

江苏省人民医院浦口分院 神经内科,江苏 南京 210031
收稿日期:2021-03-25;接收日期:2021-07-16;网络出版时间:2021-09-01
基金项目:江苏省南京市医学科技发展资金项目(Nos. YKK17257, QRX17108) 资助
作者简介:曹丽华??江苏省人民医院浦口分院副主任医师、科教科副科长,南京市卫计委十三五“青年人才”,南京市中青年“卫生优秀人才”,浦口区中青年“卫生拔尖人才”。南京市神经病学青委会委员,南京医学会认知障碍分会委员,南京医学会帕金森病学组成员,南京医学会肌电图学组成员,南京市慢病预防学会委员,江北新区神经病学分会委员。已在国内外刊物发表论文15篇,主持参与20余项国家级和省市级科研项目。获国家专利授权8项.
摘要:人类肠道菌群是数以万亿的细菌组成的高度多样化的生态系统,菌群失调与多个系统疾病有关联。肠道菌群通过菌群-肠-脑轴与神经系统多途径双向互作,能引起神经免疫炎症反应、肠黏膜和血脑屏障功能改变、直接刺激迷走神经和肠道神经系统脊神经、神经内分泌-下丘脑-垂体-肾上腺轴,造成神经系统疾病。肠道菌群的代谢产物也有一定的作用。文中综述自闭症谱系障碍、多发性硬化、帕金森病、癫痫、吉兰巴雷综合征、阿尔茨海默病、视神经脊髓炎、肝性脑病、肌萎缩侧索硬化、精神分裂症、抑郁症、慢性疲劳综合征、亨廷顿病、脑卒中等肠道菌群改变特征及其干预措施的研究进展。目前肠道菌群的研究还处在初级阶段,因果关系和机制方面的研究比较少,这对精准实施菌群临床干预措施具有重要意义,期待将来有所突破成为一些神经系统疾病治疗的新路径。
关键词:肠道菌群菌群-肠-脑轴疾病神经系统干预措施
Intestinal flora and neurological disorders
Qianqian Tang, Lihua Cao


Department of Neurology, Pukou Branch of Jiangsu Provincial People's Hospital, Nanjing 210031, Jiangsu, China
Received: March 25, 2021; Accepted: July 16, 2021; Published: September 1, 2021
Supported by: Medical Science and Technology Development Fund Project of Nanjing, Jiangsu Province, China (Nos. YKK17257, QRX17108)
Corresponding author: Lihua Cao. Tel: +86-25-58532975; E-mail: feiran519@126.com.
Abstract: The human intestinal flora is a highly diverse ecosystem composed of trillions of bacteria. The imbalance of the flora is related to a variety of diseases. The intestinal flora interacts with the nervous system bidirectionally in many ways through the gut-brain axis. It causes neuroimmune inflammatory response, dysfunction of gut mucosa and blood-brain barrier, direct stimulation of the vagus nerve, spinal nerve of the enteric nervous system, and the neuroendocrine hypothalamus-pituitary-adrenal axis, causing neurological disorders. The metabolites of the intestinal microbial community also play a role. This article summarizes the characteristics of the altered intestinal flora and intervention measures in autism spectrum disorder, multiple sclerosis, Parkinson's disease, epilepsy, Guillain-Barré syndrome, Alzheimer's disease, neuromyelitis optica, hepatic encephalopathy, amyotrophic lateral sclerosis, schizophrenia, depression, chronic fatigue syndrome, Huntington's disease and stroke. The current research on intestinal flora is still in its infancy, and very few studies were carried out on causality and the underlying mechanisms, which prevents the development of precise flora-based clinical intervention measures. It is expected the research on intestinal flora would lead to novel approaches for treatment of some neurological disorders.
Keywords: intestinal floragut-brain axisdiseasenervous systemintervention measures
人类对微生物的理解多是以感染性疾病的形式呈现的,这类疾病往往给人类带来巨大的灾难,目前世界范围内流行的新型冠状病毒肺炎疫情是这一灾难强有力的一种表现形式,人类阻止或消除传染病的脚步从未停下。随着医学的发展,人们又发现其以非感染性疾病的形式影响着人们的健康。在人体皮肤、上呼吸道、口腔、肠道、阴道居住着大量的微生物,多数微生物寄宿在肠道[1]。肠道菌群是近年来医学研究的热点,肠道菌群平衡着机体健康,肠道菌群紊乱则会引起呼吸[2]、循环[3]、泌尿[4]、生殖[5]、内分泌[6]、血液[7]、运动[8]、免疫[9]、消化[10]、神经系统疾病[11],与消化系统疾病关系的研究深入而全面,与神经系统疾病关系的研究也在快速进步,现综述肠道菌群与神经系统疾病的文献,增加与拓展一些神经系统疾病发生机制的思路,探索这类神经系统疾病诊疗干预的新路径。
1 肠道菌群概述肠道菌群是寄居于肠道内并与宿主共生的多种微生物群落的总称,有细菌、病毒和真菌等[12],其中98%为细菌,重量为0.2–2.0 kg,种类有500多种,有1014个集落形成单位,约为人体细胞数量的10倍,基因数目是人类基因的150倍[13],有5个主要细菌门:拟杆菌门、厚壁菌门、放线杆菌门、变形杆菌门和疣状杆菌门,前两个门最常见[14]。厚壁菌门/拟杆菌门比例失调或倒置提示肠道微生物菌群失衡[15]。人体肠道菌群的产生开始于胎儿期的母体子宫[16],在分娩时开始接触到宫体外微生物,分娩方式、母乳喂养、早产、宿主遗传学、抗生素暴露和母体感染等因素都影响着肠道内菌群的种类、数量和比例[17],随着年龄的增长,肠道菌群不断变化并维持着与内外界环境的动态平衡,每天更替的总数量和总质量分别约10–400万亿个和60–2 000 g,比每天更替的人体细胞数量大[18]。肠道菌群与宿主形成了平衡稳定的利益组合体,人体为肠道菌群提供生活场所,肠道菌群在人体内发挥各种生理功能,人体代谢所需的酶类35%以上是由正常肠道菌群合成的,参与3大营养物质的代谢和维生素的合成[19]。肠道菌群在肠道黏膜表面形成保护性生物屏障和维护上皮细胞之间的连接、有调节其通透性的作用[20]、促进肠道上皮细胞分泌防御素及免疫球蛋白A (Immunoglobulin A,IgA)、降低致病菌的定殖、抑制致病菌与肠道上皮细胞结合和侵入、阻止病原菌的过度增殖和病毒感染,对机体免疫系统的发育成熟和正常功能的维持具有重要意义[15],还有改善人体新陈代谢、抗炎、抗氧化和抗衰老等作用[21-22]。肠道菌群在生命早期的神经系统生长发育过程中,通过调节脑源性神经营养因子、突触素和突触后密度蛋白等对大脑结构的塑造产生作用,影响成年时的情绪、行为和感觉[23],在神经系统疾病的发生发展中发挥至关重要作用[24]。
