摘要/Abstract
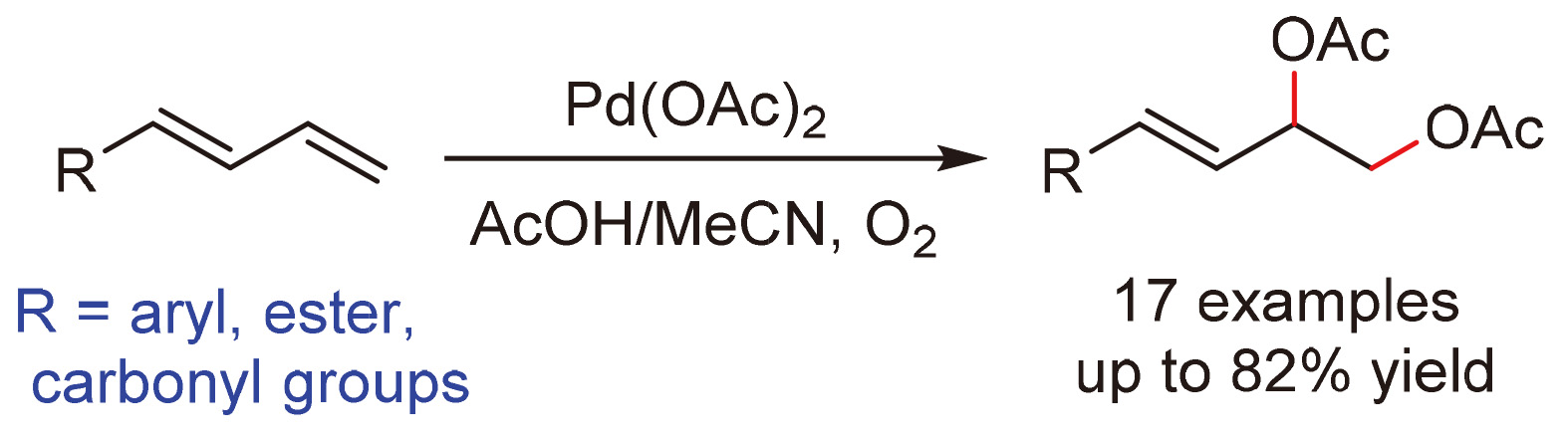
邻二醇类化合物在农药、手性医药以及精细化学品等方面具有重要的用途.发展了一种钯(II)催化共轭二烯的1,2-双乙酰氧基化的方法,利用简单易得的醋酸作为氧源,氧气作为氧化剂,高区域选择性地实现了共轭二烯的1,2-双乙酰氧基化反应.本方法对芳基、酯基和羰基等取代的共轭二烯具有良好的底物适应性,催化产物经过简单醇解或水解转化为邻二醇化合物,是一种高效合成邻二醇类化合物的新策略.
关键词: 钯, 共轭二烯, 1,2-双乙酰氧基化, 邻二醇
1,2-Diols have important applications in pesticides, chiral medicines and fine chemicals. A Pd(II)-catalyzed 1,2-diacetoxylation method using readily available acetic acid as the oxygen source and oxygen as the oxidant was developed. For the 1,2-diacetoxylation of conjugated dienes, the reaction proceeds with high 1,2-regioselectivity. This protocol has good substrate scope for conjugated dienes possessing aryl-, ester-and carbonyl groups. The catalytic products can be transformed to 1,2-diols through simple alcoholysis or hydrolysis, therefore it is an efficient method for the synthesis of 1,2-diols.
Key words: palladium, conjugated diene, 1,2-diacetoxylation, 1,2-diol
PDF全文下载地址:
点我下载PDF
