒感染和清除病毒中发挥作用[41,42]。抗体与ASFV诱导的保护性免疫应答相关,其介导的保护性反应可有效延缓疾病的进程。CD8+ T细胞反应在ASFV感染的保护性免疫反应中发挥关键的作用。ARGILAGUET等将ASFV血凝素(sHA)、p30和p54胞外域融合真核表达质粒免疫猪后无法抵御ASFV强毒株的攻击。随后,将这3种病毒抗原连接到泛素上,用其免疫可诱导特异性T细胞应答,在不产生抗体的情况下,对致死性ASFV的攻击起到部分保护,确定了T细胞反应在ASFV感染保护中的作用[43]。为进一步研究能够刺激CD8+ T细胞反应的潜在免疫保护区,LACASTA等通过表达文库构建了上千个表达质粒(ASFVUblib),并对其进行了免疫攻毒保护试验,其保护率只有60%,但免疫攻毒后存活猪无排毒现象[44],为有效疫苗的研发迈出了重要一步。事实上,ASFV的p30、p54和p72蛋白单独或与其他病毒蛋白混合免疫,均能产生较高水平的抗体,并使血液中病毒含量显著减少,如何更有效地将细胞免疫和体液免疫结合,提高保护率,是ASFV核酸疫苗研发的关键和面临的难题[45]。
3.2.3 亚单位疫苗 ASFV基因组含有多达167个开放阅读框,这种蛋白编码的复杂性使得有效保护性抗原的筛选工作十分困难。因此ASFV亚单位疫苗的研制举步维艰。目前已证实多个病毒蛋白能够诱导中和抗体,其中p54和p30参与病毒的吸附和内化过程。但表达p54和p30的重组杆状病毒不能保护猪免受ASFV强毒株的攻击[46]。此外,同时表达p30、p54、p22和p72的杆状病毒尽管能诱导产生中和抗体,但仍无法保护猪免受ASFV强毒株的攻击[47]。这些数据表明,抗体介导的中和作用在ASFV诱导的保护中并不起关键作用。在另一项研究中,表达CD2v/EP402R蛋白的杆状病毒可以对ASFV同源毒株的攻击提供部分保护[48]。这可能与诱导产生的抗体能够抑制血细胞吸附(HAD)以及暂时抑制病毒的感染有关。
3.2.4 病毒活载体疫苗 针对ASFV的病毒活载体疫苗研究相对较少,目前已经开展的研究主要选用了痘病毒和腺病毒作为载体。LOPERA-MADRID等利用反向疫苗学技术筛选了5种ASFV抗原,在HEK293细胞中表达B646L(p72)、E183L(p54)和O61R(p12),并在经过减毒的牛痘病毒安卡拉(MVA)株病毒载体中表达B646L、EP153R和EP402R(CD2v),将上述蛋白按照不同组合通过初次免疫-加强免疫(prime-boost)策略免疫猪后,可以诱导ASFV特异性抗体和T细胞反应[49]。为了鉴定更多的ASFV免疫原性基因和潜在的保护性抗原,JANCOVICH等分别在质粒载体和重组牛痘病毒中克隆了47个ASFV基因用于免疫,利用DNA初次免疫和重组牛痘病毒加强免疫猪。经Georgia 2007/1株攻毒后,免疫猪的血液和淋巴组织中的ASFV基因组水平明显降低,但猪只出现了与急性ASFV一致的临床和病理变化[45]。LOKHANDWALA等先后将ASFV的保护性抗原重组至人5型腺病毒载体和复制缺陷型腺病毒载体中,通过“鸡尾酒”式混合免疫后能够在猪体内诱导高水平的体液免疫和细胞免疫应答[50,51]。但该研究未涉及动物攻毒保护试验,因此所构建的病毒活载体疫苗的效力还有待验证。
3.2.5 ASFV减毒活疫苗和基因缺失疫苗 ASFV减毒活疫苗可以诱导针对相同基因型ASFV毒株的有效免疫保护,但是对不同基因型ASFV毒株不能提供有效保护[52,53],这可能与病毒特异性T细胞反应有关[54,55]。这也是制约ASFV疫苗研发的一个难题。自然致弱的减毒活疫苗ASFV OURT88/3株和NH/P68株可以保护相同基因型ASFV毒株攻击,但对不同基因型ASFV毒株仅有部分的保护力。同时这些减毒活疫苗免疫后引发病毒血症和肺炎、关节炎等副反应,有一定的安全隐患[52,53]。目前减毒活疫苗的研发主要集中在提高安全性和增强对不同基因型的保护力方面。缺失病毒-细胞互作基因(A224L、A238L和A276R)的重组NH/P68株虽然对相同或者不同基因型(Armenia 2007株)有60%—100%的保护力,然而该疫苗株也产生病毒血症和副作用。在猪肺泡巨噬细胞(PAM)中培养的NH/P68株能对相同或者不同基因型ASFV攻击提供良好的保护,且在免疫后不表现明显的亚临床症状,在不同的细胞中培养的ASFV免疫猪后表现出不同的保护性[56]。通过基因工程手段构建的缺失9GL和MGF360/505的Georgia 2007/1株虽然使病毒的毒力减弱,但是不能对相同基因型的亲本毒株攻击提供有效的保护[57]。目前,通过同源重组构建的Georgia 2007/1株9GL和UK基因缺失减毒活疫苗可以对相同基因型ASFV的攻击提供100%保护力[58]。为研究安全有效的ASF疫苗,各国的科学家们进行了大量的尝试。MONTEADGUDO等发现,删除ASFV BA71株的CD2v(EP402R)基因(BA712RA7)后可以显著降低其毒力,将该基因缺失病毒免疫猪只后可以对BA71的攻击提供100%的保护力,同时也可以抵抗目前在东欧流行的基因II型Georgia 2007/1株的攻击[59]。该研究结果对于研发具有交叉保护力的ASFV减毒活疫苗具有重要借鉴意义。
4 防控措施和建议
ASF疫情在我国高频度、跨区域大范围出现,并有持续蔓延之势,防控形势异常严峻。非洲猪瘟的传播方式众多,发病猪及感染猪的排泄物、分泌物、猪肉及其制品以及污染的运输车辆、饲料、人员、衣物、鞋子等均为重要的传染源,特别是感染猪的调运会加速疫情的传播。4.1 进一步加强防控措施
为了更有效地遏制当前ASF疫情的蔓延,建议尽快成立国家ASF应急中心,由中央统一协调和督导兽医防疫、检验检疫、海关、交通、公安等相关部门,联合开展ASF疫情筛查、处置、猪只调运监管、猪肉及相关产品运输检疫等措施;立即启动重大疫情I级应急响应,暂停跨省市猪只调运,严控跨区域猪只流动。同时抓紧研制符合生物安全标准的生猪调运装备,建立可监控、可追溯的生猪调运体系;完善疫情诊断机制,疫情确诊要有两个以上的有资质专业机构相互印证,防止疫情错判或漏诊,冲击养猪业和食品安全、浪费防疫资源乃至引起社会动荡,造成巨大经济和社会损失;加强对基层从业人员的培训,确保防控措施有效落地。养猪场(户)加强饲养管理,提高生物安全水平,严禁使用泔水喂猪,避免家猪与野猪接触,防止软蜱等吸血昆虫的叮咬。加强疫情监测,一旦发生疫情,立即启动应急预案,严格封锁、扑杀、消毒、移动控制,严防疫情扩散。严禁从有ASF疫情的国家或地区进口猪及其产品,对进口猪及其产品的入境运输工具进行严格监督、检查、登记和消毒,防止运输工具机械传播。对国际航班、火车、船舶的废弃物和泔水等严格进行无害化处理,防止家猪接触感染。
4.2 加快开展科学研究
组织具有高级别生物安全设施的研究机构进行联合攻关,研制更加快速高效的检测和防控技术,加强流行病学调查,追溯疫情来源,对流行毒株进行遗传和分子特征解析和致病性等研究,评估疫病传播特点和传播风险。由于目前尚无有效的ASF疫苗,开发安全有效的疫苗迫在眉睫。4.3 加强国际合作,吸取他国ASF防控的经验和教训
西班牙、海地等国家应对和根除非洲猪瘟的成功经验值得我国学习和借鉴。以西班牙为例,ASF于1960年传入西班牙。1985年之前,西班牙仅采取扑杀阳性猪群和进行消毒处理等措施控制该病,疫情未能得到有效控制。之后,西班牙颁布了非洲猪瘟根除计划,采取了一系列防控措施,包括建立流动现场兽医团队网络体系;对所有猪场进行血清学监测;提高养猪场的生物安全水平;迅速拔除所有疫点,对受威胁区进行病原学和流行病学调查,及时对生产者进行足额补偿;严格控制猪只移动。通过及时准确的监测和严格有效的封锁和扑杀等措施,在根除计划颁布后10年成功根除了该病[60]。一些发达国家之所以长期保持ASF无疫状态(或者即便疫情传入也能及时予以根除),总结起来关键在于:具有完善的监测计划和迅捷的预警响应系统;具有完善的疫情控制体系和强有力的防控技术支撑;具有严格的动物流动监管体系和动物产品可追溯体系;具有相对发达的养猪业(多以规模化猪场为主),生物安全措施比较健全[61,62]。
反观俄罗斯,ASF自2007年起流行11年,愈演愈烈,主要原因在:疫病防控体系薄弱,缺乏集中系统的防控计划,导致监测系统不完善,疫情应对滞后和疫病扩散;感染猪及其肉制品的非法贸易、泔水饲喂、发病猪的不当处置等,导致疫情大面积扩散;现代养猪业相对落后,散养户较多,加大防控难度;虽然实行扑杀政策,但经济补偿不到位,造成农场主不配合(隐瞒疫情和处置情况),贻误防控时机[61,63]。
5 结语
ASF是一种急性、出血性、致死性极强的重大疫病,一旦爆发,将重创疫区国家的养猪业和猪肉国际贸易。随着经济全球化发展,国际贸易往来日益频繁,该病呈现全球流行态势,对世界各国的养猪业构成持续的威胁。曾经离我们还很遥远的ASF瘟疫,已成为我国养猪业的重大现实的威胁,在短短二个多月内迅速蔓延至我国10多个省份,持续扩散和流行的风险极高。ASF流行病学复杂,宿主媒介涉及家猪、野猪和软蜱,在三者之间保持着复杂的传播循环。因此,ASF的预防和控制需要更好地了解该病的流行病学,从而实施有针对性的措施。目前尚无针对ASF有效的治疗方法和疫苗,因此迫切需要研究早期快速的ASF检测及防控技术,以及时发现ASFV感染猪并及时扑灭疫情。基于抗原或基因组的ASFV检测技术,可大大节省诊断时间。随着分子技术的发展,可移动式PCR、等温扩增和试纸条等检测技术等可实现快速现地诊断,且具有良好的敏感性,但这些方法还需要临床前评价和临床试验。目前商业化的ELISA试剂盒虽然可以用于检测ASFV抗体或抗原,但其特异性和敏感性还有待提高。由于ASFV具有庞大的基因组结构和复杂的免疫逃逸机制,使得研制有效的疫苗成为难题。未来需要深入解析病毒毒力相关基因、免疫保护性相关抗原、有效的抗原递送系统以及高效的免疫佐剂。同时,鉴于核酸疫苗、亚单位疫苗、病毒活载体疫苗的效力有限,应着力研制基因缺失疫苗和弱毒疫苗,解决安全性(无残余毒力、不长时间排毒)、稳定性(体内外稳定,不发生返祖突变)、免疫效力(特别是对异型的交叉保护)、鉴别诊断(不影响监测)等难题。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.virusres.2012.10.030URLPMID:23123296 [本文引用: 3]