2 肠道菌群在神经系统疾病中的作用机制肠道菌群与神经系统是双向互动多途径联系的,目前发现至少有5个路径[25]。肠道菌群失调引起神经系统疾病也必然是多途径多机制,有的存在1–2种,有的是多途径共同作用。尽管详细的机理仍待进一步探索,但目前比较明确的理论有:(图 1) ①肠黏膜、血脑屏障功能改变:肠道微生物的异常导致淀粉样蛋白和脂多糖(Lipopolysaccharide,LPS) 的分泌,增加了肠道通透性,使LPS、淀粉样蛋白等其他细胞因子进入肠壁,刺激Toll样受体4 (Toll-like receptor 4,TLR4) 和其他Toll样受体(Toll-like receptors,TLRs) 产生炎性细胞因子[26]。这些炎症因子可增加血脑屏障的通透性,进入脑组织,引起神经炎症、神经损伤,淀粉样蛋白等沉积形成斑块,导致神经元死亡,直接影响大脑功能。益生菌有加强上皮连接和保护黏膜屏障的功能,对肠膜完整性具有保护作用,使肠道降低通透性,减少肠道炎症因子进入血液循环后对神经系统的损害[27],这是阿尔兹海默病(Alzheimer’s disease,AD) 临床研究的进展之一。②神经免疫炎症反应:肠道菌群与全身免疫细胞相通,肠腔内细菌调节肠上皮屏障,渗透到肠道相关的淋巴组织(Gut-associated lymphoid tissue,GALT) 和血液中,可与各种免疫细胞相互作用。某些细菌可以刺激效应型T细胞分化,促进T细胞脑浸润,与循环中的炎性细胞因子共同破坏血脑屏障(Blood brain barrier,BBB) 的完整性,引起神经免疫炎症反应,导致疾病发生[28]。③肠道菌群直接刺激迷走神经、肠道神经系统和脊神经:无菌小鼠模型研究发现,肠道菌群参与神经发生发育、髓鞘形成和小胶质细胞的激活,还参与社交能力、内脏疼痛、免疫功能、应激敏感性、恐惧和焦虑反应中脑信号的形成,无菌小鼠的大脑无法得到正常发育[18]。迷走神经是肠道到大脑的信号通道,可以通过小分子或大分子(如α-突触核蛋白的朊样移位) 的运输,也可以通过电信号在神经元上传递。20世纪70年代,治疗胃溃疡的常规方法是切除胃中全部或部分神经来抑制胃酸分泌,在最近数十年的随访中发现,接受这种治疗方式的患者似乎不易患有帕金森病(Parkinson’s disease,PD)[29],这是一个值得继续探讨的问题。④神经内分泌-下丘脑-垂体-肾上腺轴(Hypothalamic-pituitary-adrenal axis,HPA):肠道菌群可以影响神经回路和与应激反应相关行为的组成、有助于神经内分泌的成熟。实验动物研究表明,含有肠道菌群的粪便对出生后应激反应的发展至关重要,这对HPA轴的正常发育是有意义的[30]:大脑通过激活HPA轴释放皮质醇、增加肠道通透性,调控内分泌细胞、免疫细胞、细胞因子等影响胃肠道微环境,从而影响肠道细菌的组成[31]。⑤肠道菌群代谢产物的作用:肠道微生物在肠道内产生大量代谢产物,影响肠道本身和非肠道组织。血液内一半的小分子不是由微生物产生就是由微生物调节的[32],常见的有:多巴胺、5-羟色胺(5-hydroxytryptamine,5-HT)、谷氨酸、脑源性神经营养因子、γ-氨基丁酸、短链脂肪酸(Short-chain fatty acids,SCFA)、氧化三甲胺(Trimethylamine N-oxide,TMAO) 和神经毒性代谢产物如氨气、酚、胺、酚酸、右旋乳酸等[33-35]。神经毒性代谢产物与肝性脑病的发生有密切关系[36],SCFA可刺激肠内5-HT的合成和分泌,5-HT结合受体后,起到调节血脑屏障通透性、干预神经元发育分化、通过神经信号调控情绪和调节血液循环影响大脑功能[37];SCFA通过G蛋白敏感的G蛋白偶联受体传导信号,并且循环在血液中的SCFA影响组蛋白3和4的组织特异性乙酰化,诱导基因组的表观遗传变化,参与宿主神经免疫内分泌功能、肠道稳态和能量代谢的调节[38]。小鼠实验发现,有益微生物产生一种烟酰胺代谢物,注射给易患肌萎缩侧索硬化(Amyotrophic lateral sclerosis,ALS) 的小鼠,这种分子进入了脑部,能改善ALS小鼠的症状[39]。
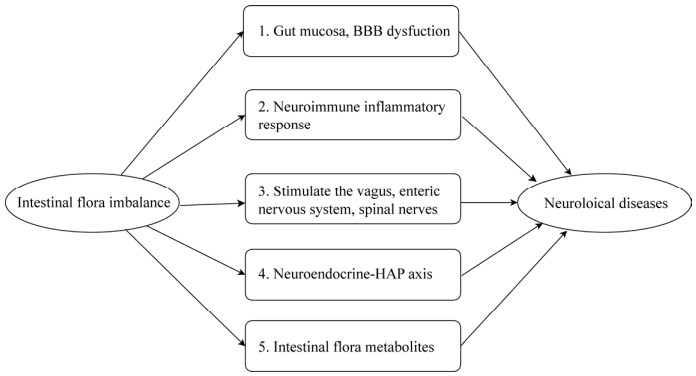 |
| 图 1 肠道菌群引起神经系统疾病的机制 Fig. 1 Mechanisms of neurological disorders caused by imbalanced intestinal flora. BBB: blood-brain barrier; HPA: hypothalamic-pituitary-adrenal. |
| 图选项 |
3 神经精神疾病3.1 自闭症谱系障碍(Autism spectrum disorder, ASD)ASD是一组神经发育障碍性疾病,其特征是在多个环境中社会交流和互动障碍,个人兴趣或活动受到限制以及重复性刻板行为[40]。病因不清楚,目前认为是遗传和环境因素综合作用的结果。儿童和青少年的ASD患病率在0.6%–1.7%之间,这是一个严重的公共卫生问题[41]。随着对ASD的深入研究,在ASD患者中观察到全身和神经炎症增加,甚至出现脑特异性自身抗体和高血清素,故提出免疫系统失调、炎症和母体因素学说[42]。越来越多的证据表明,ASD患者的肠道微生物群及其代谢产物(包括神经递质) 与健康对照相比是不同的[43],在ASD受试者中观察到相对较高水平的产生SCFA的拟杆菌门,抗炎属双歧杆菌的水平降低,梭菌属水平增加。一项系统评价纳入了18项研究[41],共有493名ASD儿童和404名对照组,ASD儿童的拟杆菌门、厚壁菌门和放线菌门菌群较对照组丰富。ASD儿童的类杆菌属、副杆菌属、梭状芽孢杆菌属、粪杆菌属和角杆菌属的比例显著增高,副球菌和双歧杆菌的比例下降。另一项系统评价结果显示[44]:ASD患儿的肠道菌群变化缺乏一致性,在一定范围内,门水平的厚壁菌群:普雷沃菌、包括产气荚膜梭菌的梭状芽胞杆菌群和双歧杆菌属与对照组有差异。国内基于操作分类单元(Operational taxonomic units,OTUs) 的16S rDNA测序结果表明[45],ASD组和健康对照组(Healthy control,HC) 在科、属、种水平上存在较大差异。ASD组类杆菌和硒单胞菌的丰度显著低于HC组。普雷沃菌科Prevotellaceae的数量ASD组明显低于HC组。在动物研究中,与常规定殖的小鼠相比,无菌雄性小鼠社会障碍出现增加,表明肠道菌群在这种行为中起重要作用[46]。大量临床观察表明,ASD多数有胃肠道功能异常,在18项原始研究中,便秘最常见,出现在12项研究中(80%),其次是腹泻,出现在8项研究中(53%)[47]。几项有关益生菌对ASD患者和ASD动物模型研究结果表明,益生菌对ASD症状有积极作用,改善神经行为症状,如焦虑或注意力不集中和/或胃肠道症状[48]。ASD的脑-肠-菌群轴有许多有待发现,关于肠道菌群干预疗法,特别是粪便微生物群移植在治疗ASD的胃肠道症状方面有广阔前景[49-50]。ASD的饮食方法,包括排除谷蛋白、酪蛋白、复合碳水化合物饮食、生酮饮食和低草酸饮食以及膳食补充剂:脂肪酸、益生元、维生素、矿物质、谷胱甘肽、植物化学物质如姜黄素、白藜芦醇、柚皮素和莱菔硫烷等临床研究结果是喜忧参半[51-52],饮食疗法廉价、易于实施是改善ASD患者生活状况的基础。迄今为止,大量研究表明肠道菌群在ASD中的重要相关性,未来的研究需要足够大的样本和标准化的方法来阐明肠道菌群和ASD之间复杂的相互关系,为精准干预提供理论基础。
3.2 多发性硬化(Multiple sclerosis, MS)MS是慢性自身免疫性中枢神经系统(Central nervous system,CNS) 变性脱髓鞘疾病,病理特征为由星形胶质细胞、小胶质细胞以及活化的免疫细胞组成的炎症和白质病变[53]。遗传、感染和环境因素(如EB病毒感染、吸烟和缺乏维生素D) 的相互作用导致免疫系统紊乱,免疫细胞进入中枢神经系统,在T细胞介导下发生MS[54-55]。研究表明,肠道菌群失调与MS的发生发展密切相关,两项小鼠实验性自身免疫性脑脊髓炎(EAE) 研究使用管饲法移植MS患者或健康人类对照的微生物群,移植MS患者微生物群的小鼠EAE发病率增加,病情更严重并且抗炎细胞因子白细胞介素10 (Interleukin 10,IL-10)的表达降低[56]。对MS患者和健康人粪便中的肠道微生物群比较发现:MS患者的假单胞菌、支原体、嗜血杆菌、布氏杆菌、多利亚菌、肠杆菌和黄杆菌富集,普氏菌、对羟基苯甲酸杆菌、阿德勒克罗伊茨菌、柯林斯氏菌、乳酸杆菌、共细菌和嗜血杆菌减少[53]。在俄罗斯人MS中芽殖菌属和未分类的瘤胃球菌属的相对丰度显著增加[57]。还有MS不同阶段肠道微生物组的微生物和功能差异的报告[58]:复发缓解型MS、继发进展型MS与HC相比,每个患者组都有许多物种的丰度发生显著变化,MS中的产生短链脂肪酸(SCFA) 的细菌减少。这些说明MS存在肠道菌群异常,通过饮食、益生菌、粪菌移植(Fecal microbiota transplantation,FMT) 等干预肠道菌群措施来改善这种异常,对MS患者可能带来益处。目前,有病例报告FMT对MS症状和疾病进展的影响,FMT后有持续性的有益效果。在一份病例系列报告中,一名MS合并复发性艰难梭菌感染的患者接受单一FMT治疗,FMT解决了复发性艰难梭菌感染,并预防了MS疾病进展超过10年,3名患者观察到重复的FMT改善了MS症状[59]。
3.3 帕金森病(Parkinson’s disease, PD)PD是第二种常见的进行性神经退行性疾病,临床表现为运动迟缓、肌强直、静止性震颤和姿势步态障碍。其特征是中脑黑质多巴胺能神经元和Meynert基底核的胆碱能神经元的功能永久性丧失,以及中枢神经系统中α-突触核蛋白的持续积累和聚集,受累的神经元出现路易小体和路易体神经轴突[60]。PD患病率为0.1%–0.2%,60岁以上达1.0%,随着年龄的增长而增加[61],我国PD人数到2030年将有500万人,几乎占到全球PD患病人数的一半。PD不仅对患者的日常活动造成损害,也带来巨大的社会和医疗负担[62]。PD病因和发病机制大多不清楚,5%–10%患者有遗传原因[61],风险因素包括年龄、男性和环境因素[63]。近来研究PD病理的一个热点是α-突触核蛋白(α-synuclein,α-Syn) 聚集成路易小体。小鼠模型表明α-Syn可以通过血脑屏障被运送到大脑[64],认为这种疾病可能始于肠道,α-Syn从肠粘膜转运到中枢神经系统,在肠道中也有Syn的聚集。实验证据也支持肠道菌群组分的改变促进α-Syn聚集体从肠道神经系统向大脑扩散形成特征性神经退行性变异,肠道菌群在PD的发病机制中发挥重要作用[65]。文献报告PD患者发生肠道菌群紊乱,益生菌可以改善PD患者的症状,认为肠道菌群可能与帕金森病发病有关[66]。与健康个体比较,PD患者肠道微生物群的变化是:紫胶菌科中布劳提亚属和蔷薇属减少,粪便杆菌也减少,乳酸杆菌科、阿克曼菌属和双歧杆菌属增加[67-68]。一项荟萃分析表明,PD患者的肠道菌群中,乳杆菌属、阿克曼菌属、双歧杆菌属富集,而毛螺菌科及粪杆菌属等重要的产短链脂肪酸菌属减少;这些变化可能导致促炎症状态,并与帕金森病患者的复发性胃肠道症状相关[69]。动物实验研究显示,接受健康小鼠粪便移植的PD模型小鼠运动功能得到改善、纹状体神经递质增加和神经炎症减少[70]。接受PD患者粪便的健康小鼠运动功能恶化,纹状体神经递质减少[71]。临床接受FMT治疗的PD患者病例报告:改善了腿震颤和其他帕金森病症状,便秘改善持续至术后3个月随访结束[72]。这些关联为帕金森病中出现的失调提供了支持,尽管微生物与人类疾病之间的方向性和因果关系尚不清楚[73]。
