摘要/Abstract
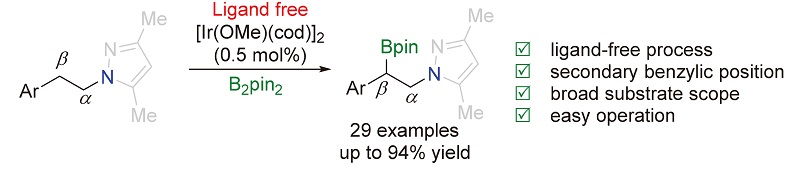
报道了无外加配体参与的以吡唑作为导向基团的铱催化的sp3碳氢键的区域选择性硼化反应. 在催化量的[Ir(OMe)(cod)]2存在下, 该反应能够顺利地将苄位的二级碳氢键转化成碳硼键. 该反应具有非常广谱的官能团兼容性, 能够以良好到优秀的产率生成相应的产物. 此外, 导向基团吡唑能够通过臭氧接转化成酰胺.
关键词: 碳氢键活化, 铱, 合成方法, 硼化
The ligand-free regioselective iridium-catalyzed C(sp3)—H bond borylation using pyrazole as the directing group is reported. The reaction occurs smoothly at the secondary benzylic position in the presence of a catalytic amount of commercially available [Ir(OMe)(cod)]2. A variety of functionalities could be well tolerated, affording corresponding products in good to excellent yields. The pyrazole could be degraded into amide by ozonolysis.
Key words: C—H activation, iridium, synthetic methods, borylation
PDF全文下载地址:
点我下载PDF