African swine fever virus used to occur primarily in Africa. There had been occasional incursions into Europe or America which apart from the endemic situation on the island of Sardinia always had been successfully controlled. But following an introduction of the virus in 2007, it now has expanded its geographical distribution into Caucasus and Eastern Europe where it has not been controlled, to date. African swine fever affects domestic and wild pig species, and can involve tick vectors. The ability of the virus to survive within a particular ecosystem is defined by the ecology of its wild host populations and the characteristics of livestock production systems, which influence host and vector species densities and interrelationships. African swine fever has high morbidity in na茂ve pig populations and can result in very high mortality. There is no vaccine or treatment available. Apart from stamping out and movement control, there are no control measures, thereby potentially resulting in extreme losses for producers. Prevention and control of the infection requires good understanding of its epidemiology, so that targeted measures can be instigated.
DOI:10.3201/eid1412.080591URLPMID:19046509 [本文引用: 3]

The virus isolate introduced to the Caucasus in 2007 is closely related to a group of viruses, genotype II, circulating in Mozambique, Madagascar, and Zambia. African swine fever (ASF) is widespread in Africa but is rarely introduced to other continents. In June 2007, ASF was confirmed in the Caucasus region of Georgia, and it has since spread to neighboring countries. DNA fragments amplified from the genome of the isolates from domestic pigs in Georgia in 2007 were sequenced and compared with other ASF virus (ASFV) isolates to establish the genotype of the virus. Sequences were obtained from 4 genome regions, including part of the geneB646Lthat encodes the p72 capsid protein, the completeE183LandCP204Lgenes, which encode the p54 and p30 proteins and the variable region of theB602Lgene. Analysis of these sequences indicated that the Georgia 2007 isolate is closely related to isolates belonging to genotype II, which is circulating in Mozambique, Madagascar, and Zambia. One possibility for the spread of disease to Georgia is that pigs were fed ASFV-contaminated pork brought in on ships and, subsequently, the disease was disseminated throughout the region.
DOI:10.1016/j.virusres.2012.12.007URLPMID:23266725 [本文引用: 2]

Since the introduction of the virus into the Republic of Georgia in 2007 African swine fever (ASF) has become a large-scale epidemic involving the domestic pig population but wild boars are involved as well. From 2008 to 2009 the ASF epidemic affected wild and domestic pigs in all the southern regions of the Russian Federation (RF). The driving force of the epidemic in its initial stages was direct contact between infected wild boars and between wild boars and traditionally free-ranging domestic pigs in backyard farms. Driving forces of the epidemic at the its first stages was direct contact of infected wild boars between each other and with traditionally free ranged domestic pigs in backyard farms. The next stage developed due to illegal movement of pig products contaminated by African swine fever virus (ASFV) from affected regions and swill feeding, and inefficient implementation of measures to prevent and control ASF. From 2010 through 2012, ASF spread to other, previously unaffected regions of the RF. Most of outbreaks in the southern regions (Krasnodar, Stavropol, Rostov regions) are secondary. Currently, the disease situation observed in endemic areas of the RF, including the southern Krasnodar and Volgograd regions and the central Tver region, is very complicated. In 2012, a large number of outbreaks in domestic pigs and in wild boars were reported. The circulating ASFV is highly virulent and has maintained its virulence throughout the epidemic since its introduction in 2007. Considering the forces currently driving the ASF epidemic circulation of ASF virus in wild boars, ineffectiveness of prevention and control measures, lack of common interest in eradicating the disease and absence of a nationally funded eradication program continued outbreaks, including those in previously unaffected regions of the RF, can be expected.
URL [本文引用: 5]
DOI:10.20506/rst.34.2.2375URLPMID:26601452 [本文引用: 2]

African swine fever (ASF), one of the most important diseases of swine, is present in many African countries, as well as in eastern Europe, Russia and Sardinia. It is caused by a complex virus, ASF virus (ASFV), for which neither vaccine nor treatment is available. ASFV affects swine of all breeds and ages, and also replicates in soft ticks of the genus Ornithodoros, facilitating ASFV persistence and reocurrence of disease. Depending on the involvement of these ticks, and the presence or not of sylvatic asymptomatic animals, several epidemiological cycles have been identified. The disease persists in East and southern African countries in a sylvatic cycle between O. porcinus (of the O. moubata species complex) and common warthogs. In some countries a domestic pig ick cycle exists, whereas in other regions, notably West Africa, the role of soft ticks has not been demonstrated, and ASFV is transmitted between domestic pigs in the absence of tick vectors. Even in several East and Central African countries which have the sylvatic or domestic cycle, the majority of outbreaks are not associated with ticks or wild suids. In Europe, O. erraticus was detected and identified as a crucial vector for ASF maintenance in outdoor pig production on the Iberian Peninsula. However, in most parts of Europe, there is a lack of information about the distribution and role of Ornithodoros ticks in ASF persistence, particularly in eastern regions. This article reviews ASF epidemiology and its main characteristics, with a special focus on the distribution and role of soft ticks in ASF persistence in different settings. Information about tick detection, control measures and future directions for research is also included.
[本文引用: 4]
DOI:10.1016/j.virusres.2012.10.020URLPMID:23142553 [本文引用: 3]

African swine fever virus (ASFV) is a large icosahedral DNA virus which replicates predominantly in the cytoplasm of infected cells. The ASFV double-stranded DNA genome varies in length from about 170 to 193kbp depending on the isolate and contains between 150 and 167 open reading frames. These are closely spaced and read from both DNA strands. The virus genome termini are covalently closed by imperfectly base-paired hairpin loops that are present in two forms that are complimentary and inverted with respect to each other. Adjacent to the termini are inverted arrays of different tandem repeats. Head to head concatemeric genome replication intermediates have been described. A similar mechanism of replication to Poxviruses has been proposed for ASFV. Virus genome transcription occurs independently of the host RNA polymerase II and virus particles contain all of the enzymes and factors required for early gene transcription. DNA replication begins in perinuclear factory areas about 6h post-infection although an earlier stage of nuclear DNA synthesis has been reported. The virus genome encodes enzymes required for transcription and replication of the virus genome and virion structural proteins. Enzymes that are involved in a base excision repair pathway may be an adaptation to enable virus replication in the oxidative environment of the macrophage cytoplasm. Other ASFV genes encode factors involved in evading host defence systems and modulating host cell function. Variation between the genomes of different ASFV isolates is most commonly due to gain or loss of members of multigene families, MGFs 100, 110, 300, 360, 505/530 and family p22. These are located within the left terminal 40kbp and right terminal 20kbp. ASFV is the only member of the Asfarviridae, which is one of the families within the nucleocytoplasmic large DNA virus superfamily.
[本文引用: 3]
DOI:10.3390/v9050103URLPMID:28489063 [本文引用: 2]