3.4 癫痫(Epilepsy)癫痫是一种大脑疾病,与兴奋性和抑制性脑网络的不平衡有关[74],导致神经元电活动异常引发肌肉阵发性收缩,表现为一个人无缘由地反复出现癫痫发作的倾向[75],也是一种慢性神经系统疾病,影响全球超过5 000万人,占全球经济疾病负担的0.5%,是世卫组织重点关注的五大神经精神疾病之一[76]。60%的癫痫为特发性,被认为是遗传因素和环境因素共同作用的结果,目前有很多治疗药物,约有三分之一癫痫患者产生耐药而使治疗失败[77]。作为癫痫的替代疗法:生酮饮食(Ketogenic-diet,KD),多年来一直用于治疗难治性癫痫患儿,是一种成熟的非药物疗法[78],采用生酮饮食治疗的癫痫患者减少了癫痫发作次数,这与生酮饮食改变肠道菌群组成和功能相关[79],说明癫痫与肠道微生物群失调相关,难治性癫痫患者厚壁菌相对于拟杆菌的丰度增加,微生物群的α多样性差异也很大[80]。有研究发现耐药患者与非耐药患者相比,厚壁菌/拟杆菌比值增加,后者与健康人相似,α多样性可能是由于稀有细菌负荷加大而增加,双歧杆菌和乳酸杆菌的增加与癫痫发作减少4次或4次以下相关[81]。通过16S rDNA测序,对原发性局灶性癫痫患者(n=30) 和健康对照组(HC) (n=10) 的肠道菌群组成进行了分析和比较[82]:癫痫患者的蛋白质细菌门(25.4%) 高于HC (1.5%),蛋白细菌门中弯曲杆菌属、德尔夫特菌属、嗜血杆菌属、月屈光属、奈瑟菌属在癫痫患者中明显高于健康志愿者(P < 0.05)。10.6%的癫痫患者检出梭杆菌门,而HC中未检出,梭杆菌门的属为瘦肉杆菌属和梭杆菌属。有研究认为癫痫患者粪便微生物群组成发生明显改变,肠道共生菌群因临床表型不同而发生改变,癫痫患者的α多样性指数明显低于HC (P < 0.05)。不同临床预后患者和HC粪便微生物群落结构和组成存在差异(P < 0.05)。癫痫患者的微生物组变化包括放线杆菌和疣状杆菌增多,普雷沃特菌属、布氏杆菌、双歧杆菌增多,门级蛋白细菌减少,在属水平上耐药癫痫患者在放线杆菌属、疣状杆菌属、硝基螺旋菌属以及布劳特氏菌属、双歧杆菌属、黑线下属、戴阿利斯特杆菌属和厌氧菌属富集(Kruskal-Wallis检验:P < 0.05) 可作为疾病诊断的潜在生物标志物[83]。研究发现益生菌对癫痫有积极作用[84]。世界首例使用FMT来缓解肠道和神经系统症状的患者是一名有克罗恩病的22岁女孩,既往已有17年的癫痫病史,在FMT前,患者在未使用丙戊酸钠治疗的情况下频繁发作,FMT后,患者在未使用抗癫痫药的情况下20多个月无发作。该病例报告说明FMT能有效防治癫痫,通过重塑肠道菌群可能是一种治疗癫痫的新方法[85]。
3.5 阿尔茨海默病(Alzheimer’s disease, AD)AD是一种进行性CNS神经退行性疾病[86-87],其特征是记忆力减退、人格和行为改变等全面性痴呆表现,患者在疾病后期无法进行正常的日常活动[88],是遗传因素和环境因素相互作用的结果[89],是痴呆症最普遍的形式。随着人类老年化进程加快,AD的发病率每年都在增加,全球约有5 000万人,65–75岁人群有10%,80岁及以上老年人高达32%受到影响[90]。世界卫生组织(World health organization,WHO) 推测到2050年AD患者的数量可能会增加3倍[88]。AD持续增长的发病率和沉重的负担使得其成为全球公共卫生问题。目前认为AD主要神经病理改变是细胞外β淀粉样蛋白沉积为神经炎斑块和细胞内高磷酸化tau蛋白积聚为神经原纤维缠结[91],导致神经炎症、氧化应激、线粒体功能障碍、酶系统失调和神经元死亡[88],神经炎症被认为在AD中起着关键作用[92]。近年来的研究发现,肠道菌群失调可能引发人体低水平炎症,包括神经炎症,这些均扩展了对AD的认识。研究表明,在AD患者中,厚壁菌门减少,拟杆菌及布劳特氏菌属增加,α多样性降低,AD患者与非痴呆年龄匹配的对照进行比较时,肠道微生物群组成具有SCFA产生细菌丰度减少和促炎细菌丰度增加[93]。根据载脂蛋白E (Apolipoprotein E,ApoE) 基因型选择的患者肠道微生物群组成的横断面研究表明,ApoE 4携带者与其他ApoE基因型相比,产生丁酸盐的肠道细菌(包括梭菌) 的丰度降低,粪便SCFA水平降低[94]。有人观察到野生型小鼠表现出使用广谱抗生素后认知功能下降,随后接受来自衰老抗性小鼠的粪便治疗后,空间学习和记忆得到改善[95]。临床报告一位82岁男性病例,AD病史5年,接受FMT治疗2个月后症状改善,随访4–6个月持续好转[96]。这些表明肠道微生物在AD中的潜在作用,AD患者肠道微生物群发生了改变,是因是果还需要临床实验验证,对AD患者进行FMT的随机对照试验(Randomized controlled trial,RCT) 研究。肠道微生物群-脑轴促进AD发病和进展是一个假说,未来的研究要确定影响AD肠道微生物组变化的独立环境因素。
3.6 视神经脊髓炎(Neuromyelitis optica, NMO)NMO是CNS自身免疫性炎性脱髓鞘疾病,表现横纹肌炎和视神经炎,严重者发生致残性瘫痪和视力丧失,是临床罕见病[97],病变部位局限于脊髓、脑干和视神经,其特征是存在中性粒细胞和嗜酸性粒细胞,以及抗体和补体的沉积[98],病因发病机理不明,有遗传因素和环境因素(植物、细菌或病毒) 诱发特异性水通道蛋白-4 (Aquaporin-4,AQP4) 免疫球蛋白G (Immunoglobulin G,IgG) 抗体,产生自身免疫损害[99]。有证据显示,肠道微生物群失调和肠黏膜屏障破坏与NMO的关联[100],NMO组和健康对照组的肠道菌群组成明显不同,NMO患者的致病菌属黄杆菌属和链球菌的丰度增加。粪便杆菌、毛螺菌、普雷沃菌、布劳提亚菌、玫瑰杆菌、罗姆布茨菌、粪球菌和融合杆菌丰度下降,NMO患者的肠道菌群的“光合作用” “光合作用蛋白”和“硫胺代谢” 3种代谢途径显著下调[101];产气荚膜梭菌是与NMO高度相关的细菌,和肠道中某些梭状芽孢杆菌可以调节T细胞和Th17细胞之间的平衡[102]。这些初步发现与NMO之间的发生机制需要进一步研究,未来通过调节肠道菌群可能是NMO的一种潜在干预策略。由于病人极少,进行临床试验和动物模型实验也很困难。
3.7 吉兰巴雷综合征(Guillain-Barré syndrome, GBS)GBS是最常见和最严重的周围神经病,症状严重程度因人而异,20%–30%的病例伴发呼吸衰竭、全世界每年约有10万人患上这种疾病[103]。静脉注射免疫球蛋白或血浆置换治疗是支持治疗的最佳管理方法,大多数病人预后良好,也有可能发生永久性残疾[103]。发病机制被认为是感染或其他免疫刺激自身免疫反应引起的,发病前多有胃肠道空肠弯曲杆菌感染。动物实验表明空肠弯曲菌感染后,抗神经节苷脂自身抗体显著升高,辅助型T细胞2 (T helper 2 cell,Th2) 依赖性空肠弯曲菌反应显著升高,这些反应与优势拟杆菌和厚壁菌群有关,说明肠道微生物群可能是控制GBS易感性的一个因素[104]。小鼠动物模型中发现,空肠弯曲杆菌菌株和肠道微生物群均影响炎症和GBS的发展,并且表明空肠弯曲杆菌感染后,益生菌可改善炎症和自身免疫疾病[105],由于临床GBS病例较少,只有动物研究,目前未见患者的肠道菌群文献报道。
3.8 肝性脑病(Hepatic encephalopathy, HE)HE是由肝功能不全、急性肝衰竭和门体分流等引起脑功能障碍,表现为广泛的神经或精神异常,从亚临床改变到昏迷[106-107];是由严重肝脏疾病所致的代谢紊乱为基础的中枢神经系统功能失调综合征,是预后不良的标志,严重影响患者的生活质量,经常给亲属和护理人员带来沉重负担[108];也是肝硬化住院治疗的原因和晚期最严重的并发症之一[109]。HE发病机制复杂,是多种因素的相互作用,如肠道功能失调、肠道通透性亢进和神经炎症,其中肠道菌群失调及其有害微生物副产物(如氨、吲哚、氧吲哚和内毒素) 起着至关重要的作用[110]。这些有毒代谢物在肠道中浓度增加后被吸收入血,病变肝脏又无法清除这些产物,对脑组织产生损害,出现各种各样的神经精神异常。大量的临床和动物研究结果显示,肝性脑病患者的肠道菌群发生了改变,肝性脑病肝硬化患者与对照组间的肠道菌群差异大于非肝性脑病肝硬化患者与对照组间的差异,虽然肝性脑病和非肝性脑病的肝硬化患者的粪便菌群构成没有明显的不同,但这两者的肠黏膜菌群构成存在明显差异,与非肝性脑病肝硬化患者相比,肝性脑病肝硬化患者肠黏膜的罗氏菌属减少,肠球菌、韦永球菌、巨型球菌和伯克菌增多,且这些细菌数量的增多伴随着患者认知功能的减退[111]。对轻微型肝性脑病(Minimal hepatic encephalopathy,MHE) 患者的粪便和唾液微生物群进行检测发现粪便和唾液样本中乳酸杆菌科的相对丰度较高,而在没有MHE的人群中毛螺菌科的相对丰度更高,logistic回归分析显示,粪便和唾液中的毛螺菌科属(瘤胃球菌属和梭状芽孢杆菌属) 与良好认知相关[112]。目前HE的一线临床治疗方案,即HE的主要治疗手段,无论是利福昔明等抗生素治疗,还是口服乳果糖、乳梨糖等益生元或者用微生态调节剂直接补充益生菌,都是通过减少致病菌数量、血液内毒素和氨氮水平等调节肠道菌群的组成来发挥治疗作用,能显著改善HE患者的神经精神症状,提高生活质量[113-114]。目前有较多的益生菌用于HE的治疗和预防,有效性的证据需进一步观察。益生菌的有效性有严格菌株、种属和剂量依赖性,有必要进行高质量的随机化、标准化程序的临床试验,才能获得益生菌的有效性和安全性数据[115]。
