摘要/Abstract
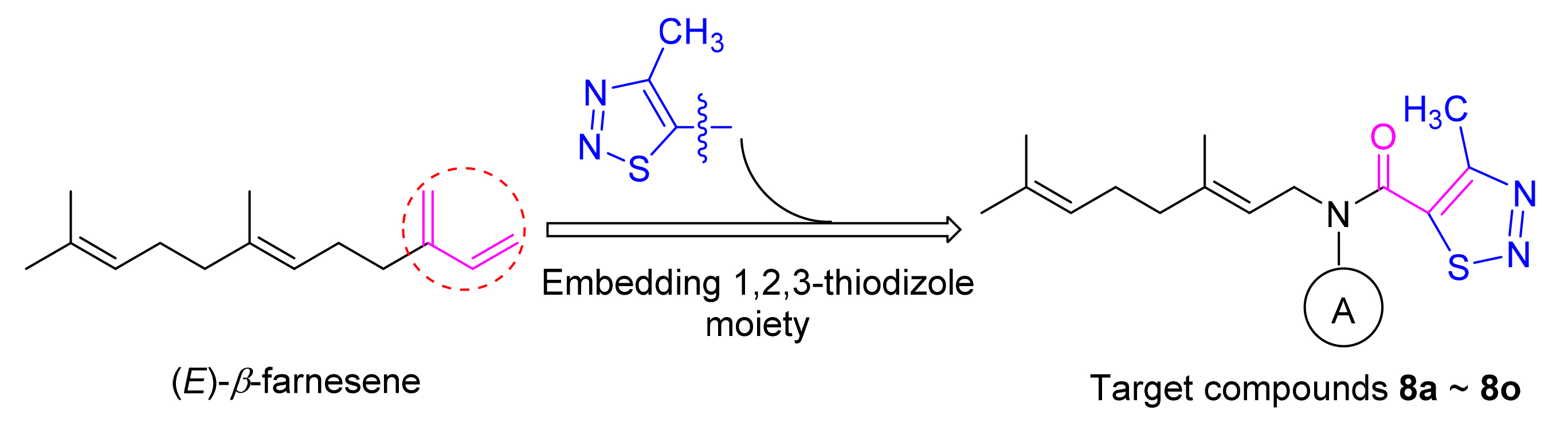
为了发现控制蚜虫的新型高活性化合物,以蚜虫报警信息素(E)-β-farnesene(EBF)为先导化合物,用1,2,3-噻二唑环替代EBF结构中不稳定的共轭双键,设计、合成了15个EBF类似物,所有化合物结构均通过1H NMR、13C NMR、IR及HRMS确证.生物活性测试结果表明:化合物对桃蚜表现出较好的驱避活性,其中以N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(2-萘基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8d)、N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(4-甲基-吡啶-2-基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8l)和N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(5-甲基-吡啶-2-基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8m)最为显著,驱避率分别为63.1%、61.3%和63.4%;化合物对桃蚜的毒杀活性均高于EBF,其中N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(4-氰基-苯基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8b)、N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(6-甲基-吡啶-2-基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8g)、N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(吡啶-2-基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8j)、N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(3-甲基-吡啶-2-基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8k)、8l、8m和N-((E)-3,7-二甲基-2,6-辛二烯-1-基)-N-(5-甲基-1,3,4-噻二唑-2-基)-4-甲基-1,2,3-噻二唑-5-甲酰胺(8o)的杀蚜活性比较突出(LC50分别是10.2、9.0、25.1、31.7、8.4、12.8和9.6 μg/mL),但活性略低于对照药剂吡蚜酮(LC50值7.1 μg/mL).
关键词: 1,2,3-噻二唑, EBF类似物, 合成, 驱避活性, 杀虫活性
In order to discover novel compounds with high-activity to control aphid, aphid alarm pheromone (E)-β-farnesene (EBF) was chosen as lead compound and 15 EBF analogues were designed and synthesized by replacing unstable conjugated double bond of EBF with 1,2,3-thiadiazole. Their structures were confirmed by 1H NMR, 13C NMR, IR and HRMS analysis. Repellent activity results indicated that analogues displayed better repellent activity against Myzus persicae (Sulzer). Among which compounds N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(2-naphthyl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8d), N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(4-methyl-pyridine-2-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8l) and N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(5-methyl-pyridine-2-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8m) exhibited excellent repellent activity of 63.1%, 61.3% and 63.4% respectively. The aphicidal bioassay results showed that most analogues exhibited considerable aphicidal activity against Myzus persicae. Especially, analogues N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(4-CN-phenyl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8b), N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(6-methyl-pyridine-2-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8g), N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(2-pyridyl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8j), N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(3-methyl-pyridine-2-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8k), 8l, 8m and N-((E)-3,7-dimethyl-2,6-octadien-1-yl)-N-(5-methyl-1,3,4-thiadiazole-2-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide (8o) exhibited high activity with LC50 values of 10.2, 9.0, 25.1, 31.7, 8.4, 12.8 and 9.6 μg/mL, respectively, which were higher than the lead compound (E)-β-farnesene, but lower than commercial insecticide pymetrozine with LC50 of 7.1 μg/mL.
Key words: 1,2,3-thiadiazole, EBF analogues, synthesis, repellent activity, insecticidal activity
PDF全文下载地址:
点我下载PDF