African swine fever (ASF) is a highly contagious viral disease of swine which causes high mortality, approaching 100%, in domestic pigs. ASF is caused by a large, double stranded DNA virus, ASF virus (ASFV), which replicates predominantly in the cytoplasm of macrophages and is the only member of theAsfarviridaefamily, genusAsfivirus. The natural hosts of this virus include wild suids and arthropod vectors of theOrnithodorosgenus. The infection of ASFV in its reservoir hosts is usually asymptomatic and develops a persistent infection. In contrast, infection of domestic pigs leads to a lethal hemorrhagic fever for which there is no effective vaccine. Identification of ASFV genes involved in virulence and the characterization of mechanisms used by the virus to evade the immune response of the host are recognized as critical steps in the development of a vaccine. Moreover, the interplay of the viral products with host pathways, which are relevant for virus replication, provides the basic information needed for the identification of potential targets for the development of intervention strategies against this disease.
DOI:10.1111/tbed.12700URLPMID:28921895 [本文引用: 1]

react-text: 156 African Swine Fever is a notifiable devastating hemorrhagic fever with high mortality rates in pigs. It affects all members of the Suidae family and is one of the most important pig diseases due to…" /react-text react-text: 157 /react-text [more]
DOI:10.1016/j.virusres.2015.01.022URLPMID:25662020 [本文引用: 1]

The main cellular target for African swine fever virus (ASFV) is the porcine macrophage. However, existing data about the early phases of infection were previously characterized in non-leukocyte cells such as Vero cells. Here, we report that ASFV enters the natural host cell using dynamin-dependent and clathrin-mediated endocytosis. This pathway is strongly pH-dependent during the first steps of infection in porcine macrophages. We investigated the effect of drugs inhibiting several endocytic pathways in macrophages and compared ASFV with vaccinia virus (VV), which apparently involves different entry pathways. The presence of cholesterol in cellular membranes was found to be essential for a productive ASFV infection while actin-dependent endocytosis and the participation of phosphoinositide-3-kinase (PI3K) activity were other cellular factors required in the process of viral entry. These findings improved our understanding of the ASFV interactions with macrophages that allow for successful viral replication.
DOI:10.1128/JVI.01557-09URL [本文引用: 1]

African swine fever virus (ASFV) is a large DNA virus that enters host cells after receptor-mediated endocytosis and depends on acidic cellular compartments for productive infection. The exact cellular mechanism, however, is largely unknown. In order to dissect ASFV entry, we have analyzed the major endocytic routes using specific inhibitors and dominant negative mutants and analyzed the consequences for ASFV entry into host cells. Our results indicate that ASFV entry into host cells takes place by clathrin-mediated endocytosis which requires dynamin GTPase activity. Also, the clathrin-coated pit component Eps15 was identified as a relevant cellular factor during infection. The presence of cholesterol in cellular membranes, but not lipid rafts or caveolae, was found to be essential for a productive ASFV infection. In contrast, inhibitors of the Na(+)/H(+) ion channels and actin polymerization inhibition did not significantly modify ASFV infection, suggesting that macropinocytosis does not represent the main entry route for ASFV. These results suggest a dynamin-dependent and clathrin-mediated endocytic pathway of ASFV entry for the cell types and viral strains analyzed.
[本文引用: 1]
DOI:10.1016/S0368-1742(21)80031-4URL [本文引用: 1]
[本文引用: 1]
DOI:10.4102/jsava.v80i2.172URLPMID:19831264 [本文引用: 1]

African swine fever is one of the most important and serious diseases of domestic pigs. Its highly contagious nature and ability to spread over long distances make it one of the most feared diseases, since its devastating effects on pig production have been experienced not only in most of sub-Saharan Africa but also in western Europe, the Caribbean, Brazil and, most recently, the Caucasus. Unlike most diseases of livestock, there is no vaccine, and therefore prevention relies entirely upon preventing contact between the virus and the susceptible host. In order to do so it is necessary to understand the way in which the virus is transmitted and spreads. By implementing strict biosecurity measures that place barriers between the source of virus and the pigs it is possible to prevent infection. However, this has implications for free-ranging pig husbandry systems that are widespread in developing countries. Attempts to produce a vaccine are ongoing and new technology offers some hope for the future, but this will not remove the necessity for implementing adequate biosecurity on pig farms.
DOI:10.1016/S0378-1135(98)00187-4URLPMID:9659687 [本文引用: 1]

Abstract Warthog (Phacochoerus aethiopicus), giant forest hog (Hylochoerus meinertzhageni) and bushpig (Potamochoerus porcus) are known to be susceptible to infection with African swine fever (ASF) virus. Little however, is known about the ecology of the disease in the bushpig. This study has shown that the bushpig remains viraemic for between 35 and 91 days following infection during which time it is able to infect the tick vector O. moubata. These ticks were able to transmit the disease to pigs. The virus persists in the lymphatic tissues for less than 34 weeks. Bushpigs infected with LIL 20/l virus but not VIC T90/l virus transmitted infection to in-contact pigs. Infected domestic pigs did not transmit the infection to in-contact bushpigs. ASF virus was able to replicate in in vitro cultures of bushpig leucocytes and endothelial cells. Recovered bushpigs could be reinfected with some strains of virus but not others. While it has been demonstrated that bushpigs remain carriers of ASFV following infection a complete understanding of their significance in the epidemiology of the disease awaits further investigations of their association with O. moubata.
DOI:10.1016/0167-5877(84)90050-3URL [本文引用: 1]

African swine fever (ASF) virus is maintained in Africa primarily by a cycle of infection between wart hogs and soft ticks (Ornithodoros moubata) in which ticks may become infected by feeding on young wart hogs, which are viraemic for only a short period after birth. Infected ticks constitute a permanent reservior of infection for domestic pigs, which may become infected by the bite of infected ticks or the ingestion of infected wart hog tissues. In the Mediterraean region, ASF in enzootic in Spain, Portugal and Sardinia. The main factors contributing to persistence in the Iberian peninsula are the difficulty of detecting disease caused by the newer, less virulent viruses, extensive systems of husbandry, increased production and trade in pigs, the excess concentration of pig farms in certain areas and the presence of soft tick vectors (Ornithodoros erraticus) in the south-west.
URLPMID:4283999 [本文引用: 2]

Heuschele WP, Coggins L.
[本文引用: 1]
DOI:10.1016/S0167-5877(96)01088-4URLPMID:9329109 [本文引用: 1]

Abstract The animal health hazards associated with the importation of pork and pork products include four viral agents: foot and mouth disease, classical swine fever (hog cholera), African swine fever, and swine vesicular disease viruses. The safety of importing pork from a zone infected with one or more of these diseases can be adequately determined only through risk assessment. This also applies for the safety of importing pork products which have undergone some form of processing (fully cooked pork products are not counted here). For each disease, the agent (pH and temperature lability), target organs, agent survival in pork and pork products, and agent quantification are discussed. Agent quantification is an input of the risk assessment which measures the viral titres in waste pork and pork products in relation to the oral infective dose estimated for each disease. Two other viral diseases, transmissible gastroenteritis of pigs and porcine reproductive and respiratory syndrome, are presented to illustrate why these two diseases are not hazards when associated with pork and pork products.
DOI:10.1007/s11262-009-0444-0URLPMID:20052526 [本文引用: 1]

Samples collected from wild and domestic suids in Nigeria, over a 3-year period (2003-2006), were evaluated for African swine fever (ASF) virus genome presence by targeting three discrete genome regions, namely the 478-bp C-terminal p72 gene region advocated for genotype assignment, a 780-bp region spanning the 5'-ends of the pB125R and pB646L (p72) genes and the hypervariable central variable region (CVR) encoded within the 9RL ORF (pB602L). ASF virus (ASFV) presence was confirmed in 23 of the 26 wild and domestic pigs evaluated. No evidence of ASF infection was found in two warthogs from Adamawa State; however, one bushpig from Plateau State was positive. Nucleotide sequences of the 478-bp and 780-bp amplicons were identical across all ASFV-positive samples sequenced. However, five discrete CVR variants were recovered, bringing the total number identified to date, from Nigeria, to six. The largest of the CVR variants, termed 'Tet-36' was identical to a virus causing outbreaks in neighbouring Benin in 1997, indicating a prolonged persistence of this virus type in Nigeria. Co-circulation of three tetramer types (Tet-36, Tet-27 and Tet-20) was found in Plateau State in July 2004, whilst in Benue State, two tetramer types (Tet-20 and Tet-21) were present in August 2005. Despite simultaneous field presence, individual co-infection was not observed. This study has reaffirmed the epidemiological utility of the CVR genome region for distinguishing between geographically and temporally constrained genotype I viruses, and has revealed the presence of multiple ASFV variants in Nigeria.
DOI:10.1007/s11262-008-0293-2URLPMID:19009341 [本文引用: 1]

Complete sequencing of p54- gene from 67 European, American, and West and East African Swine Fever virus (ASFV) isolates revealed that West African and European ASFV isolates classified within the predominant Genotype I according to partial sequencing of p72 were discriminated into four major sub-types on the basis of their p54 sequences. This highlighted the value of p54 gene sequencing as an additional, intermediate-resolution, molecular epidemiological tool for typing of ASFV viruses. We further evaluated p54-based genotyping, in combination with partial sequences of two other genes, for determining the genetic relationships and origin of viruses responsible for disease outbreaks in Kenya. Animals from Western and central Kenya were confirmed as being infected with ASFV using a p72 gene-based PCR assay, following outbreaks of severe hemorrhagic disease in domestic pigs in 2006 and 2007. Eleven hemadsorbing viruses were isolated in macrophage culture and genotyped using a combination of full-length p54 -gene sequencing, partial p72 -gene sequencing, and analysis of tetrameric amino acid repeat regions within the variable region of the B602L gene (CVR). The data revealed that these isolates were identical in their p72 and p54 sequence to viruses responsible for ASF outbreaks in Uganda in 2003. There was a minor difference in the number of tetrameric repeats within the B602L sequence of the Kenyan isolates that caused the second Kenyan outbreak in 2007. A practical implication of the genetic similarity of the Kenyan and Ugandan viral isolates is that ASF control requires a regional approach.
DOI:10.1007/s00705-002-0946-8URLPMID:12664294 [本文引用: 1]