3.9 肌萎缩侧索硬化(Amyotrophic lateral sclerosis, ALS)ALS是一种不能根治的神经退行性疾病,影响上下运动神经元,一般发病后2–5年内因呼吸衰竭死亡[116]。病因及发病机制不明,可能是一个复杂的基因环境相互作用的结果[117]。越来越多的证据表明,肠道菌群组成的不平衡可能是导致ALS发生的环境因素之一[118]。在神经退行性疾病的动物模型中,存在慢性神经炎症;临床ALS患者中也可以检测到这种炎症,其有促进ALS的作用,可能与疾病的发生发展有关[119]。一项病例对照研究,比较66名ALS患者和61名健康对照组(HC) 的肠道微生物群的结果表明:与HC相比,ALS患者中主要产丁酸菌、直肠真杆菌和肠道罗斯拜瑞氏菌的相对丰度显著降低。调整年龄、性别和便秘并没有实质性改变结果。与HC相比,ALS中能够产生丁酸盐的8种优势菌种的总丰度也显著降低(P < 0.001)。结论:ALS患者肠道完整性和炎症调节重要的几种产丁酸菌的水平较低[120]。ALS易感Sod1转基因小鼠实验证明嗜黏蛋白-阿克曼菌属改善,而瘤胃球菌属和狄氏副拟杆菌加剧ALS的症状[121]。国内的研究报告:ALS患者中厚壁菌门/拟杆菌门比率、甲烷短杆菌属呈现增强趋势,而有益微生物(粪杆菌属和拟杆菌属) 的相对丰度呈现显著降低趋势,ALS患者和健康人的人体内毒素、SCFA、亚硝态氮/硝态氮(NO2-N/NO3-N) 和γ-氨基丁酸(γ-aminobutyric acid,GABA) 的平均浓度分别为64.2/65.3 EU/mL、57.5/55.3 μg/mL、5.7/5.3 ng/mL和6.1/5.4 μmol/L,说明ALS患者胃肠道的消化代谢功能也可能下降[122]。瑞典2 484例ALS患者巢式病例对照研究发现:使用抗生素,特别是重复使用抗生素,可能会导致ALS的后续风险增加[123]。6个月的益生菌治疗没有使患者肠道微生物群的生物多样性更接近对照受试者,对ALS的疾病进展基本没有影响[118]。这说明需要进一步研究ALS的肠道微生物组成及新的干预措施。
3.10 精神分裂症(Schizophrenia)精神分裂症是一种慢性衰退性精神疾病[124],也是导致全世界2 100万人的严重身体和社会疾病[125],主要特征是基本个性改变,思维、情感、人格和行为的分裂障碍以及精神活动的不协调,临床症状有妄想、幻觉、思维异常、冷漠、退缩、迟钝等[126]。病因学复杂,可能是相互作用的遗传和环境影响的各种组合的产物[127]。认为精神分裂症存在多巴胺、谷氨酸和γ-氨基丁酸(GABA) 等神经递质系统的异常[128-129]。神经发育以及炎性免疫方面的研究是其热门话题,越来越多的证据表明肠道菌群有影响神经发育和调节人体的免疫炎性反应的作用,肠道菌群与精神分裂症之间存在密切联系[130]。有文献报道:与健康组肠道菌群比较,精神分裂症患者的肠道微生物群中含有许多兼性厌氧菌,如发酵乳杆菌、屎肠球菌、奥氏嗜碱杆菌和坂崎克罗诺杆菌/土杆菌,在属水平上表现出更大的α多样性和更高的β多样性[127]。还有文献报道,25例精神分裂症患者其厌氧球菌属、嗜血杆菌属、萨特雷拉属、巨球菌属、粪球菌属、布氏杆菌属、瘤胃球菌属明显丰富,25例健康对照组只有嗜血杆菌属、萨特雷拉属丰富,两组菌群总丰度没有差异[131]。在一项关于精神分裂症辅助益生菌的随机、安慰剂对照试验中,患者的胃肠功能有所改善,但精神症状的严重程度没有变化[132],后来发现益生菌只能改善侵袭性酵母非阳性感染的患者中相关精神症状[133]。精神分裂症肠道菌群改变的数据差异,可能存在其他外部因素的干扰,需要进一步的实验研究和更多的临床试验来验证调节肠道微生物治疗患者的有效性和安全性,以改善该类疾病的处理[134]。
3.11 抑郁症(Depression)抑郁症是指情绪低落和/或对日常活动失去兴趣或乐趣至少2周,还包括一些症状,如沮丧、无价值感和/或绝望、精力下降、食欲减退、睡眠障碍、精神运动功能改变和自杀倾向和/或行动[135-136],全世界抑郁症的总患病率在6%–17%之间,约有8.4亿人受此影响,女性、老年人发病率高[137-138],是一种病因尚未完全阐明的多因素复杂的慢性病,患病人数不断增加。据估计,到2030年,抑郁症将成为中高收入国家疾病负担的主要原因[139]。最近研究发现肠道菌群组成与抑郁症发生有关系,肠道菌群-肠-脑轴被认为通过迷走神经调节各种中枢过程,它的代谢产物和免疫介质能触发神经传递、神经炎症和行为的变化[140]。抑郁症患者与健康对照组相比[141],微生物群落的α-多样性和β-多样性存在差异。在门水平上,硬壁菌、拟杆菌和蛋白细菌的丰度不一致。抑郁症患者中观察到放线杆菌和梭杆菌门的高丰度。在科水平上,放线菌科、红蝽杆菌科、双歧杆菌科、梭菌科Ⅺ、卟啉单胞菌科、梭状芽胞杆菌科、乳酸杆菌科、链球菌科、真杆菌科、嗜热厌氧菌科、梭杆菌科、诺卡氏菌科、链霉菌科的丰度较高,而脉管菌科、普雷沃菌科、类杆菌科、针叶藻科、示波螺旋藻科、泥鳅科和甲壳藻科的丰度较低。在属水平上,高丰度的有颤螺旋菌属、布劳特氏菌属、霍尔德曼氏菌、梭状芽胞菌属ⅪⅩ、厌氧棒杆菌属、Anaerofilum、链球菌属、Gellia、血尿杆菌属、副拟杆菌属、伊格尔兹氏菌属、克雷伯氏菌属、帕拉普氏菌属、韦荣氏球菌属、梭状芽胞菌属Ⅳ、丹毒丝菌属、真杆菌、微单胞菌属、脱硫弧菌属、Parasutterlla、放线菌、细辛杆菌、阿托伯菌、欧尔森氏菌和低丰度的粪球菌属、乳酸杆菌、大肠杆菌/志贺氏菌、梭状芽孢杆菌、小杆菌属、霍华德菌、杆菌属和萨特氏菌属。对抑郁症患者粪便中SCFA进行分析,非抑郁症组所有的浓度都较高,抑郁症组中只有已酸升高,低水平SCFA可能引起中枢神经系统功能失调和神经炎症,导致抑郁症状[142]。益生菌干预临床研究:一项益生菌植物乳杆菌299v (LP299v) 随机、双盲、安慰剂对照对抑郁症患者的心理和免疫调节作用研究发现,益生菌组中尿氨酸浓度降低,与对照组相比,益生菌组在注意力和知觉以及语言学习任务方面的得分显著提高[143]。一项为期12周的益生菌植物乳杆菌的随机、双盲、安慰剂对照试验,益生菌有减少压力和焦虑,改善认知和记忆的效果[144]。以上资料说明抑郁症患者的肠道菌群发生了明显改变。一些益生菌对抑郁症患者治疗有效果,需要进一步的临床研究,才能把微生物组学的发现转化为实践策略。
3.12 慢性疲劳综合征(Chronic fatigue syndrome, CFS)CFS又称慢性疲劳免疫功能障碍综合征、病毒后疲劳综合征、肌痛性脑脊髓炎(Myalgic ceaneomyelitis,ME)[145],是一种病因不明的致残性和衰弱性神经免疫性疾病[146-147],有长期疲劳症状,伴有肌肉和关节疼痛、头痛,淋巴结压痛,反复咽痛,认知和注意力、记忆和睡眠方面有明显问题,以及体力活动后恶化的一种独特的综合征[148]。CFS的临床诊断需有至少6个月疾病状态史,排除其他疲劳病因后才能确定,至今也没有很好的治疗方法,对患者的日常、职业和社会功能造成了毁灭性影响[149]。早期文献报道与健康人群相比,慢性疲劳综合征患者的肠道菌群发生明显变化,比如大肠杆菌和双歧杆菌数量减少,肠球菌和需氧菌数量增多[150]。近来对细菌核糖体RNA (Ribosomal RNA,rRNA)标记的深度测序,CFS和对照组的总体微生物组成在门和科的水平上存在差异,与对照组相比CFS显示厚壁菌的相对丰度较低,变形杆菌的相对丰度较高,瘤胃球菌科较低,毛螺旋菌科相似,双歧杆菌科较低,CFS组和对照组在类杆菌科(35%对43%)、立根菌科(3%对4%) 和普氏菌科(3.2%对0.7%) 存在一些差异,患者血液中LPS、脂多糖结合蛋白(Lipopolysaccharide binding protein,LBP) 和可溶性细菌脂多糖的膜受体14 (Soluble cluster of differentiation 14,sCD14) 水平升高[151]。应用红霉素与益生菌交替使用两周共4周的治疗方案治疗44例CFS患者,意向治疗组的治疗效果显著,改善了一些临床症状和认知功能(注意力、处理速度、认知灵活性、故事记忆力)。随着时间的推移,情绪、疲劳保持稳定[152]。最近一项系统评价,CFS患者的肠道菌群与健康对照组进行了比较,每项研究中都报告了差异,所有研究的结果都不一致。7项中有3项被认为具有统计学意义,认为目前还没有足够的证据表明肠道菌群在CFS/ME的发病机制中的作用。建议使用一致的标准诊断CFS,在采集样本前通过控制影响微生物组分的因素来减少混杂变量,包括更严重的CFS/ME病例[153]。CFS是受许多因素,包括环境和遗传、免疫等问题的影响,除了肠道细菌群失调,肠道病毒和一些营养缺乏(维生素C、维生素B复合物、钠、镁、锌、叶酸、L-肉碱、L-色氨酸、必需脂肪酸和辅酶Q10)在CFS症状的严重程度和恶化中起重要作用[148, 154],这是肠道菌群相关研究的挑战。
3.13 亨廷顿病(Huntington’s disease, HD)HD是一种常染色体显性遗传性神经退行性疾病[155],致病基因为Huntington基因,其编码的亨廷顿蛋白在全身各个器官包括中枢神经系统中广泛表达[156]。临床主要表现为舞蹈样不自主动作、精神障碍和进行性痴呆,是隐匿、缓慢进行性加重的过程,症状的发生和严重程度受到包括饮食、体力活动和压力在内的环境因素的综合影响[157]。动物实验研究:HD R6/2小鼠模型发现肠通透性增加和紧密连接蛋白的表达下降,存在肠道菌群失调;与野生型小鼠相比,R6/2小鼠类杆菌的相对丰度较高,厚壁菌属的相对丰度较低,与拟杆菌、副杆菌、乳酸杆菌、共细菌和肠杆菌科有明显的相关性[155]。用16S rRNA扩增子测序HD的R6/1转基因小鼠模型中的肠道微生物组,与同窝野生型对照组比较,12周龄HD小鼠的微生物群组成存在显著差异,类杆菌门增加和厚壁菌门减少,雄性HD小鼠的微生物多样性增加,但雌性HD小鼠的多样性没有差异[158]。丁酸盐代谢途径升高,肠道中保护性SCFA丁酸盐的产生增加[159]。无菌动物研究表明肠道菌群对调节髓鞘形成有一定的作用[160]。临床研究发现,HD肠道菌群β多样性存在显著差异,α多样性显著降低,男性在广古菌门、厚壁菌门、疣微菌门和酸乳杆菌科、艾克曼菌、拟杆菌科、双歧杆菌科、克里斯滕森菌科、梭菌科、红蝽杆菌科、伊格尔兹氏菌科、肠杆菌科、丹毒丝菌科、黄杆菌科、毛螺菌科、甲烷杆菌科、消化球菌科、消化链球菌科及理研菌科上有明显差异,这些事实说明,肠道菌群及其功能的改变是影响HD的一个因素[161]。纠正失衡的肠道菌群对HD有无治疗作用需要不断的探索,同基因治疗一样任重而道远。
