摘要/Abstract
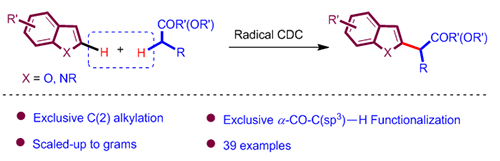
报道了一种小分子酮、酯与富电子杂环芳烃的高度选择性的自由基脱氢交叉偶联反应. 酯、酮作为溶剂, 过氧化物加热条件下发生裂解与酯、酮作用产生α羰基碳中心自由基, 进而与富电子杂环芳烃发生交叉脱氢偶联, 得到一系列C-2官能化富电子杂环产物. 该反应成功地运用自由基的极性效应, 从而精确控制自由基反应的化学选择性. 此外, 该体系还具有反应迅速、操作简便、官能团兼容性较好、区域选择性单一等优点. 预期它将在合成有机化学上得到较广泛的应用.
关键词: 自由基, C—H活化, 酮酯, 交叉脱氢偶联, 富电子杂环芳烃
The cross dehydrogenative coupling (CDC) via highly selective C—H bond functionalization represents one of the most atom-economical, environmentally-benign and efficient synthetic strategies. For a long time, the cleavage of C—H bonds initiated by free radicals has been regarded as unselective and useless. However, more and more studies have shown that free radical mediated strategies could also achieve C—H bond functionalization in high selectivity recently. In general, it’s well-known that nucleophilic free radical species tend to extract hydrogen atoms on electron-deficient C—H bonds, while electrophilic free radicals abstract hydrogen atoms on electron-rich C—H bonds. A recent study by our group shows that after thermal decomposition of peroxy tert-butyl ether, the electron-rich methyl radicals are produced. Then the radical cleavage of the C(sp 3)—H bond in ketone/ester would happen prior to the α-carbonyl-C—H bond. Subsequently, the electrophilic α-carbonyl-C-centered radical selectively reacted with electron-rich olefins to afford new C—C bonds. Here, a free-radical initiated highly selective cross dehydrogenative coupling reaction of simple ketones and esters with electron-rich heteroarenes was demonstrated. The ketones and esters were used as solvent, and they would afford the corresponding α-carbonyl C-centered radicals, which then add to heteroaromatics leading to a series of C(2)-functionalized heterocycles. The chemoselectivity of this system was well-controlled by application of the polar effect of free radicals. In addition, this protocol features fast, simple operation, good functional group tolerance and site specific etc. The potential of this method was demonstrated through the synthesis of non-steroidal anti-inflammatory and analgesic drug tolmetin. It is expected to have wide applications in synthetic organic chemistry. Typical reaction conditions are as follows: a mixture of heteroarenes (1 equiv., 0.20 mmol), TBPA (3 equiv., 0.06 mmol) and ketones/esters (6 mL) was heated under reflux at 130 ℃ for about 1 h. After completion of the reaction, the crude product was cooled to room temperature, the excess solvent was recovered by rotary evaporator and the residue was further purified by column chromatography on silica gel to obtain the desired product (eluent: petroleum ether/ethyl acetate).
Key words: free radical, C—H activation, ketone and ester, cross dehydrogenative coupling (CDC), electron-rich heteroarene
PDF全文下载地址:
点我下载PDF