A PCR-based sequencing method was developed which permits detection and characterization of African swine fever virus (ASFV) variants within 5 and 48 , respectively, of receipt of a clinical specimen. Amplification of a 478 p fragment corresponding to the C-terminal end of the p72 gene, confirms virus presence with genetic characterization being achieved by nucleotide sequence determination and phylogenetic analysis. The method was applied to 55 viruses including those representative of the major ASF lineages identified previously by restriction fragment length polymorphism (RFLP) analysis. Results confirmed that the p72 genotyping method identifies the same major viral groupings. Characterization of additional viruses of diverse geographical, species and temporal origin using the PCR-based method indicated the presence of ten major ASF genotypes on the African continent, the largest of which comprised a group of genetically homogeneous viruses recovered from outbreaks in Europe, South America, the Caribbean and West Africa (the ESAC-WA genotype). In contrast, viruses from southern and East African countries were heterogeneous, with multiple genotypes being present within individual countries. This study provides a rapid and accurate means of determining the genotype of field and outbreak strains of ASF and is therefore useful for molecular epidemiological clarification of ASF.
DOI:10.1016/j.vetmic.2006.11.007URLPMID:17174485 [本文引用: 1]

African swine fever (ASF) is a highly lethal and economically significant disease of domestic pigs in the southern African sub-region, where outbreaks regularly occur. There is anecdotal evidence suggesting that trans-boundary movement of infected animals may have played a role in precipitating widespread outbreaks in the past, however, since the 1970s outbreaks have generally been more localised, particularly in those countries where control of animal movement is strictly regulated. The origin and relatedness of regional ASF outbreaks was investigated here by means of a two-step genetic characterisation approach whereby p72 gene sequencing was used to delineate genotypes, prior to intra-genotypic resolution of viral relationships by central variable region (CVR) characterisation of the 9RL ORF. In this manner, regional virus heterogeneity and epidemiological links between outbreaks could be assessed for the first time through phylogenetic analysis of the C-terminal end of the p72 gene of viruses recovered from domestic pig outbreaks in southern Africa between 1973 and 1999. The phylogeny revealed the presence of 14 distinct p72 genotypes of which 6 (genotypes XVII XII) were considered novel. Eight of these were country-specific with the remaining six having a trans-boundary distribution. CVR products were heterogeneous in size ranging from 377 bp to 533 bp across the 14 southern African genotypes. Within-genotype CVR comparisons revealed the presence of a genotype XIX virus with an extended field presence in South Africa (1985 1996) and permitted discrimination between three genotype VII viruses that were identical across the p72 gene.
DOI:10.1007/s00705-005-0602-1URLPMID:16052280 [本文引用: 1]

African swine fever (ASF) a lethal, viral hemorrhagic disease of domestic pigs, first reported from East Africa in 1921, is still widespread in this region. In order to assess field heterogeneity at the regional level, nucleotide sequences corresponding to the C-terminal end of the p72 gene were determined for 77 ASF viruses of diverse temporal and species origin occurring in eight East African countries. The number of sites completely conserved across all East African sequences characterized in this study was 84.2% and 86.8% on nucleotide and amino acid level, respectively. Phylogenetic analysis of a homologous 404 p region revealed the presence of thirteen East African genotypes, of which eight appear to be country specific. An East African, pig-associated, homogeneous virus lineage linked to outbreaks in Mozambique, Zambia and Malawi over a 23 year period was demonstrated. In addition, genotype I (ESACWA) viruses were identified in East African sylvatic hosts for the first time which is significant as this genotype was previously thought to be restricted to the West African region where it occurs only in domestic pigs. The presence of discrete epidemiological cycles in East Africa and recovery of multiple genotypes affirms the epidemiological complexity of ASF in this region.
DOI:10.1016/j.vetmic.2004.09.003URLPMID:15504588 [本文引用: 1]

In 1998, domestic pigs originating from villages within a 40 km radius of Ulongwe in the northern Tete Province of Mozambique were held in a quarantine facility for a 3-month period prior to their importation into South Africa. Eight of a total of 25 pigs died within the first 3 weeks of quarantine of what appeared clinically and on post mortem examination to be African swine fever (ASF). Organs were collected and preserved in formol-glycerosaline and the presence of ASF virus in these specimens was confirmed by three independent polymerase chain reaction (PCR) tests. Two gene regions were characterised, namely the C-terminus end of the major immunodominant protein VP72 and the central variable region (CVR) of the 9RL open reading frame (ORF). Results confirmed the presence of two genetically distinct viruses circulating simultaneously within a single outbreak focus. However, despite the pigs being housed within the same facility, no evidence of co-infection was observed within individual animals. Comparison of the two 1998 virus variants with viruses causing historical outbreaks of the disease in Mozambique revealed that these viruses belong to two distinct genotypes which are unrelated to viruses causing outbreaks between 1960 and 1994. In addition, the CVR and p72 gene regions of one of the 1998 Mozambique virus variants (variant-40) was shown to be identical to the virus recovered from an ASF outbreak in Madagascar in the same year, whilst the other (variant-92) was identical to a 1988 pig isolate from Zambia.
DOI:10.1007/s00705-016-3069-3URLPMID:27714502 [本文引用: 2]

Abstract Due to the current unavailability of vaccines or treatments for African swine fever (ASF), which is caused by African swine fever virus (ASFV), rapid and reliable detection of the virus is essential for timely implementation of emergency control measures and differentiation of ASF from other swine diseases with similar clinical presentations. Here, an improved PCR assay was developed and evaluated for sensitive and universal detection of ASFV. Primers specific for ASFV were designed based on the highly conserved region of the vp72 gene sequences of all ASFV strains available in GenBank, and the PCR assay was established and compared with two OIE-validated PCR tests. The analytic detection limit of the PCR assay was 60 DNA copies per reaction. No amplification signal was observed for several other porcine viruses. The novel PCR assay was more sensitive than two OIE-validated PCR assays when testing 14 strains of ASFV representing four genotypes (I, V, VIII and IX) from diverse geographical areas. A total of 62 clinical swine blood samples collected from Uganda were examined by the novel PCR, giving a high agreement (59/62) with a superior sensitive universal probe library-based real-time PCR. Eight out of 62 samples tested positive, and three samples with higher Ct values (39.15, 38.39 and 37.41) in the real-time PCR were negative for ASFV in the novel PCR. In contrast, one (with a Ct value of 29.75 by the real-time PCR) and two (with Ct values of 29.75 and 33.12) ASFV-positive samples were not identified by the two OIE-validated PCR assays, respectively. Taken together, these data show that the novel PCR assay is specific, sensitive, and applicable for molecular diagnosis and surveillance of ASF.
DOI:10.1016/j.virusres.2012.10.022URLPMID:23131492 [本文引用: 1]

The rapid and reliable detection of African swine fever virus (ASFV) is essential both for timely implementation of control measures to prevent the spread of disease, and to differentiate African swine fever (ASF) from other pig disease with similar clinical presentations. Many virological tests are currently available for the detection of ASFV (live virus), antigen and genome, including virus isolation, ELISA, fluorescent antibody, polymerase chain reaction (PCR) and isothermal assays. In recent years real-time PCR (rPCR) has become one of the most widely used formats for virological diagnosis providing sensitive, specific and swift detection and quantification of ASFV DNA. The ability to integrate rPCR into automated platforms increases sample throughput and decreases the potential for cross-contamination. In more recent years isothermal assays, which are a lower-cost alternative to PCR more suitable for use in non-specialised or mobile laboratories, have been developed for the detection of ASFV, however these assays have not been fully validated for routine use in the field. The performance of all virological detection assays in ASF diagnostics, as well as prospects for improving diagnostic strategies in the future, are discussed and reviewed in this chapter.
DOI:10.1128/JCM.00857-15URLPMID:4508403 [本文引用: 1]

Abstract This study represents a complete comparative analysis of the most widely used African swine fever (ASF) diagnostic techniques in the European Union (EU) using field and experimental samples from animals infected with genotype II ASF virus (ASFV) isolates circulating in Europe. To detect ASFV, three different PCRs were evaluated in parallel using 785 field and experimental samples. The results showed almost perfect agreement between the Universal ProbeLibrary (UPL-PCR) and the real-time ( = 0.94 [95% confidence interval {CI}, 0.91 to 0.97]) and conventional ( = 0.88 [95% CI, 0.83 to 0.92]) World Organisation for Animal Health (OIE)-prescribed PCRs. The UPL-PCR had greater diagnostic sensitivity for detecting survivors and allows earlier detection of the disease. Compared to the commercial antigen enzyme-linked immunosorbent assay (ELISA), good-to-moderate agreement ( = 0.67 [95% CI, 0.58 to 0.76]) was obtained, with a sensitivity of 77.2% in the commercial test. For ASF antibody detection, five serological methods were tested, including three commercial ELISAs, the OIE-ELISA, and the confirmatory immunoperoxidase test (IPT). Greater sensitivity was obtained with the IPT than with the ELISAs, since the IPT was able to detect ASF antibodies at an earlier point in the serological response, when few antibodies are present. The analysis of the exudate tissues from dead wild boars showed that IPT might be a useful serological tool for determining whether or not animals had been exposed to virus infection, regardless of whether antibodies were present. In conclusion, the UPL-PCR in combination with the IPT was the most trustworthy method for detecting ASF during the epidemic outbreaks affecting EU countries in 2014. The use of the most appropriate diagnostic tools is critical when implementing effective control programs. Copyright 2015, American Society for Microbiology. All Rights Reserved.
DOI:10.1016/S0166-0934(02)00189-1URLPMID:12445938 [本文引用: 1]

