摘要/Abstract
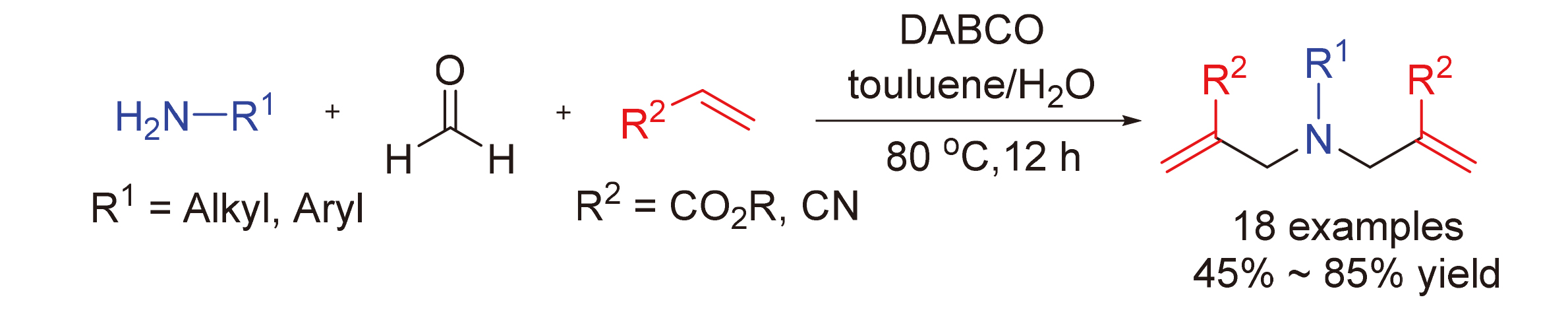
aza-Morita-Baylis-Hillman反应是一类非常重要的构建C—C键的人名反应, 被广泛应用于合成化学和药物化学领域. 报道了一类新颖的1,4-二氮杂二环[2.2.2]辛烷(DABCO)介导的二次aza-Morita-Baylis-Hillman串联反应. 该反应利用伯胺与甲醛能原位生成亚胺正离子的特征, 在甲苯与水的混合溶剂中, 实现了DABCO诱导的缺电子烯烃与亚胺正离子间的二次Mannich反应, 最终以中等到良好的产率获得了一系列氨基衍生的1,6-二烯化合物. 实验结果显示该三组分反应体系适用于一系列的苄胺、烷基胺和芳基胺底物, 有效避免了传统aza-Morita-Baylis-Hillman反应对底物胺的束缚, 为多样性1,6-二烯类化合物的合成提供了更加简洁的方法.
关键词: aza-Morita-Baylis-Hillman反应, 串联反应, 伯胺, 1,6-二烯
Aza-Morita-Baylis-Hillman reaction plays specific roles in the construction of C—C bond, and their applications in synthetic chemistry and pharmaceutical chemistry have also been well-documented. Herein, a novel 1,4-diazabicyclo- [2.2.2]octane (DABCO) mediated doubleaza-Morita-Baylis-Hillman cascade strategy toward synthetically important aza-MBH adducts is reported. Complementary to classical aza-MBH reaction, this protocol employs a wide variety of primary amines including benzyl, alkyl and aryl moieties as the reaction partners, giving an efficient alternative to produce amino derived 1,6-dienes in moderate to high yields in the presence of formaldehyde and α,β-unsaturated carbonyl compounds. Rather surprisingly, using an aqueous medium has proven to be successful in promoting the reaction efficiency and achieving a higher yield of target products. One-pot operation, high chemoselectivity, short reaction time, and broad substrate scope of primary amines exemplified the significant advances and practicability of this protocol.
Key words: aza-Morita-Baylis-Hillman reaction, domino reaction, primary amine, 1,6-diene
PDF全文下载地址:
点我下载PDF