3.14 脑卒中(Stroke)脑卒中是脑组织局部或整体血液暂时或永久供应中断而引起的脑损伤,可导致永久性神经功能缺损或死亡[162]。神经病理学分为缺血性脑卒中(85%) 和出血性脑卒中(15%)[163]。脑卒中是一种致命的神经系统疾病,给社会带来了沉重的经济负担[164],已成为全球健康问题,是目前全球第二大死亡原因和第三大致残原因[163]。先前的研究发现,90%的脑卒中病例可能与不良饮食、吸烟和低体力劳动等行为因素及代谢因素包括肥胖、高血压、糖尿病等有关,近来发现有些胃肠道疾病及肠道菌群也可能是脑卒中的危险因素[165-166]。两项临床队列研究结果:脑缺血迅速诱发肠缺血,并通过自由基反应产生过量的硝酸盐,导致肠道菌群扩张引起肠道生态失调。肠杆菌科富集通过增强全身炎症加剧脑梗塞,并且是中风患者原发性不良结果的独立危险因素。施用氨基胍或超氧化物歧化酶以减少硝酸盐产生或施用钨酸盐以抑制硝酸盐呼吸均导致肠杆菌科过度生长受到抑制,全身炎症减少和脑梗塞减轻[167]。这为临床防治干预,提供了具有转化价值的新思路。动物实验表明:与接受老年小鼠的粪菌比较,接受年轻小鼠的粪菌的脑卒中小鼠改善了几项行为测试、降低了死亡率和梗塞面积,减少了促炎细胞因子[168];用抗生素混合物治疗的小鼠在脑卒中急性期显示梗塞体积显著减少。在用野生型微生物群重新定殖的小鼠中,神经保护作用被消除。用氨苄青霉素或万古霉素而不是新霉素进行单一抗生素治疗足以减少中风后3 d的梗塞体积并改善运动感觉功能。这种神经保护作用与特定的微生物群体相关,而不是与总细菌密度相关,说明肠道微生物群对缺血性卒中短期和长期预后具有重要作用,调节特定微生物酶途径相关的微生物组可能为高风险患者提供预防策略[169]。在局部脑缺血后的猴子中,观察到粪便杆菌、链球菌、乳酸杆菌和颤螺菌属的相对水平降低[170]。丁酸盐是粪便杆菌和颤螺菌属代谢产物之一,是一种短链脂肪酸,有维持肠屏障完整性、抑制促炎细胞因子产生的重要作用[171]。对30例脑缺血性卒中(CI) 患者和30例健康对照者粪便肠道微生物群进行分析,CI患者和健康对照之间的微生物α-多样性和结构相似,但CI患者的肠道微生物群具有更多的SCFA生产菌,包括Odoribacter、艾克曼菌、瘤胃菌科-UCG-005和食物谷菌属。Norank-O-柔膜细菌目-RF9、肠杆菌、瘤胃菌科-UCG-002与低密度脂蛋白(LDL) 呈负相关,而高密度脂蛋白(High-density lipoprotein,HDL) 与瘤胃球菌属-1属呈显著正相关。肠杆菌属与美国国立卫生研究院卒中量表(National institute of health stroke scale,NIHSS)、改良Rankin量表(Modified rankin scale,MRS) 呈显著负相关[172]。脑卒中有很多可控和不可控的危险因素,肠道菌群改变模式差异大,除了肠道菌群,代谢产物TMAO、SCFA、继发性胆汁酸等与脑血管疾病有关,要根据肠道微生物群特征及其脑卒中接受体微生物群变化个性化进行调节才能获得益处,建议患病前后健康的生活方式、益生菌营养可以降低肠道生态失调导致中风后认知障碍(包括痴呆) 的风险[173]。
以上14种神经精神疾病的肠道菌群变化特征见表 1。
表 1 14种神经精神疾病肠道菌群变化特征Table 1 Characteristics of altered intestinal flora in 14 neuropsychiatric diseases
| Disease types | Alterations of intestinal flora | Animals | Patients | References |
| Autism spectrum disorder | Increase in Bacteroides, Parabacter, Clostridium, Faeculus, Ceratobacter Decrease in Prevotella, Paracoccus, Bifidobacterium | Yes | [41, 45] | |
| Multiple sclerosis | Increase in Pseudomonas, Mycoplasma, Haemophilus, Brucella, Doria, Enterobacter, Flavobacterium Decrease in Prevotella, Bacillus p-hydroxybenzoic acid, Kreuzia Adler, Collins bacteria, Lactic acid Bacillus, Adlercreutzia, Collinsella co-bacteria, Haemophilus Increased in Gemmiger sp., unclassified Ruminococcaceae in Russian patients | Yes | [53, 57] | |
| Parkinson’s disease | Increase in Lactobacillaceae family, Akkermansia sp., Bifidiobacterium sp. Decrease in Blautia sp., Lachnospiraceae family, Lachnospiraceae family | Yes | [67-68] | |
| Epilepsy | Increase in Campylobacter, Delft, Haemophilus, Lunaropterus, Neisseria, Actinobacteria, Verrucobacteria, Prevotella, Brucella Increase in α-diversity patients with drug-resistant epilepsy: Increase in Bifidobacteria, Actinobacillus, Verrucobacterium, Nitrospirillum, Blauterella, Bifidobacterium, Nigella, Dealisteria, anaerobic Bacteria | Yes | [82-83] | |
| Alzheimer’s disease | Increase in Bacteroides and Blautia, pro-inflammatory bacteria Decrease in phylum Firmicutes, α-diversity, SCFA-producing bacteria | Yes | [93] | |
| Neuromyelitis optica | Increase in pathogenic microorganisms of the genus Flavonifractor and Streptococcus Decrease in Faecal bacillus, Lachnospiracea, Prevotella, Brautia, Rose bacillus | Yes | [101] | |
| Guillain-Barré Syndrome | Romboutsia, Faecoccus and Fusion bacilli Increase in Bacteroides Firmicutes, Campylobacter jejuni strains | Yes | [104-105] | |
| Hepatic ecephalopathy | Increase in Enterococcus, Veillonococcus, Megacoccus, Burkholderia, Lactobacillus Decrease in Rootella | Yes | [111-112] | |
| Amyotrophic lateral sclerosis | Increase in ratio Firmicutes/Bacteroidetes, Methanobrevibacter Decrease in butyric acid producing bacteria Eubacterium rectale, Roseburia intestinalis, beneficial microorganisms (faecalibacterium and Bacteroides) | Yes | Yes | [120, 122] |
| Schizophrenia | Increase in Lactobacillus fermentum, Enterococcus faecium, Alcaliophilus auseri and Kronobacter Sakazaki, Anaerococcus, Haemophilus, Satrera, Macrococcus, Faecococcus, Brandt Bacillus and Rumenococcus, Larger α-diversity, higher β-diversity | Yes | [127, 131] | |
| Depression | Increase in Actinomycetes, Clostridium. Actinomycetes, Coriobacteria, Bifidobacteria, Clostridiales insertae sedis Xi, Porphyrinmonosporaceae, Clostridium, Lactobaceae, Streptococcaceae, Eubacidae, Thermophilic Anaerobaceae, Clostridae, Nocardiaceae, Streptomycedae Decrease in Vascular, Prevoodoceae, Bacteroideae, Coniophytae, Spirulinaceae, Spirulinaceae, loach family Crustaceaceae, SCFA in feces | Yes | [141-142] | |
