摘要/Abstract
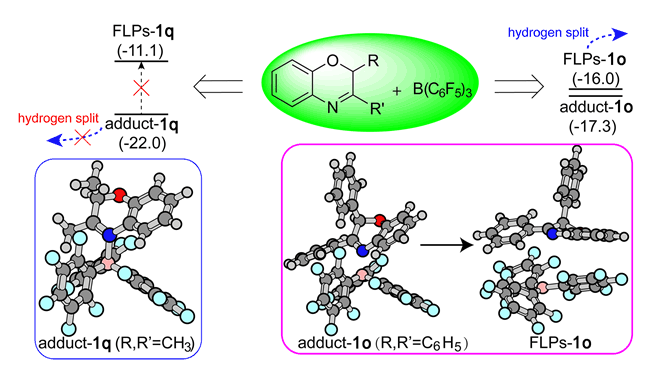
在受阻路易斯酸碱对(FLPs)催化的2,3-二取代2H-1,4-苯并噁嗪氢化反应中,3号位取代基不同会导致反应效率极大改变,因此我们选取反应活性具有较大差别的三种底物作为模型化合物对其反应机理进行了研究,建立了氢化反应势能面.发现当B(C6F5)3与2,3-二苯基2H-1,4-苯并噁嗪或2-甲基-3-苯基2H-1,4-苯并噁嗪混合后,会形成FLPs与路易斯酸碱加合物的混合物.而将B(C6F5)3与2,3-二甲基2H-1,4-苯并噁嗪混合后主要形成没有催化活性的路易斯酸碱加合物,因其能量低于FLPs,在催化体系中不容易转化为FLPs,这导致三种模型化合物在FLPs催化的氢化反应中效率不同.进一步的取代基电子效应及位阻效应计算表明:B(C6F5)3与2-甲基-3-取代2H-1,4-苯并噁嗪混合后形成的路易斯酸碱加合物和FLPs化合物之间稳定性差别源于3位取代基空间位阻不同.
关键词: 受阻路易斯酸碱对, 路易斯酸碱加合物, 氢化反应, 势能面
Due to the different reactivity of hydrogenation reaction by metal-free FLPs catalyst for 2,3-disubstituted 2H-1,4-benzoxazine, we explored the reaction mechanism by density functional theory calculations. We have chosen three kinds of substrates with different hydrogenation reactivity as the prototype substrates and toluene as the solvent to calculate the potential energy profile for the FLPs-catalysed hydrogenation reaction at M06-2X/6-311++G(d,p) level with polarized continuum model (PCM) to simulate the solvent effect. From the potential energy profile, we found that when B(C6F5)3 encounters with 2,3-diphenyl 2H-1,4-benzoxazine (1o) or 2-methyl-3-phenyl 2H-1,4-benzoxazine (1p) in toluene, it mainly generates the mixture of Lewis acid-base adducts and Frustrated Lewis Pairs, which has almost similar stability suggesting the transformation of each other by intermolecular rearrangement. However, it reveals big difference when the B(C6F5)3 encounters with 2,3-dimethyl 2H-1,4-benzoxazine (1q), where the Lewis acid-base adducts is the preference rather than the mixture of Lewis acid-base adducts and Frustrated Lewis Pairs or Frustrated Lewis Pairs since the lower stability energy. Due to the big energy gap (10.9 kcal/mol) between Lewis acid-base adducts and Frustrated Lewis Pairs, the generated Lewis acid-base adducts could not transform into Frustrated Lewis Pairs in the FLPs-catalysed hydrogenation of 1q at 298 K. That is the main reason why 1q is an inert substrate for the hydrogenation catalysed by FLPs. Natural Bond Orbital, Mulliken charge analysis and the proton affinity energy of N4 site was carried out to assess the electric effect of substituent at C3 on N4 site. It reveals negligible effect of substituent at C3 on N4 charge (basicity) and thus proposes that steric hindrance effect is the major factor affecting the stability energy of Lewis acid-base adducts and Frustrated Lewis Pairs. This is confirmed further by calculative investigation about the substituent effect (-CH2CH3, -CH(CH3)2 and C(CH3)3) on the stability of Lewis acid-base adducts and Frustrated Lewis Pairs in 2-methyl-3-substituted 2H-1,4-benzoxazine, where with the increased steric hindrance effect, Lewis acid-base adducts tend to have similar stability with Frustrated Lewis Pairs even though less stability. These results clearly illustrate the elusive phenomenon in our previous experiment and may provide new insight for the design of another novel FLPs-catalysed hydrogenation reaction.
Key words: Frustrated Lewis Pairs, Lewis acid-base adducts, hydrogenation reaction, potential energy profile
PDF全文下载地址:
点我下载PDF