A closed-tube polymerase chain reaction (PCR) was developed to allow the rapid detection of African swine fever virus (ASFV) DNA. This assay targets the VP72 gene of ASFV and uses the 5′-nuclease assay (TaqMan03) system to detect PCR amplicons, avoiding tube opening and potential cross-contamination of post-PCR products. An artificial mimic was engineered with the TaqMan03 probe site replaced by a larger irrelevant DNA fragment allowing discrimination from ASFV by using two-colour TaqMan03 probe reporters. When added to the samples, successful amplification of this mimic demonstrated the absence of substances inhibitory to PCR, thereby validating negative results. Assay sensitivity was confirmed by obtaining positive signals with a representative selection of ASFV isolates. Many of the clinical and post-mortem features of ASF resemble those of classical swine fever (CSF) and porcine dermatitis and nephropathy syndrome (PDNS). Therefore, fast and reliable detection of ASFV is essential not only for the implementation of control measures to prevent the spread of ASF, but also in the differential diagnosis from CSF and PDNS. This assay should prove to be a valuable tool in the laboratory diagnosis of ASF and will complement existing molecular methods to provide rapid differential diagnosis in cases of suspected swine fever.
DOI:10.1016/j.jviromet.2006.11.018URLPMID:17196673 [本文引用: 1]

Rapid and reliable detection of viral pathogens is critical for the management of the diseases threatening the economic competitiveness of the swine farming industry worldwide. Molecular beacon assays are one type of real-time polymerase chain reaction (PCR) technology capable of fast, specific, sensitive, and reliable viral detection. In this paper, the development of molecular beacon assays as novel tools for the rapid detection of Aujeszky's disease virus, African swine fever virus, porcine circovirus type 2 and porcine parvovirus is described. The assays are capable of rapidly detecting 2 10 1 copies of target and are linear between 2 10 9 and 2 10 2 copies. They can detect virus specifically in clinical samples such as whole blood, serum and tissue. In comparison to conventional PCR they are either as sensitive or more sensitive. As such these molecular beacon assays represent a powerful tool for the detection of these viruses in swine.
DOI:10.1016/j.jviromet.2010.12.023URLPMID:21192983 [本文引用: 1]

A multiplex RT-PCR (mRT-PCR) assay was developed and evaluated for its ability to detect multiple viral infections of swine simultaneously. One pair of primers was selected carefully for each of the following three RNA viruses: porcine reproductive and respiratory syndrome virus (PRRSV), classical swine fever virus (CSFV), and porcine teschovirus (PTV). Each target produced a specific amplicon with a size of 451 bp (PRRSV), 343 bp (CSFV), or 163 bp (PTV). The sensitivity of the mRT-PCR using purified plasmid constructs containing the specific viral target fragments was 2.02 10 2, 2.90 10 3, and 6.16 10 3 copies for PRRSV, CSFV, and PTV, respectively. Among 69 clinical samples from Heilongjiang, Jilin, and Henan provinces, co-infection by PRRSV and CSFV was 4.4%, co-infection by PRRSV and PTV was 11.6%, co-infection by PTV and CSFV was 13.0%, and co-infection by the three viruses was 8.7%. In conclusion, the mRT-PCR should be useful for routine molecular diagnosis and epidemiology.
[本文引用: 1]
DOI:10.1515/pjvs-2015-0093URLPMID:26812812 [本文引用: 1]

In this assay, we developed and evaluated a multiplex PCR (mPCR) for its ability in detecting multiple infections of swine simultaneously. Four pairs of primers were used to detect five viruses. Specific primers were designed for classical swine fever virus (CSFV), African swine fever virus (ASFV) and pseudorabies (PRV). A pair of primers was designed prudently for two different types of porcine reproductive and respiratory syndrome virus that respectively were porcine reproductive and respiratory syndrome virus (PRRSV), highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV). The detection limits of the mPCR were 1.09×104, 1.50×103, 2.10×103, 1.30×103and 8.97×102copies/reaction for CSFV, ASFV, HP-PRRSV, PRRSV and PRV, respectively. A total of 49 clinical specimens were tested by the mPCR, and the result showed that co-infection by two or three viruses was 51%. In conclusion, the PCR is a useful tool for clinical diagnosis of not only single infections but also mixed infections in swines.
[本文引用: 1]
DOI:10.1016/j.jviromet.2009.11.034URLPMID:202020202020202020202020 [本文引用: 1]

A loop-mediated isothermal amplification (LAMP) assay was developed for the detection of African swine fever virus (ASFV). This assay targets the topoisomerase II gene of ASFV and its specificity was confirmed by restriction enzyme digestion of the reaction products. The analytical sensitivity of this ASFV LAMP assay was at least 330 genome copies, and the test was able to detect representative isolates of ASFV ( n = 38) without cross-reacting with classical swine fever virus. The performance of the LAMP assay was compared with other laboratory tests used for ASF diagnosis. Using blood and tissue samples collected from pigs experimentally infected with ASFV (Malawi isolate), there was good concordance between the LAMP assay and real-time PCR. In addition to detecting the reaction products using either agarose gels or real-time PCR machines, it was possible to visualise dual-labelled biotin and fluorescein ASFV LAMP amplicons using novel lateral flow devices. This assay and detection format represents the first step towards developing a practical, simple-to-use and inexpensive molecular assay format for ASF diagnosis in the field which is especially relevant to Africa where the disease is endemic in many countries.
DOI:10.1016/j.snb.2017.12.087URL [本文引用: 1]
DOI:10.1007/BF02251387URLPMID:4934981 [本文引用: 1]

Immunofluorescence (IF) has improved the diagnosis of African swine fever by its speed and simplicity. The tests used for its diagnosis and for its differentiation from classical swine fever are described. The virus characteristics on which the tests are based are: production of haemadsorption (HAD) followed by cytolysis on leukocyte cultures 93% of positive specimens produce HAD in first passa...
DOI:10.1016/j.vaccine.2014.05.051URLPMID:24877766 [本文引用: 1]

African swine fever (ASF) is among the most devastating viral diseases of pigs. In recent years, the disease has spread alarmingly. Despite intensive research activities, promising vaccine candidates are still lacking. For this reason, a study was undertaken to re-assess inactivated ASFV preparations with state-of-the-art adjuvants. Inactivated preparations of ASF virus (ASFV) “Armenia08” were adjuvanted with either Polygen64 or Emulsigen03-D, respectively, and used to immunize six weaner pigs two times with a three-week interval. Six weeks after the first immunization, animals were challenged with the homologues highly virulent ASFV. Although ASFV-specific antibodies were detectable in all but one vaccinated animal prior to challenge, no protective effect of immunization was observed. All animals developed acute-lethal ASF and had to be euthanized within eleven days post challenge. A slightly accelerated clinical course in vaccinees could even indicate an antibody dependent enhancement, which could also influence efficacy of other vaccine approaches.
DOI:10.1016/j.virusres.2012.11.009URLPMID:23201582 [本文引用: 1]

African swine fever virus (ASFV) infection usually results in an acute haemorrhagic disease with a mortality rate approaching 100% in domestic pigs. However, pigs can survive infection with less-virulent isolates of ASFV and may become chronically infected. Surviving animals are resistant to challenge with homologous or, in some cases, closely related isolates of the virus indicating that pigs can develop protective immunity against ASFV. During asymptomatic, non-virulent ASFV infections natural killer cell activity increases in pigs, suggesting this cell type plays a role in ASFV immunity. Furthermore, depletion of CD8+ lymphocytes from ASFV immune pigs demolishes protective immunity against related virulent viruses. This suggests that ASFV specific antibody alone is not sufficient for protection against ASFV infection and that there is an important role for the CD8+ lymphocyte subset in ASFV protective immunity. These results were supported by DNA immunization studies, demonstrating a correlation between the protection afforded against lethal challenge and the detection of a large number of vaccine-induced antigen-specific CD8+ T-cells. Peripheral blood mononuclear cells (PBMCs) from ASF immune pigs protected from clinical disease show higher proportions of ASFV specific CD4+CD8high+ double positive cytotoxic T cells than PBMCs from ASF immune but clinically diseased pig. The frequency of ASFV specific IFN纬 producing T cells induced by immunization correlates to the degree of protection from ASFV challenge, and this may prove to be a useful indicator of any potential cross-protection against heterologous ASFV isolates.
DOI:10.1016/j.virusres.2012.10.012URLPMID:23159730 [本文引用: 1]

Almost all viruses can be neutralized by antibodies. However, there is some controversy about antibody-mediated neutralization of African swine fever virus (ASFV) with sera from convalescent pigs and about the protective relevance of antibodies in experimentally vaccinated pigs. At present, there is no vaccine available for this highly lethal and economically relevant virus and all classical attempts to generate a vaccine have been unsuccessful. This failure has been attributed, in part, to what many authors describe as the absence of neutralizing antibodies. The findings of some studies clearly contradict the paradigm of the impossibility to neutralize ASFV by means of monoclonal or polyclonal antibodies. This review discusses scientific evidence of these types of antibodies in convalescent and experimentally immunized animals, the nature of their specificity, the neutralization-mediated mechanisms demonstrated, and the potential relevance of antibodies in protection.
DOI:10.1371/journal.pone.0040942URLPMID:23049728 [本文引用: 1]

