摘要/Abstract
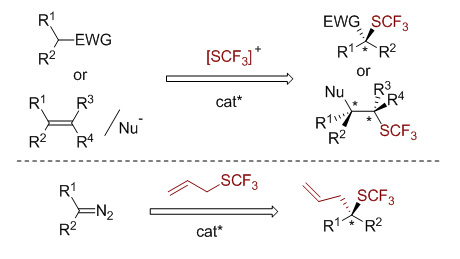
三氟甲硫基吸电子能力强、脂溶性高,在药物分子内引入三氟甲硫基可以显著地提高化合物的细胞膜穿透性与代谢稳定性.而不同立体异构的药物分子会有不同的药理活性,因此不对称三氟甲硫基化反应在近几年内逐渐受到化学家们的重视.目前,这一领域还处于发展的初步阶段,本文将就目前为止已经发展的不对称三氟甲硫基化方法做简单的总结,包括使用亲电的三氟甲硫基试剂和使用含三氟甲硫基的合成砌块这两种不同的策略.最后对不对称三氟甲硫基化领域所存在的挑战进行简单的展望.
关键词: 三氟甲硫基化, 不对称催化, 有机氟化合物, 立体选择性, 有机合成
Fluorine-containing groups can modulate the physicochemical and biological properties of organic molecules. Consequently, the synthesis of fluorinated organic molecules has attracted considerable attention in the field of pharmaceuticals, agrochemicals and material sciences. Among fluorine-containing groups, the trifluoromethylthio group has the highest Hansch’s hydrophobicity parameter and remarkable electron-withdrawing character. The incorporation of a trifluoromethylthio group into organic molecules can significantly enhance their membrane permeability and metabolic stability because of its high lipophilicity and strong electron-withdrawing effect. As a result, various methods have been involved to synthesize SCF3-containing compounds using electrophilic or nucleophilic trifluoromethylthio reagents. On the other hand, the chirality of pharmaceutical molecules has an important effect on their properties, and different stereoisomers of a pharmaceutical molecules always have dramatically different pharmaceutical activities. Thus, the asymmetric trifluoromethylthiolation of organic molecules is of growing interest in recent years. Up to now, this field is still in the stage of initial development. In this perspective article, we will briefly summarize the methods of asymmetric trifluoromethylthiolation of organic molecules that have been reported so far. Two different strategies including the use of electrophilic trifluoromethylthiolating reagents and the use of trifluoromethylthio-containing building blocks will be introduced. Employing electrophilic trifluoromethylthiolating reagents, the enantioselective trifluoromethylthiolation of β-ketoesters, oxindoles as well as alkenes have been developed using Cinchona alkaloid, copper(Ⅱ) or indane-based chiral sulfide/selenide as the catalyst. Alternatively, using trifluoromethylthiolated building blocks is another approach to establish chiral centers bearing the trifluoromethylthio group. In this approach, an asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of a sulfonium ylide generated from SCF3-containing sulfide and metal carbene has been disclosed using chiral Rh(Ⅱ) and Cu(I) as the catalyst. Finally, we will discuss the challenges of the asymmetric trifluoromethylthiolation of organic molecules in the future.
Key words: trifluoromethylthiolation, asymmetric catalysis, organofluorine compounds, stereoselectivity, organic synthesis
PDF全文下载地址:
点我下载PDF
