摘要/Abstract
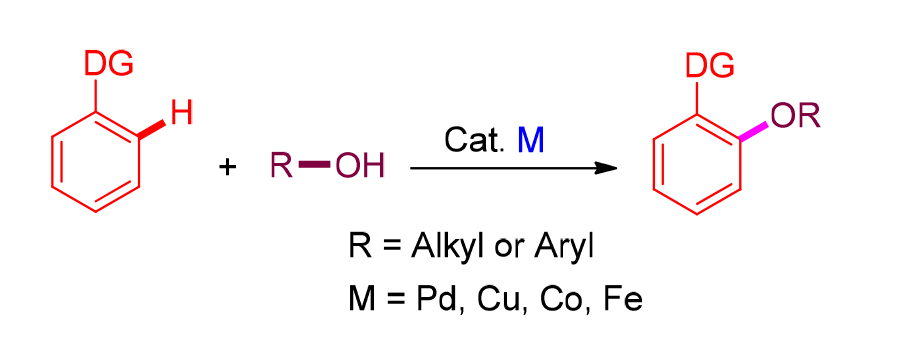
芳香醚结构是许多天然产物以及药物分子的重要骨架, 同时也是非常重要的有机合成中间体. 通常, 芳香醚由含离去官能团的芳烃与醇或酚偶联制得. 此类方法由于需对底物进行预官能团化, 因此存在着低原子及步骤经济性不足等问题. 近年来, 利用过渡金属催化的C—H键活化策略, 通过芳烃与醇或酚的脱氢偶联实现芳基醚的制备, 越来越受到有机化学家关注. 综述了最近通过脱氢偶联制备芳香醚化合物的研究进展. 围绕各类方法的底物适应范围和反应机理等进行详细论述, 并就该领域的局限性和未来的发展前景进行总结和展望.
关键词: 芳香醚, 脱氢偶联, C—H键活化, 苯酚, 醇
Aryl ethers are important central motifs that are abundant in many natural products and drug molecules, as well as versatile building blocks for organic synthesis. Aryl ethers were usually synthesized through the coupling reactions between leaving group substituted arenes and alcohols. However, the introduction of leaving group requires extra synthetic operation and produces lots of wastes. Over the past decade, the method for the synthesis of aryl ethers via C—H alkoxylation or aryloxylation has received much attention due to its potential as an atom and step efficient methodology. Herein, the research advances on the synthesis of aryl ethers through dehydrogenative coupling are reviewed. The detailed substrate scopes and reaction mechanisms, as well as the limitations of current procedures and the prospects for the future, are discussed.
Key words: aryl ether, dehydrogenative coupling, C—H activation, phenol, alcohol
PDF全文下载地址:
点我下载PDF
