摘要/Abstract
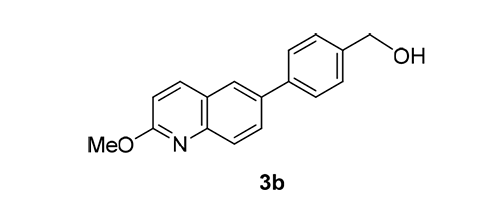
人DNA拓扑异构酶Ⅱα(topoisomerase Ⅱα,Topo Ⅱα)是重要的抗肿瘤药物研究靶标之一.前期研究发现对联三苯类化合物对Topo Ⅱα有抑制作用,并且抑制人乳腺导管癌细胞增殖.本研究通过对联三苯类化合物的骨架跃迁,设计合成了19个6-取代芳基-2-甲氧基喹啉类衍生物3a~3s,包括取代苯基、氮硫杂环和萘环等.体外人三阴乳腺癌MDA-MB-231细胞株生长抑制实验和Topo Ⅱα抑制实验结果表明,6-(4-羟甲基苯基)-2-甲氧基喹啉(3b)有明显细胞毒性和Topo Ⅱα抑制活性(IC50=9.9 μmol·L-1).研究结果为研发新型Topo Ⅱα抑制剂提供了新方向,对肿瘤的预防和治疗具有重要意义.
关键词: 拓扑异构酶Ⅱα, 合成, 结构优化, 抗肿瘤活性
Human DNA Topoisomerase Ⅱα (Topo Ⅱα) is one of the important therapeutic targets for the treatment of cancers. Our previous study showed that p-terphenyls have inhibitory effects on Topo Ⅱα and inhibit the proliferation of human breast ductal carcinoma cells. In this study, nineteen 6-substituted aryl-2-methoxyquinolines (3a~3s) were designed, synthesized and evaluated for their cytotoxicity against the growth of human triple negative breast cancer MDA-MB-231 cell line and inhibitory activity against Topo Ⅱα. Among these compounds, 6-(4-(hydroxymethyl)phenyl)-2-methoxyquinoline (3b) showed the most potent activity (IC50=9.9 μmol·L-1). These results have important significance for the further study of aryl quinoline TopoⅡα inhibitors.
Key words: Topoisomerase Ⅱα, Synthesis, Structure optimization, Antitumor activity
PDF全文下载地址:
点我下载PDF
