摘要/Abstract
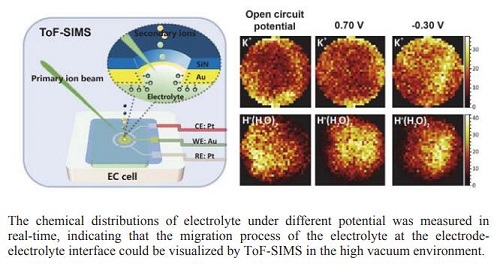
电子转移过程是电化学反应的核心,而构成电极反应的前提则是电活性物质在电极表面的迁移扩散.对电位调控下电解质迁移过程的观测,有助于对电极表面电化学反应机理进行更加深入的理解.以氯化钾溶液在金电极表面的迁移过程为研究模型,通过制备可用于高真空环境中的微流控电化学池,实现液体电化学飞行时间-二次离子质谱(ToF-SIMS)联用,用于氯化钾溶液在不同电极电位条件下迁移过程的实时监测.通过化学成像呈现不同电位下氯化钾溶液在金电极附近的分布情况,观测到K+与H+(H2O)n在施加-0.30 V电压后,在电极附近存在聚集,施加0.70 V电压后,电极附近处,两物种浓度明显降低.通过液体电化学ToF-SIMS联用,可实现电极调控作用下电解质迁移过程的原位监测,展现了带电粒子在电场作用下的宏观迁移过程,为电极-电解质界面的原位实时监测提供可视化的研究手段.
关键词: 电极电位调控, 迁移过程可视化, 化学成像, 飞行时间-二次离子质谱
The essence of electrochemical reaction was the electron transfer process on the electrode while the premise for electrochemical reaction happening was the migration and diffusion of electro-active species. Interpreting the monitoring process of electrode towards the electro-active species during the electrochemical reaction would really do favor to the further understanding of the electrochemical evolution process at the electrode-electrolyte interface. In this work, time-of-flight secondary ion mass spectrometry (ToF-SIMS) was adopted for the in-situ monitoring of the electrode-electrolyte interface during the electrochemical reaction with the cooperation of a microfluidic electrochemical cell which was constructed for the liquid sample analysis under the high vacuum environment. With the application of the primary ion beam on the silicon nitride membrane of the microfluidic cell, a micro-hole with the diameter of 2 μm would be fabricated for the direct monitoring of the electrode-electrolyte interface. The migration process of KCl aqueous solution in the confined micropore under the monitoring of gold electrode was investigated by ToF-SIMS here. The direct observation of K+(H2O)n and H+(H2O)n in the electrolyte provided information of the electrode-electrolyte interface at molecular level. Besides, the chemical distributions of K+ and H+(H2O)n under different potential were also studied to verify the feasibility of pore-confined ToF-SIMS in visualizing electrochemical evolution process on the electrode-electrolyte interface. The chemical distributions of K+ and H+(H2O)n obtained by ToF-SIMS showed that K+ would enrich to the surface of gold electrode when the negative potential was applied but diffuse to the bulk solution when positive potential was applied. For H+(H2O)n, they would be repulsed away from the electrode when the positive potential was applied and enrich to the surface of electrode when the negative potential was applied. The potential-dependent behaviors of K+ and H+(H2O)n indicated that visualization of the migration process of electrolyte on the electrode-electrolyte interface was realized, which may help the further study of the evolution at electrode-electrolyte interface during the electrochemical reaction, providing new insight into the revealment of the mechanism of electrochemical reaction.
Key words: electrode monitoring, visualizing migration process, chemical imaging, time-of-flight secondary ion mass spectrometry
PDF全文下载地址:
点我下载PDF