| Chronic fatigue syndrome | Increase in Proteus, Enterococci and aerobic bacteria, the levels of LPS LBP and sCD14 in the blood Decrease in Escherichia coli and Bifidobacteria Firmicutes, Rumencoccus | Yes | [150-151] | |
| Huntington’s disease | Increase in Bacteroides, β-diversity Decrease in Firmicutes, α-diversity | Yes | Yes | [155, 161] |
| Stroke | Increase in Odoribacter, Akkermansia, Ruminococcaceae-UCG-005 and Victivallis Decrease in Faecalis, Streptococci, lactobacilli, Oscillospira | Yes | Yes | [170] |
表选项
4 肠道菌群干预神经系统疾病的研究肠道菌群干预措施有饮食、抗生素、益生菌、益生元、合生元和粪菌移植(FMT) 等。
4.1 饮食饮食是微生物群组成的主要因素,不同的时间、饮食偏好及食材影响我们的肠道生态系统[174-175]。宿主生理、免疫状态和代谢能力对细菌定殖和特定微生物物种存在也有调节作用[176]。饮食多样性对人体健康的重要性毋庸置疑。世界上有数不清的饮食模式,研究表明地中海饮食对人体有潜在的健康益处:地中海饮食具有抗炎和调节肠道菌群的作用,降低患者厚壁菌/拟杆菌比率[177]。食用谷类、坚果、蔬菜、豆类、水果和鱼类可以降低神经退行性疾病、精神疾病的发病率[178]。生酮饮食增加血清酮体和减少神经元凋亡[179],生酮饮食癫痫患者可以减少癫痫发作次数,这与生酮饮食改变肠道菌群组成和功能相关[180]。高膳食纤维饮食提供的膳食纤维主要由厚壁菌和其他细菌发酵,增加SCFA的产量,如醋酸盐、丙酸盐和丁酸盐[181],丁酸是肠道菌群和宿主代谢健康的重要中介,影响体重、身体组成和糖稳态,改善高脂饮食诱导的肥胖和高血糖/高胰岛素血症[182],对改善中风后的恢复有利。
4.2 益生菌、益生元和合生元益生菌是活的非致病微生物,当给予足够的量(至少106个活的CFU/g) 时,通过改善肠道内的微生物平衡并参与新陈代谢,对宿主有益[176]。益生菌的作用[115]:通过加强上皮连接和保持黏膜屏障功能显示出对胃黏膜完整性的细胞保护作用,增强肠屏障功能、调节肠道蠕动。补充益生菌既可以增加骨密度,又可以预防原发性(雌激素缺乏症) 和继发性骨质疏松症,降胆固醇,抗致癌,抗诱变和抗过敏,通过肠腔和肠壁的空间竞争和竞争营养物质与病原菌产生拮抗作用,生产抗菌剂、有机酸和细菌素,刺激肠道菌群产生黏蛋白阻止病原体的植入,增强肠道系统的免疫调节,抑制细菌毒素的产生。对ASD患者和ASD动物模型研究结果表明,益生菌对ASD症状有积极作用,改善神经行为症状,如焦虑或注意力不集中和/或胃肠道症状[183]。在一项队列研究中观察到用嗜酸乳杆菌Rosell-11益生菌治疗ASD儿童2个月,能改善患儿沟通、交流及反社会行为[184]。乳酸杆菌和双歧杆菌是治疗肝性脑病最有效的菌种,但丁酸梭状芽胞杆菌、大肠杆菌Nissle 1917、唾液链球菌和布拉氏酵母菌也被使用[185],酪酸梭菌和婴儿双歧杆菌有望作为轻度肝性脑病的辅助治疗[186-187]。对21项试验的荟萃分析,与安慰剂/未治疗相比,益生菌对肝性脑病的治疗有利,与乳果糖相当[187]。除了治疗肝性脑病,益生菌还可作为预防措施,防止肝性脑病复发[188]。临床应用益生菌可以改善神经系统疾病症状,但结果不一致[189-191]。有效性需要进一步观察,虽然安全性较好,但要注意其不良反应的发生,有人归纳为4大类:A、全身感染,B、有害的代谢活动,C、过度的免疫刺激,D、不希望的基因转移[192],谨慎应用于免疫功能低下的患者。益生元是在小肠中不被消化的碳水化合物,在结肠中有活性,被结肠中细菌发酵,影响SCFA的产生,调节黏蛋白的产生和肠道相关淋巴组织的局部炎症反应,从而刺激巨噬细胞的吞噬作用[193]。在对13项脑中风临床试验的荟萃分析中,益生元治疗没有降低重症监护病房和住院死亡率,但降低了重症监护病房的肺炎发病率和住院时间[194]。乳果糖和乳糖醇被视为益生元,有利于耐酸、非脲酶产生细菌的定殖,可以降低血氨浓度,降低结肠pH值、改善胃肠转运和增加粪便氮排泄[195],因其安全有效,目前在临床上广泛应用于各种类型的肝性脑病,已成为治疗肝性脑病的药物之一。合生元是益生菌+益生元,有1+1大于2的期望,因益生菌、益生元的研究还在探索中,故合生元的应用资料匮乏。
4.3 抗生素早先发现口服万古霉素治疗8周后ASD和胃肠道症状暂时改善[196],目前只有病例观察,抗生素治疗可以改变一些神经系统疾病的病程[197-198],如PD、癫痫。抗生素在急性缺血性脑卒中(Acute ischemic stroke,AIS) 的应用,没有明确的证据支持,在AIS后的最初几个小时内进行预防性抗生素治疗使病人获益,对227名患者的随机对照试验,90 d的功能结果和死亡率都没有有利影响[199]。另一项对1 224名AIS后有吞咽困难的患者中使用预防性抗生素,没有降低肺炎的发病率[200]。有前瞻性随机研究报告证实预防性抗生素治疗总体感染率有所下降,但这并不影响AIS后肺炎的发生率或2个月时的功能转归得分[201]。抗生素是通过杀灭细菌来影响肠道菌群的组成,应根据抗生素的类型和微生物组在神经系统疾病中的具体作用进行选择,才能提高其治疗作用,由于抗生素没有针对特定类型的细菌,临床治疗效果自然不理想,这取决于未来对肠道菌群作用的研究,一旦确立肠道菌群在神经系统疾病中的作用,通过使用抗生素来调节肠道菌群,就会产生很好的临床治疗效果[198]。
4.4 粪菌移植(Fecal microbiota transplantation, FMT)FMT是将供者粪便微生物群从健康人群转移到患者身上[202],研究表明,FMT可广泛影响受者的肠道菌群,是调节肠道微生物群的最有效选择,是治疗复发性艰难梭菌感染的有效方法[203]。被确定为纠正神经精神障碍患者和AIS患者的失调的可能策略[204]。移植富含SCFA (尤其是丁酸) 的粪便导致微生物群重塑,增加了乳酸菌种类,改善了肠道微生物群,从而积极调节了脑缺血反应[205]。在临床环境中,粪便微生物群移植已被证明对肠道菌群失调有效,治疗复发性艰难梭菌感染有效,也有引起两名接受粪菌移植者发生继发性大肠杆菌菌血症的报道[206]。在一项开放标签临床试验中[207-208],18名ASD患者每天接受FMT 7–8周,胃肠道和ASD的行为症状得到改善,一直持续到治疗后2年。一项CP101安慰剂对照随机FMT随机对照试验(RCT) 正在进行中[24]。
目前,只有两个病例系列报告FMT对MS症状和疾病进展的影响,两病例系列声称都有持续性FMT后的有益效果,一个次要的进步是多发性硬化患者同时接受单一FMT治疗复发性艰难梭菌感染。FMT解决了复发性艰难梭菌感染,也预防了MS疾病进展超过10年[209]。在病例系列中有3名患者观察到重复的FMT改善MS症状。临床试验列出一个正在进行MS患者接受FMT治疗的RCT,一个RCT非随机对照试验[24]。
关于FMT治疗PD,是病例报告[72],接受FMT治疗的PD患者随访12周,改善腿震颤和其他帕金森病症状,便秘也有缓解。一个随机对照试验和一个非随机对照试验FMT在PD患者中的试验正在进行中[24]。
关于FMT治疗癫痫的病例报告[210],显示全身性癫痫和克罗恩病接受了3次FMT治疗。在FMT治疗前,患者在未使用丙戊酸钠治疗的情况下频繁发作,FMT治疗后,患者在未使用抗癫痫药的情况下无发作20个月,而且克罗恩病活动指数改善。一个注册的FMT干预癫痫患者单组评估研究正在进行[24]。
目前还没有关于人类AD、GBS、ALS和脑卒中患者的FMT研究发表,但是临床试验网站显示AD、ALS患者正在进行FMT的RCT[24]。FMT的AD动物实验研究,在无菌AD小鼠模型中,分别进行正常肠道菌群和AD肠道菌群的移植都引起增加病理学,后者更明显[211],在抗生素降低认知功能的小鼠中,FMT能逆转这种损害[212]。GBS的动物模型研究表明,特定的肠道微生物群组成可增加空肠弯曲杆菌感染后GBS的易感性[104]。阿莫西林/克拉维酸治疗敏感小鼠组与不敏感的小鼠组比较,敏感小鼠组显示梗塞体积减小,缺血性脑损伤和感觉运动障碍也减轻[213],用正常的微生物群灌胃导致梗塞体积减小[214]。与接受老年小鼠的粪菌比较,接受年轻小鼠的粪菌的中风小鼠改善了几项行为测试、降低死亡率和梗塞面积,减少了促炎细胞因子[215]。
肠道菌群主要干预措施在神经系统疾病中的应用现况见表 2。
表 2 肠道菌群干预在神经系统疾病中应用现况Table 2 Application of intestinal flora intervention in neurological disorders
| Neuropsychological diseases | Intestinal flora intervention | Animal model | Case reports | In clinical trials | References | ||||
| Diet | Probiotics | Prebiotics | Antibiotics | FMT | |||||
| Multiple sclerosis | √ | √ | √ | √ | √ | √ | [24, 60, 118, 123] | ||
| Parkinson’s disease | √ | √ | √ | √ | √ | [24, 71-72] | |||
| Epilepsy | √ | √ | √ | √ | √ | √ | [24, 180, 197, 211] | ||
| Alzheimer’s disease | √ | √ | √ | √ | √ | [24, 95-96, 212] | |||
| Stroke | √ | √ | √ | √ | √ | √ | [115, 168, 182, 201, 214] | ||