Abstract The lack of available vaccines against African swine fever virus (ASFV) means that the evaluation of new immunization strategies is required. Here we show that fusion of the extracellular domain of the ASFV Hemagglutinin (sHA) to p54 and p30, two immunodominant structural viral antigens, exponentially improved both the humoral and the cellular responses induced in pigs after DNA immunization. However, immunization with the resulting plasmid (pCMV-sHAPQ) did not confer protection against lethal challenge with the virulent E75 ASFV-strain. Due to the fact that CD8(+) T-cell responses are emerging as key components for ASFV protection, we designed a new plasmid construct, pCMV-UbsHAPQ, encoding the three viral determinants above mentioned (sHA, p54 and p30) fused to ubiquitin, aiming to improve Class I antigen presentation and to enhance the CTL responses induced. As expected, immunization with pCMV-UbsHAPQ induced specific T-cell responses in the absence of antibodies and, more important, protected a proportion of immunized-pigs from lethal challenge with ASFV. In contrast with control pigs, survivor animals showed a peak of CD8(+) T-cells at day 3 post-infection, coinciding with the absence of viremia at this time point. Finally, an in silico prediction of CTL peptides has allowed the identification of two SLA I-restricted 9-mer peptides within the hemagglutinin of the virus, capable of in vitro stimulating the specific secretion of IFN when using PBMCs from survivor pigs. Our results confirm the relevance of T-cell responses in protection against ASF and open new expectations for the future development of more efficient recombinant vaccines against this disease.
DOI:10.1016/S1590-8658(00)80141-3URLPMID:4249112 [本文引用: 1]

African swine fever is one of the most devastating pig diseases, against which there is no vaccine available. Recent work from our laboratory has demonstrated the protective potential of DNA vaccines encoding three African swine fever viral antigens (p54, p30, and the hemagglutinin extracellular domain) fused to ubiquitin. Partial protection was afforded in the absence of detectable antibodies prior to virus challenge, and survival correlated with the presence of a large number of hemagglutinin-specific CD8(+) T cells in blood. Aiming to demonstrate the presence of additional CD8(+) T-cell determinants with protective potential, an expression library containing more than 4,000 individual plasmid clones was constructed, each one randomly containing a Sau3AI restriction fragment of the viral genome (p54, p30, and hemagglutinin open reading frames [ORFs] excluded) fused to ubiquitin. Immunization of farm pigs with the expression library yielded 60% protection against lethal challenge with the virulent E75 strain. These results were further confirmed by using specific-pathogen-free pigs after challenging them with 10(4) hemadsorbing units (HAU) of the cell culture-adapted strain E75CV1. On this occasion, 50% of the vaccinated pigs survived the lethal challenge, and 2 out of the 8 immunized pigs showed no viremia or viral excretion at any time postinfection. In all cases, protection was afforded in the absence of detectable specific antibodies prior to challenge and correlated with the detection of specific T-cell responses at the time of sacrifice. In summary, our results clearly demonstrate the presence of additional protective determinants within the African swine fever virus (ASFV) genome and open up the possibility for their future identification.African swine fever is a highly contagious disease of domestic and wild pigs that is endemic in many sub-Saharan countries, where it causes important economic losses and is currently in continuous expansion across Europe. Unfortunately, there is no treatment nor an available vaccine. Early attempts using attenuated vaccines demonstrated their potential to protect pigs against experimental infection. However, their use in the field remains controversial due to safety issues. Although inactive and subunit vaccines did not confer solid protection against experimental ASFV infection, our DNA vaccination results have generated new expectations, confirming the key role of T-cell responses in protection and the existence of multiple ASFV antigens with protective potential, more of which are currently being identified. Thus, the future might bring complex and safe formulations containing more than a single viral determinant to obtain broadly protective vaccines. We believe that obtaining the optimal vaccine formulation it is just a matter of time, investment, and willingness.
DOI:10.1128/JVI.02219-17URLPMID:29386289 [本文引用: 2]

African swine fever virus (ASFV) causes an acute hemorrhagic fever in domestic pigs, with high socioeconomic impact. No vaccine is available, limiting options for control. Although live attenuated ASFV can induce up to 100% protection against lethal challenge, little is known of the antigens which induce this protective response. To identify additional ASFV immunogenic and potentially protective antigens, we cloned 47 viral genes in individual plasmids for gene vaccination and in recombinant vaccinia viruses. These antigens were selected to include proteins with different functions and timing of expression. Pools of up to 22 antigens were delivered by DNA prime and recombinant vaccinia virus boost to groups of pigs. Responses of immune lymphocytes from pigs to individual recombinant proteins and to ASFV were measured by interferon gamma enzyme-linked immunosorbent spot (ELISpot) assays to identify a subset of the antigens that consistently induced the highest responses. All 47 antigens were then delivered to pigs by DNA prime and recombinant vaccinia virus boost, and pigs were challenged with a lethal dose of ASFV isolate Georgia 2007/1. Although pigs developed clinical and pathological signs consistent with acute ASFV, viral genome levels were significantly reduced in blood and several lymph tissues in those pigs immunized with vectors expressing ASFV antigens compared with the levels in control pigs. IMPORTANCEThe lack of a vaccine limits the options to control African swine fever. Advances have been made in the development of genetically modified live attenuated ASFV that can induce protection against challenge. However, there may be safety issues relating to the use of these in the field. There is little information about ASFV antigens that can induce a protective immune response against challenge. We carried out a large screen of 30% of ASFV antigens by delivering individual genes in different pools to pigs by DNA immunization prime and recombinant vaccinia virus boost. The responses in immunized pigs to these individual antigens were compared to identify the most immunogenic. Lethal challenge of pigs immunized with a pool of antigens resulted in reduced levels of virus in blood and lymph tissues compared to those in pigs immunized with control vectors. Novel immunogenic ASFV proteins have been identified for further testing as vaccine candidates.
DOI:10.1006/viro.1998.9068URLPMID:9568043 [本文引用: 1]

Abstract The nature of the initial interactions of African swine fever (ASF) virus with target cells is only partially known, and to date only the ASF virus protein p12 has been identified as a viral attachment protein. More recently, antibodies to viral proteins p54 and p30 have been shown to neutralize the virus, inhibiting virus binding and internalization, respectively. Therefore, we investigated the role of these proteins in the receptor-mediated ASF virus endocytosis in swine macrophages, the natural host cells. Proteins p54 and p30, released from ASF virus particles after treatment of virions with a nonionic detergent, bound to virus-sensitive alveolar pig macrophages. Binding of these proteins was found to be specifically inhibited by neutralizing antibodies obtained from a convalescent pig or from pigs immunized with recombinant p54 or p30 proteins. The baculovirus-expressed proteins p54 and p30 retained the same biological properties as the viral proteins, since they also bound specifically to these cells, and their binding was equally inhibited by neutralizing antibodies. Binding of 35S-labeled recombinant p54 and p30 proteins to macrophages was specifically competed by an excess of unlabeled p54 and p30, respectively. However, cross-binding inhibition was not observed, suggesting the existence of two different saturable binding sites for these proteins in the susceptible cells. In addition, protein p54 blocked the specific binding of virus particles to the macrophage, while protein p30 blocked virus internalization. Both proteins independently prevented virus infection and in a dose-dependent manner, suggesting that binding interactions mediated by both proteins are necessary to give rise to a productive infection. The relevance of blockade of virus-cell interactions mediated by p54 and p30 in the protective immune response against ASF virus was then investigated. Immunization of pigs with either recombinant p54 or p30 proteins induced neutralizing antibodies which, as expected, inhibited virus attachment or internalization, respectively. However, immunized pigs were not protected against lethal infection and the disease course was not modified in these animals. In contrast, immunization with a combination of p54 and p30 proteins simultaneously stimulated both virus neutralizing mechanisms and modified drastically the disease course, rendering a variable degree of protection ranging from a delay in the onset of the disease to complete protection against virus infection. In conclusion, the above results strongly suggest that proteins p54 and p30 mediate specific interactions between ASF virus and cellular receptors and that simultaneous interference with these two interactions has a complementary effect in antibody-mediated protection.
DOI:10.1016/j.virol.2003.11.011URLPMID:14980493 [本文引用: 1]

Although antibody-mediated immune mechanisms have been shown to be important in immunity to ASF, it remains unclear what role virus neutralizing antibodies play in the protective response. Virus neutralizing epitopes have been identified on three viral proteins, p30, p54, and p72. To evaluate the role(s) of these proteins in protective immunity, pigs were immunized with baculovirus-expressed p30, p54, p72, and p22 from the pathogenic African swine fever virus (ASFV) isolate Pr4. ASFV specific neutralizing antibodies were detected in test group animals. Following immunization, animals were challenged with 10 4 TCID 50 of Pr4 virus. In comparison to the control group, test group animals exhibited a 2-day delay to onset of clinical disease and reduced viremia levels at 2 days postinfection (DPI); however, by 4 DPI, there was no significant difference between the two groups and all animals in both groups died between 7 and 10 DPI. These results indicate that neutralizing antibodies to these ASFV proteins are not sufficient for antibody-mediated protection.
DOI:10.1099/jgv.0.000490URLPMID:27114233 [本文引用: 1]

