摘要/Abstract
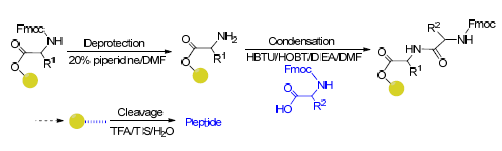
人体中寡肽转运蛋白对于小肽和药物的转运极为关键,而保守序列对于维持其结构与功能有着重要作用.为了深入了解寡肽转运蛋白中特征肽段的作用,促进寡肽在药学、医学等领域的应用.采用Fmoc固相合成法合成了寡肽转运蛋白2的特征基元Ⅲ(FYLSINAGS)及其四个突变体,经反相高效液相色谱纯化后,对产物进行了质谱检测.用紫外、荧光光谱及结构模拟方法研究了寡肽与DNA的相互作用.实验结果与结构模拟结果表明,FYGSINAGS碳端的丝氨酸、肽段中丝氨酸残基的数目和位置对于寡肽的结构及其与DNA的相互作用影响较大.将寡肽C-末端的丝氨酸突变为疏水性氨基酸有利于寡肽螺旋结构的形成,丝氨酸全部突变后寡肽与DNA的嵌入作用增强.因而寡肽转运蛋白的特征基元Ⅲ中丝氨酸残基,尤其是C末端的丝氨酸是比较重要的功能性氨基酸残基.
关键词: 寡肽, 固相合成, 相互作用, 特征基元
The peptide transporter family in human body is critical for the transport of peptides and drugs, and the conservative sequences in the peptide transporters play an important role in maintaining its structure and function. In order to understand the function of consensus peptides in peptide transporter and promote the application of oligopeptides in pharmaceutical and medical fields, the signature motif Ⅲ (FYLSINAGS) and its four mutants were synthesized by Fmoc solid phase synthesis method. The products were identified using mass spectrometry, and purified by RP-HPLC. The interaction between peptide and DNA was detected by UV and fluorescence spectrometry. The experimental and structural simulation results showed that the dominant role of electrostatic interaction and intercalation between oligopeptide FYLSINAGG and DNA was related to the concentration of oligopeptide FYLSINAGG, the interaction of FYGLINAGG containing helix structure and DNA was enhanced for the generation of complex, and the interaction between FYGLINKGG hasing helix structure or FYGLINSGG and DNA was attenuated. These results indicated that the serine residue in C-terminal of FYGSINAGS, and the number and position of serine in peptides had great influence on the structure of oligopeptide and its interaction with DNA. The mutation of serine into hydrophobic amino acids is beneficial to form helical structure of oligopeptides and enhance the embedded intercalation with DNA. Thus, serine residues in signature motif are important functional residues, especially at the C-terminus.
Key words: oligopeptide, solid phase synthesis, interaction, signature motif
PDF全文下载地址:
点我下载PDF