| Chronic fatigue syndrome | √ | √ | √ | √ | [96, 152] | ||||
| Hepatic ecephalopathy | √ | √ | √ | √ | √ | √ | [96, 185-188, 195] | ||
| Amyotrophic lateral sclerosis | √ | √ | √ | √ | [24, 118, 123] | ||||
| Schizophrenia | √ | √ | √ | [132-133] | |||||
| Depression | √ | √ | √ | [96, 143-144] | |||||
| Autism spectrum disorder | √ | √ | √ | √ | √ | √ | √ | √ | [24, 178, 183-184, 196] |
| Guillain-Barré Syndrome | √ | [103] | |||||||
| FMT: fecal microbiota transplantation; √ indicates that there are applications and clinical literature. | |||||||||
表选项
5 存在问题与未来趋势肠道菌群对人体健康的重要性早在100年前就被科学家认识,由于当时研究手段有限而限制了其发展,普通细菌培养无法培养出肠道菌群中的厌氧菌,厌氧菌在肠道菌群中数量大且是共生关系。现在通过厌氧培养获得肠道微生物的菌种,从菌株水平上研究肠道微生物的功能和作用;采用DNA指纹图谱的分析方法及第二、三代通量测序技术方法,对样本中的微生物DNA进行测定,揭示肠道菌群的种类以及它们之间的相对丰度和进化关系;实时荧光定量PCR方法,定量研究肠道菌群,探讨肠道菌群的多样性,研究肠道菌群与环境之间的关系,极大地促进肠道菌群的研究[216]。由此可见,需要不断研发新技术及其在菌群研究中的应用,才能全面了解肠道菌群对人体的影响,现在对肠道菌群的研究还处在初级阶段,多集中在研究正常菌群、异常菌群,以及什么菌对什么菌有抑制或促进作用等,机制方面的研究比较少,只有突破机制研究,才能精准调节肠道菌群[217]。除了加强肠道菌群中细菌之间联系的研究,还要对肠道内病毒、真菌等微生物进行研究;深入研究肠道微生物代谢产物对人体影响的机理和医学转化;加强影响肠道菌群多因素研究,如年龄、饮食、种族、环境、运动等;因果关系研究是菌群研究的重点,也是难点之一,目前的文献以相关性研究居多,出现临床干预效果相互矛盾就不难理解;临床干预试验的有效性和安全性应采用RCT的方法,RCT得出的结果结论可靠,临床应用证据等级高。
参考文献
| [1] | Cuomo A, Maina G, Rosso G, et al. The microbiome: a new target for research and treatment of schizophrenia and its resistant presentations? A systematic literature search and review. Front Pharmacol, 2018, 9: 1-8. DOI:10.3389/fphar.2018.00001 |
| [2] | Li CX, Liu HY, Lin YX, et al. The gut microbiota and respiratory diseases: new evidence. J Immunol Res, 2020, 2020: 1-12. |
| [3] | Novakovic M, Rout A, Kingsley T, et al. Role of gut microbiota in cardiovascular diseases. World J Cardiol, 2020, 12(4): 110-122. DOI:10.4330/wjc.v12.i4.110 |
| [4] | Pluznick JL. The gut microbiota in kidney disease. Science, 2020, 369(6510): 1426-1427. DOI:10.1126/science.abd8344 |
| [5] | AlHilli MM, Bae-Jump V. Diet and gut microbiome interactions in gynecologic cancer. Gynecol Oncol, 2020, 159(2): 299-308. DOI:10.1016/j.ygyno.2020.08.027 |
| [6] | Li R, Li YF, Li C, et al. Gut microbiota and endocrine disorder. Adv Exp Med Biol, 2020, 1238: 143-164. |
| [7] | Saghafian-Hedengren S, S?derstr?m I, Sverremark- Ekstr?m E, et al. Insights into defective serological memory after acute lymphoblastic leukaemia treatment: the role of the plasma cell survival niche, memory B-cells and gut microbiota in vaccine responses. Blood Rev, 2018, 32(1): 71-80. DOI:10.1016/j.blre.2017.08.009 |
| [8] | Collins FL, Rios-Arce ND, Schepper JD, et al. The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr, 2017, 5(4): 1-26. |
| [9] | De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol, 2019, 195(1): 74-85. |
| [10] | Giuffrè M, Campigotto M, Campisciano G, et al. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature. Am J Physiol Gastrointest Liver Physiol, 2020, 318(5): 889-906. DOI:10.1152/ajpgi.00161.2019 |
| [11] | Zhu SB, Jiang YF, Xu KL, et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation, 2020, 17(1): 1-20. DOI:10.1186/s12974-019-1655-5 |
| [12] | Kc D, Sumner R, Lippmann S. Gut microbiota and health. Postgrad Med, 2020, 132(3): 274. DOI:10.1080/00325481.2019.1662711 |
| [13] | Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr, 2015, 174(2): 151-167. DOI:10.1007/s00431-014-2476-2 |
| [14] | Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology, 2018, 154(1): 28-37. DOI:10.1111/imm.12896 |
| [15] | Moon J, Yoon CH, Choi SH, et al. Can gut microbiota affect dry eye syndrome?. Int J Mol Sci, 2020, 21(22): 8443-8471. DOI:10.3390/ijms21228443 |
| [16] | Sender R, Milo R. The distribution of cellular turnover in the human body. Nat Med, 2021, 27(1): 45-48. DOI:10.1038/s41591-020-01182-9 |
| [17] | Cryan JF, O'Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol, 2020, 19(2): 179-194. DOI:10.1016/S1474-4422(19)30356-4 |
| [18] | Bi Y, Tu Y, Zhang N, et al. Multiomics analysis reveals the presence of a microbiome in the gut of fetal lambs. Gut, 2021, 70(5): 853-864. DOI:10.1136/gutjnl-2020-320951 |
| [19] | Moszak M, Szulińska M, Bogdański P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders—a review. Nutrients, 2020, 12(4): 1096-1126. DOI:10.3390/nu12041096 |
| [20] | Farré R, Fiorani M, Abdu Rahiman S, et al. Intestinal permeability, inflammation and the role of nutrients. Nutrients, 2020, 12(4): 11855-1203. |