African swine fever (ASF) is an emerging disease threat for the swine industry worldwide. No ASF vaccine is available and progress is hindered by lack of knowledge concerning the extent of ASFV strain diversity and the viral antigens conferring type-specific protective immunity in pigs. Available data from vaccination/challenge experiments in pigs indicate that ASF protective immunity may be haemadsorption inhibition (HAI) serotype-specific. Recently, we have shown that two ASFV proteins, CD2v (EP402R) and C-type lectin (EP153R), are necessary and sufficient for mediating HAI serological specificity (Malogolovkin et al., 2015).. Here, using ASFV inter-serotypic chimeric viruses and vaccination/challenge experiments in pigs, we demonstrate that serotype-specific CD2v and/or C-type lectin proteins are important for protection against homologous ASFV infection. Thus, these viral proteins represent significant protective antigens for ASFV that should be targeted in future vaccine design and development. Additionally, these data support the concept of HAI serotype-specific protective immunity.
DOI:10.1016/j.vetimm.2017.01.004URLPMID:28241999 [本文引用: 1]

A reverse vaccinology system, Vaxign, was used to identify and select a subset of five African Swine Fever (ASF) antigens that were successfully purified from human embryonic kidney 293 (HEK) cells and produced in Modified vaccinia virus Ankara (MVA) viral vectors. Three HEK-purified antigens [B646L (p72), E183L (p54), and O61R (p12)], and three MVA-vectored antigens [B646L, EP153R, and EP402R (CD2v)] were evaluated using a prime-boost immunization regimen swine safety and immunogenicity study. Antibody responses were detected in pigs following prime-boost immunization four weeks apart with the HEK-293-purified p72, p54, and p12 antigens. Notably, sera from the vaccinees were positive by immunofluorescence on ASFV (Georgia 2007/1)-infected primary macrophages. Although MVA-vectored p72, CD2v, and EP153R failed to induce antibody responses, interferon-gamma (IFN- + ) spot forming cell responses against all three antigens were detected one week post-boost. The highest IFN- + spot forming cell responses were detected against p72 in pigs primed with MVA-p72 and boosted with the recombinant p72. Antigen-specific (p12, p72, CD2v, and EP153R) T-cell proliferative responses were also detected post-boost. Collectively, these results are the first demonstration that ASFV subunit antigens purified from mammalian cells or expressed in MVA vectors are safe and can induce ASFV-specific antibody and T-cell responses following a prime-boost immunization regimen in swine.
DOI:10.1128/CVI.00395-16URLPMID:5098023 [本文引用: 1]

Abstract The African Swine Fever Virus (ASFV) causes a fatal hemorrhagic disease in domestic swine and, at present, no treatment or vaccine is available. Natural and gene-deleted, live attenuated strains protect against closely related virulent strains, however, they are yet to be deployed and evaluated in the field to rule out chronic persistence and potential for reversion to virulence. Previous studies suggest that antibodies play a role in protection, but induction of cytotoxic T-lymphocytes (CTLs) could be the key to complete protection. Hence, generation of an efficacious subunit vaccine depends on identification of CTL targets along with a suitable delivery method that will elicit effector CTLs capable of eliminating ASFV-infected host cells and confer long-term protection. To this end, we evaluated the safety and immunogenicity of an adenovirus-vectored ASFV multi-antigen cocktail formulated in two different adjuvants and at two immunizing doses in swine. Immunization with the cocktail rapidly induced unprecedented ASFV antigen-specific antibody and cellular immune responses against all the antigens. The robust antibody responses underwent rapid isotype-switching within one week post-priming, steadily increased over a two-month period and underwent rapid recall upon boost. Importantly, the primed antibodies strongly recognized the parental ASFV (Georgia 2007/1) by IFA and Western Blot. Significant antigen-specific IFN- + responses were detected post-priming and post-boosting. Furthermore, this study is the first to demonstrate induction of ASFV antigen-specific CTL responses in commercial swine using Ad-ASFV multi-antigens. The relevance of the induced immune responses in regards to protection need to be evaluated in a challenge study.
DOI:10.1371/journal.pone.0177007URLPMID:5421782 [本文引用: 1]

African Swine Fever Virus (ASFV) is a high-consequence transboundary animal pathogen that often causes hemorrhagic disease in swine with a case fatality rate close to 100%. Lack of treatment or vaccine for the disease makes it imperative that safe and efficacious vaccines are developed to safeguard the swine industry. In this study, we evaluated the immunogenicity of seven adenovirus-vectored novel ASFV antigens, namely A151R, B119L, B602L, EP402R PRR, B438L, K205R and A104R. Immunization of commercial swine with a cocktail of the recombinant adenoviruses formulated in adjuvant primed strong ASFV antigen-specific IgG responses that underwent rapid recall upon boost. Notably, most vaccinees mounted robust IgG responses against all the antigens in the cocktail. Most importantly and relevant to vaccine development, the induced antibodies recognized viral proteins from Georgia 2007/1 ASFV-infected cells by IFA and by western blot analysis. The recombinant adenovirus cocktail also induced ASFV-specific IFN- -secreting cells that were recalled upon boosting. Evaluation of local and systemic effects of the recombinant adenovirus cocktail post-priming and post-boosting in the immunized animals showed that the immunogen was well tolerated and no serious negative effects were observed. Taken together, these outcomes showed that the adenovirus-vectored novel ASFV antigen cocktail was capable of safely inducing strong antibody and IFN- +cell responses in commercial swine. The data will be used for selection of antigens for inclusion in a multi-antigen prototype vaccine to be evaluated for protective efficacy.
DOI:10.1099/0022-1317-82-3-513URLPMID:11172092 [本文引用: 2]

African swine fever virus ASFV/NH/P68 is a naturally occurring, non-haemadsorbing and non-fatal isolate. Longitudinal clinical and immunological studies on 31 pigs inoculated oronasally or intramuscularly with this isolate defined two discrete groups of animals: those developing ASF chronic type lesions and those remaining asymptomatic. Animals developing lesions had viraemia and fever late after infection, NK activity levels close to that of control animals and high levels of anti-ASFV specific antibodies together with a marked hypergammaglobulinaemia involving IgG1, IgG2, IgM and IgA immunoglobulin isotypes. Pigs remaining asymptomatic after infection, on the other hand, did not have viraemia or fever after day 14 post-infection and had elevated NK cell activity, but normal plasma Ig concentrations and relatively low specific anti-virus antibody concentrations throughout the duration of the experiments. Importantly, the latter group of pigs virus were resistant to subsequent challenge with the highly virulent ASFV/L60 isolate and survived with no major changes in any of the parameters examined and referred to above. Finally, lymphoproliferative responses to the mitogens concanavalin A, phytohaemagglutinin and pokeweed mitogen were not depressed in either of the two clinically defined groups of pigs. Thus further studies with this infection model may provide new insights on mechanisms of protective immunity to ASFV.
DOI:10.1111/tbed.12303URLPMID:25691347883 [本文引用: 2]

Summary The attenuated African swine fever virus genotype I strain OURT88/3 has previously been shown to induce protection of European breeds of domestic pigs against challenge with virulent isolates. To determine whether protective immune responses could also be induced in indigenous breeds of pigs from the Kinshassa region in Democratic Republic of Congo, we immunized a group of eight pigs with OURT88/3 strain and challenged the pigs 3 weeks later with virulent genotype I strain OURT88/1. Four of the pigs were protected against challenge. Three of the eight pigs died from African swine fever virus and a fourth from an unknown cause. The remaining four pigs all survived challenge with a recent virulent genotype I strain from the Democratic Republic of Congo, DRC 085/10. Control groups of non-immune pigs challenged with OURT88/1 or DRC 085/10 developed signs of acute ASFV as expected and had high levels of virus genome in blood.
DOI:10.1016/j.vaccine.2011.04.052URLPMID:3120964 [本文引用: 1]

African swine fever (ASF) is an acute haemorrhagic disease of domestic pigs for which there is currently no vaccine. We showed that experimental immunisation of pigs with the non-virulent OURT88/3 genotype I isolate from Portugal followed by the closely related virulent OURT88/1 genotype I isolate could confer protection against challenge with virulent isolates from Africa including the genotype I Benin 97/1 isolate and genotype X Uganda 1965 isolate. This immunisation strategy protected most pigs challenged with either Benin or Uganda from both disease and viraemia. Cross-protection was correlated with the ability of different ASFV isolates to stimulate immune lymphocytes from the OURT88/3 and OURT88/1 immunised pigs.
DOI:10.1111/j.1365-3083.1992.tb02854.xURLPMID:1738818 [本文引用: 1]

Peripheral blood mononuclear cells (PBMC) from inbred pigs that were immunized with autologous macrophages infected with the African swine fever (ASF) virus BA71V, a non-virulent virus isolate, proliferated and produced interleukin-2 in response to homologous and heterologous isolates of the ASF virus. They produced, however, interferon (IFN) only when challenged in vitro with homologous or attenuated isolates of the ASF virus, but not with heterologous or virulent isolates. The IFN was pH 2 labile and was neutralized by specific serum to porcine recombinant IFN gamma.
DOI:10.1016/j.vaccine.2018.03.040URLPMID:29609966 [本文引用: 1]

