摘要/Abstract
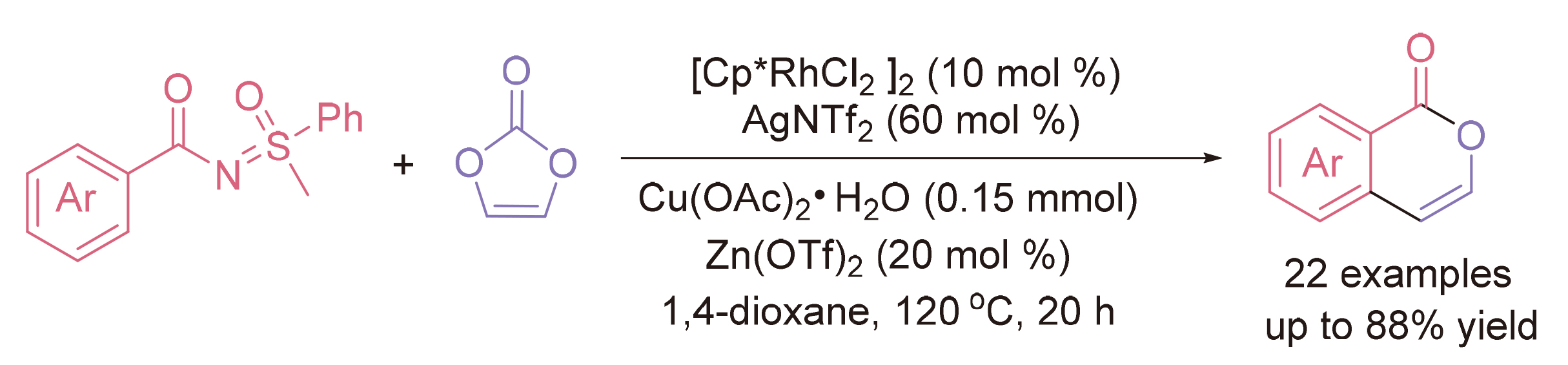
异香豆素是一种重要的药物分子, 目前已经开发了许多合成途径, 主要集中在苯甲酸或其他物质与炔烃的环化.亚磺酰基苯甲酰胺在螯合辅助的芳烃邻位C—H官能化中可以轻松地制备, 碳酸亚乙烯酯可以建立无氧化剂的催化体系, 使碳酸作为唯一的副产物. 在铑催化剂的存在下, 亚磺酰基苯甲酰胺和碳酸亚乙烯酯之间形成了环化, 通过导向基团辅助的邻位C—H偶联和分子内醇解, 以中等至良好的产率获得未取代的异香豆素.
关键词: 铑, 亚磺酰基苯甲酰胺, 碳酸亚乙烯酯
Isocoumarins are an important pharmaceutical compounds. Many synthetic pathways have been developed, mainly focusing on the cyclization of benzoic acid or other with alkynes. Sulfinylidene benzamides were facilely prepared and developed in the chelation-assisted arene ortho-C—H functionalization, and vinylene carbonate can establish an oxidant-free catalytic system, giving carbonic acid as a sole side product. In the presence of rhodium catalyst, an annulation between sulfinylidene benzamides and vinylene carbonate was developed, proceeding with directing group-assisted ortho-C—H coupling and intramolecular alcoholysis toward nonsubstituted isocoumarins in moderate to good yields.
Key words: rhodium, sulfinylidene benzamides, vinylene carbonate
PDF全文下载地址:
点我下载PDF
