摘要/Abstract
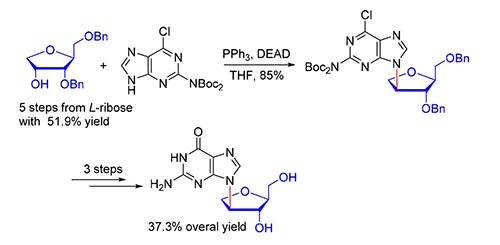
发展了一条改进的L-鸟嘌呤异核苷全合成路线.以L-核糖为起始原料,合成了3,5-O-二苄基-1-脱氧-L-核糖,再与碱基N2,N2-二叔丁氧羰基-6-氯鸟嘌呤发生关键的Mitsunobu反应来合成异核苷6.经过9步反应,以37.3%的总收率合成了L-鸟嘌呤异核苷,其中Mitsunobu反应构建异核苷键具有立体专一性、高产率、条件温和、区域选择性高等优点.该方法可以作为鸟嘌呤异核苷的通用合成路线.
关键词: 核苷, 全合成, 糖基化, Mitsunobu反应, 异核苷
An improved route for the total synthesis of iso-L-guanosine was developed. Using L-ribose as the starting material, 3,5-O-dibenzyl-1-deoxy-L-ribose was firstly synthesized. Then, Mitsunobu reaction between N2,N2-bis(tert-butyloxycarbonyl)-6-chloro-guanine and 3,5-O-dibenzyl-1-deoxy-L-ribose afforded isonucleoside 6. Finally, iso-L-guanosine was synthesized in 9 steps with 37.3% overall yield. Adopting Mitsunobu reaction as the key step, it has the merits of high steroseletivity and regioselectivity, mild reaction condition, and high yield. Currently developed approach could be used as a general synthetic strategy for the synthesis other related guanine isonucleosides.
Key words: nucleosides, total synthesis, glycosidation, Mitsunobu reaction, isonucleosides
PDF全文下载地址:
点我下载PDF
