摘要/Abstract
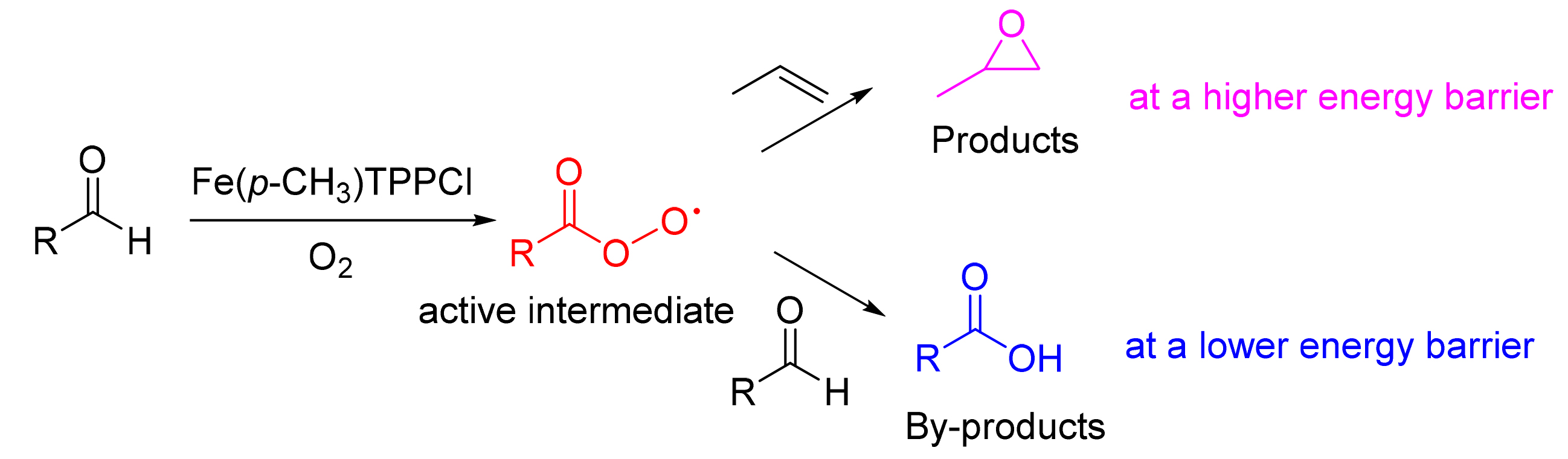
根据密度泛函理论,对分别以丙烯醛、丙醛、巴豆醛、正丁醛和异丁醛为共还原剂时,丙烯环氧化过程中可能存在的反应路径的能垒进行了理论计算,通过分析对比确定了最优反应路径为过氧酰基与丙烯进行自由基加成,然后分解为酯基自由基和环氧丙烷.此外得到了醛类的自氧化过程以及反应过程中的脱羧反应对环氧化反应有着一定影响的结论,与实验数据进行对比,二者结果比较相符.对共还原剂醛类的筛选具有一定的指导作用.
关键词: 理论计算, 丙烯环氧化, 共还原剂
On the basis of density functional theory, theoretical calculation is conducted to obtain the free energy barriers of the reaction path, which may exist in the process of propylene epoxidation with co-reductant acrolein, propanal, crotonaldehyde, N-butyl aldehyde and isobutyl aldehyde respectively. And the optimal reaction path was determined to be free radical addition of peroxy acyl and propylene through analysis and comparison. In addition, the conclusion that auto-oxidation process of aldehydes and the decarboxylation in the reaction process have a certain influence on the epoxidation reaction can be conducted, and the results are in good consistent with the experimental data. It has a certain guiding effect on the choosing of co-reducing aldehydes.
Key words: theoretical calculation, propylene epoxidation, co-reductant
PDF全文下载地址:
点我下载PDF
