摘要/Abstract
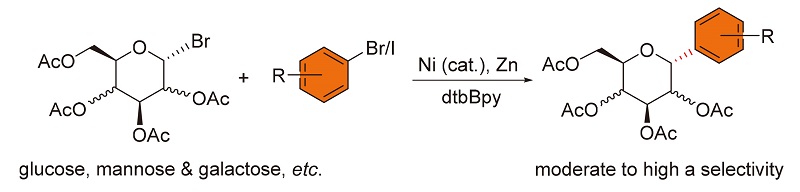
报道了在温和条件下通过镍和联吡啶催化的还原偶联反应, 由C(1)-卤代糖苷和缺电子的芳基溴化物制备芳香糖苷类化合物的方法. 运用该种方法可以由中等或高的α-选择性得到芳香碳糖苷类产物. 包括富电子的芳香碘化物在内的多种卤代芳烃底物适用于该反应, 产率在40%~95%之间. 该方法可以放大到克级规模, 并在此条件下将镍催化剂的用量减少到2 mol%.
关键词: 镍催化, 还原偶联, α-选择性制备, 芳香碳糖苷, 克级反应
The preparation of C-aryl glycosides via mild Ni/bipyridine-catalyzed reductive arylation of C(1)-glycosyl halides with electron-deficient aryl bromides was developed. Moderate to high α-selectivities were achieved for C-glycosides. A broad range of aryl halides including electron-rich aryl iodides were employed to yield C-aryl glycosides in 40%~5% yields. This method can be scaled up on a gram scale by lowering the loading of nickel catalyst to 2 mol%.
Key words: nickel-catalysis, reductive coupling, α-selective preparation, C-aryl glycoside, gram scale
PDF全文下载地址:
点我下载PDF
