摘要/Abstract
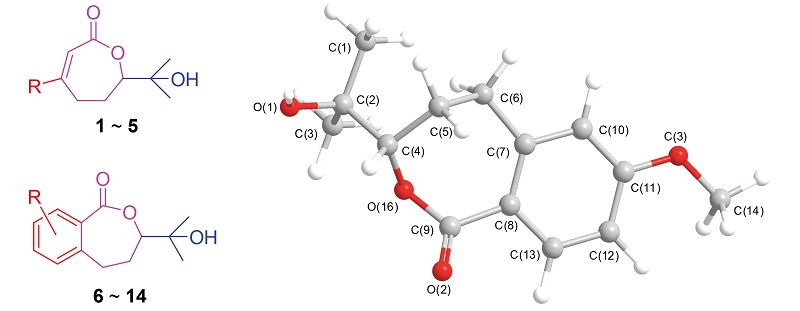
以环氧化-内酯化和Sharpless不对称双羟基化反应作为关键步骤, 以55%~90%的总收率实现了消旋及光活性3,7-二甲基-7-羟基-2-辛烯-6-内酯类似物的合成. 它们的结构经过1H NMR, 13C NMR, HR-ESI-MS和X射线衍射的表征. 对它们的杀菌活性进行了评价, 结果表明活性最好的化合物3-苯基-7-甲基-7-羟基-2-辛烯-6-内酯(4)和3-(呋喃-2-基)-7-甲基-7-羟基-2-辛烯-6-内酯(5)对6种植物病原菌的EC50值为0.5~20.0 μg/mL, 可以作为先导结构进行进一步结构优化.
关键词: 3,7-二甲基-7-羟基-2-辛烯-6-内酯, 环氧化-内酯化反应, 不对称双羟基化反应, 杀菌活性
The synthesis of racemic and optical 3,7-dimethyl-7-hydroxy-2-octen-6-olide analogues has been achieved via epoxidation-lactonization procedure and Sharpless asymmetric dihydroxylation as the key steps in 55%~90% overall yields, respectively. Their structures were fully characterized by 1H NMR, 13C NMR, HRMS data, and X-ray diffraction analysis. Their antifungal activities were evaluated, and showed that the EC50values of the most active compounds 3-phenyl-7- methyl-7-hydroxy-2-octen-6-olide (4) and 3-(furan-2-yl)-7-methyl-7-hydroxy-2-octen-6-olide (5) were in the range of 0.5~20.0 μg/mL against the tested six phytopathgens, and they were the lead structures to be optimized.
Key words: 3,7-dimethyl-7-hydroxy-2-octen-6-olide, epoxidation-lactonization, asymmetric dihydroxylation, antifungal activity
PDF全文下载地址:
点我下载PDF