African swine fever virus (ASFV) induces a variety of immune responses and clinical forms in domestic pigs. As it is the only member of the Asfarviridae family, ASFV encodes many novel genes not encoded by other virus families. Among these genes, A238L may regulate the synthesis of pro-inflammatory cytokines, controlled mainly by NFkappaB and NFAT pathways. In this study, we inoculated two... [Show full abstract]
DOI:10.1016/j.virusres.2016.05.014URL [本文引用: 1]
DOI:10.1128/JVI.01760-16URLPMID:27795430 [本文引用: 1]

Abstract African swine fever virus (ASFV) is the etiological agent of a contagious and often lethal viral disease of domestic pigs that has significant economic consequences for the swine industry. The control of African Swine Fever (ASF) has been hampered by the unavailability of vaccines. Successful experimental vaccines have been derived from naturally occurring, cell culture-adapted, or genetically modified live attenuated ASFV. Recombinant viruses harboring engineered deletions of specific virulence-associated genes induce solid protection against challenge with parental viruses. Deletion of the 9GL (B119L) gene in highly virulent ASFVs Malawi Lil-20/1 (Mal) and Pretoriuskop/96/4 (Δ9GL viruses) resulted in complete protection when challenged with parental isolates. When similar deletions were created within the ASFV Georgia 2007 (ASFV-G) genome, attenuation was achieved but the protective and lethal doses were too similar. To enhance attenuation of ASFV-G, we deleted another gene, UK (DP96R), which was previously shown to be involved in attenuation of the ASFV E70 isolate. Here we report the construction of a double gene deletion recombinant virus, ASFV-G-Δ9GL/ΔUK. When administered intramuscularly (IM) to swine there is no induction of disease even at high doses (10 6 HAD 50 ). Importantly, animals infected with 10 4 HAD 50 of ASFV-G-Δ9GL/ΔUK were protected as early as 14 days post-inoculation when challenged with ASFV-G. Presence of protection correlates with appearance of serum anti-ASFV antibodies but not with virus specific circulating ASFV-specific INF-γ producing cells. ASFV-G-Δ9GL/ΔUK is the first rationally designed experimental ASFV vaccine that protects against the highly virulent ASFV Georgia 2007 isolate as soon as 2 weeks post-vaccination. IMPORTANCE: Currently there is no commercially available vaccine against African swine fever. Outbreaks of this disease are devastating to the swine industry, and are caused by circulating strains of African swine fever virus. Here we report about a putative vaccine derived from a current circulating strain but containing two deletions in two separate areas of the virus, allowing for increased safety. Using this genetically modified virus, we are able to vaccine and protect swine from developing ASF. We were able to achieve protection from disease as early as two weeks after vaccination, even when pigs were exposed to a higher than normal concentration of ASFV. Copyright 08 2016, American Society for Microbiology. All Rights Reserved.
DOI:10.1128/JVI.01058-17URL [本文引用: 1]

African swine fever is a highly contagious viral disease of mandatory declaration to the World Organization for Animal Health (OIE). The lack of available vaccines makes its control difficult; thus, African swine fever virus (ASFV) represents a major threat to the swine industry. Inactivated vaccines do not confer solid protection against ASFV. Conversely, live attenuated viruses (LAV), either naturally isolated or obtained by genetic manipulation, have demonstrated reliable protection against homologous ASFV strains, although little or no protection has been demonstrated against heterologous viruses. Safety concerns are a major issue for the use of ASFV attenuated vaccine candidates and have hampered their implementation in the field so far. While trying to develop safer and efficient ASFV vaccines, we found that the deletion of the viral CD2v (EP402R) gene highly attenuated the virulent BA71 strainin vivo. Inoculation of pigs with the deletion mutant virus BA71 CD2 conferred protection not only against lethal challenge with the parental BA71 but also against the heterologous E75 (both genotype I strains). The protection induced was dose dependent, and the cross-protection observedin vivocorrelated with the ability of BA71 CD2 to induce specific CD8+T cells capable of recognizing both BA71 and E75 virusesin vitro. Interestingly, 100% of the pigs immunized with BA71 CD2 also survived lethal challenge with Georgia 2007/1, the genotype II strain of ASFV currently circulating in continental Europe. These results open new avenues to design ASFV cross-protective vaccines, essential to fight ASFV in areas where the virus is endemic and where multiple viruses are circulating. IMPORTANCEAfrican swine fever virus (ASFV) remains enzootic in most countries of Sub-Saharan Africa, today representing a major threat for the development of their swine industry. The uncontrolled presence of ASFV has favored its periodic exportation to other countries, the last event being in Georgia in 2007. Since then, ASFV has spread toward neighboring countries, reaching the European Union's east border in 2014. The lack of available vaccines against ASFV makes its control difficult; so far, only live attenuated viruses have demonstrated solid protection against homologous experimental challenges, but they have failed at inducing solid cross-protective immunity against heterologous viruses. Here we describe a new LAV candidate with unique cross-protective abilities: BA71 CD2. Inoculation of BA71 CD2 protected pigs not only against experimental challenge with BA71, the virulent parental strain, but also against heterologous viruses, including Georgia 2007/1, the genotype II strain of ASFV currently circulating in Eastern Europe.
DOI:10.1016/j.jcpa.2014.09.003URLPMID:25443146 [本文引用: 1]

African swine fever (ASF) is one of the most important infectious diseases of swine and has major negative consequences for affected countries. ASF is present in many sub-Saharan countries, Sardinia and several countries of eastern and central Europe, where its continuous spread has the swine industry on heightened alert. ASF is a complex disease for which no vaccine or treatment is available, so its control is based on early detection and rapid control of spread. For a robust and reliable early detection programme it is essential to be able to recognize the clinical signs and pathological changes of ASF, keeping in mind that in most cases the first introductions don't show high mortality nor characteristic clinical signs or lesions, but fever and some hemorrhagic lymph nodes. Knowledge of the main characteristics of this infection, including its current distribution and routes of transmission, is also essential for preventing and controlling ASF. This review addresses each of these topics and aims to update knowledge of the disease in order to improve early detection of ASF in the field and allow implementation of public health programmes.
DOI:10.1016/j.vetmic.2012.11.030URLPMID:23265248 [本文引用: 2]

Since African swine fever (ASF) was re-introduced into Eastern Europe in April 2007, the disease has spread through five countries, drastically changing the European ASF situation. This re-introduction has significant implications for the affected countries, and it puts the European Union (EU) at serious risk of ASF introduction. Numerous factors are complicating the control of ASF in the Russian Federation and neighboring areas, particularly the absence of a coordinated control program, the abundance of backyard pig units with low or no biosecurity and the traditional use of swill feeding. All these risk factors are driven in turn by socio-economic, political and cultural factors. Moreover, the lack of clear information regarding the current situation of ASF in the Trans-Caucasus countries such as Armenia and Georgia may be increasing the risk of ASF spread into neighboring areas. The ASF situation in Eastern Europe poses a constant risk of ASF entry into the EU, especially via routes that are difficult to control, such as wild boar movements, illegal movement of animals and animal products and movements of contaminated vehicles or other fomites. This paper reviews and discusses current ASF epidemiology in Eastern Europe, the factors that may contribute to disease endemicity in the area, the current challenges for disease control, and the risk of introduction into the EU.
DOI:10.1186/s40813-015-0013-yURLPMID:5382474 [本文引用: 1]

African Swine Fever (ASF) is an important contagious haemorrhagic viral disease affecting swine whose notification is mandatory due to its high mortality rates and the great sanitary and socioeconomic impact it has on international trade in animal and swine products. This disease only affects porcine species, both wild and domestic, and produces a variety of clinical signs such as fever and functional disorders of the digestive and respiratory systems. Lesions are mainly characterized by congestive-haemorrhagic alterations. ASF epidemiology varies significantly between countries, regions and continents, since it depends on the characteristics of the virus in circulation, the presence of wild hosts and reservoirs, environmental conditions and human social behaviour. Furthermore, a specific host will not necessarily always play the same active role in the spread and maintenance of ASF in a particular area. Currently, ASF is endemic in most sub-Saharan African countries where wild hosts and tick vectors (Ornithodoros) play an important role acting as biological reservoirs for the virus. In Europe, the disease has been endemic since 1978 on the island of Sardinia (Italy) and since 2007, when it was first reported in Georgia, in a number of Eastern European countries. It is also endemic in certain regions of the Russia Federation, where domestic pig and wild boar populations are widely affected. By contrast, in the affected eastern European Union (EU) countries where ASF is currently as epidemic, the on-going spread of the disease affects mainly wild boar populations located in restricted areas and, to a much less extent, domestic pigs. Unlike most livestock diseases, no vaccine or specific treatment is currently available for ASF. Therefore, disease control is mainly based on early detection and the application of strict sanitary and biosecurity measures. Epidemiology of ASF is very complex by the existence of different virus circulating, reservoirs and a number of scenarios, and the on-going spread of the disease through Africa and Europe. Survivor pigs can remain persistently infected for months which may contribute to virus transmission and thus the spread and maintenance of the disease, thereby complicating attempts to control it.
DOI:10.1016/j.tvjl.2017.12.025URLPMID:29486878 [本文引用: 1]

Abstract African swine fever (ASF) recently has spread beyond sub-Saharan Africa to the Trans-Caucasus region, parts of the Russian Federation and Eastern Europe. In this new epidemiological scenario, the disease has similarities, but also important differences, compared to the situation in Africa, including the substantial involvement of wild boar. A better understanding of this new situation will enable better control and prevent further spread of disease. In this article, these different scenarios are compared, and recent information on the pathogenesis of ASF virus strains, the immune response to infection and prospects for developing vaccines is presented. Knowledge gaps and the prospects for future control are discussed.