| [21] | Walrath T, Dyamenahalli KU, Hulsebus HJ, et al. Age-related changes in intestinal immunity and the microbiome. J Leukoc Biol, 2020, 10: 1002. |
| [22] | Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol, 2017, 595(2): 489-503. DOI:10.1113/JP273106 |
| [23] | Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol, 2015, 28(2): 203-209. |
| [24] | Vendrik KEW, Ooijevaar RE, De Jong PRC, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol, 2020, 10: 98. DOI:10.3389/fcimb.2020.00098 |
| [25] | Wang HX, Wang YP. Gut microbiota-brain axis. Chin Med J, 2016, 129(19): 2373-2380. DOI:10.4103/0366-6999.190667 |
| [26] | Leblhuber F, Ehrlich D, Steiner K, et al. The immunopathogenesis of Alzheimer's disease is related to the composition of gut microbiota. Nutrients, 2021, 13(2): 361-394. DOI:10.3390/nu13020361 |
| [27] | Stavropoulou E, Bezirtzoglou E. Probiotics in medicine: a long debate. Front Immunol, 2020, 11: 2192. DOI:10.3389/fimmu.2020.02192 |
| [28] | Logsdon AF, Erickson MA, Rhea EM, et al. Gut reactions: how the blood-brain barrier connects the microbiome and the brain. Exp Biol Med (Maywood), 2018, 243(2): 159-165. DOI:10.1177/1535370217743766 |
| [29] | Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol, 2015, 78(4): 522-529. DOI:10.1002/ana.24448 |
| [30] | Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary- adrenal system for stress response in mice. J Physiol, 2004, 558(Pt 1): 263-275. |
| [31] | Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun, 2014, 38: 1-12. DOI:10.1016/j.bbi.2013.12.015 |
| [32] | Willyard C. How gut microbes could drive brain disorders. Nature, 2021, 590(7844): 22-25. DOI:10.1038/d41586-021-00260-3 |
| [33] | Zhu SB, Jiang YF, Xu KL, et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation, 2020, 17(1): 1-20. DOI:10.1186/s12974-019-1655-5 |
| [34] | Averina OV, Zorkina YA, Yunes RA, et al. Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci, 2020, 21(23): 9234. DOI:10.3390/ijms21239234 |
| [35] | Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes, 2020, 11(2): 135-157. DOI:10.1080/19490976.2019.1638722 |
| [36] | Mancini A, Campagna F, Amodio P, et al. Gut: liver: brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct, 2018, 9(3): 1373-1388. DOI:10.1039/C7FO01528C |
| [37] | Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology, 2021, 160(5): 1486-1501. DOI:10.1053/j.gastro.2020.10.066 |
| [38] | Van Der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol, 2021. |
| [39] | Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature, 2019, 572(7770): 474-480. DOI:10.1038/s41586-019-1443-5 |
| [40] | American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington: American Psychiatric Association, 2013. |
| [41] | Iglesias-Vázquez L, Van Ginkel Riba G, Arija V, et al. Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients, 2020, 12(3): 792. DOI:10.3390/nu12030792 |
| [42] | Fattorusso A, Di Genova L, Dell'Isola GB, et al. Autism spectrum disorders and the gut microbiota. Nutrients, 2019, 11(3): 521. DOI:10.3390/nu11030521 |
| [43] | Ma B, Liang J, Dai M, et al. Altered gut microbiota in Chinese children with autism spectrum disorders. Front Cell Infect Microbiol, 2019, 9: 40. DOI:10.3389/fcimb.2019.00040 |
| [44] | Ho LKH, Tong VJW, Syn N, et al. Gut microbiota changes in children with autism spectrum disorder: a systematic review. Gut Pathog, 2020, 12: 6. DOI:10.1186/s13099-020-0346-1 |
| [45] | Sun H, You Z, Jia L, et al. Autism spectrum disorder is associated with gut microbiota disorder in children. BMC Pediatr, 2019, 19(1): 516. DOI:10.1186/s12887-019-1896-6 |
| [46] | Desbonnet L, Clarke G, Shanahan F, et al. Microbiota is essential for social development in the mouse. Mol Psychiatry, 2014, 19(2): 146-148. DOI:10.1038/mp.2013.65 |
| [47] | Lefter R, Ciobica A, Timofte D, et al. A descriptive review on the prevalence of gastrointestinal disturbances and their multiple associations in autism spectrum disorder. Medicina, 2019, 56(1): 11. DOI:10.3390/medicina56010011 |
| [48] | Shaaban SY, El Gendy YG, Mehanna NS, et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci, 2018, 21(9): 676-681. DOI:10.1080/1028415X.2017.1347746 |
| [49] | Nitschke A, Deonandan R, Konkle AT. The link between autism spectrum disorder and gut microbiota: a scoping review. Autism, 2020, 24(6): 1328-1344. DOI:10.1177/1362361320913364 |
| [50] | Saurman V, Margolis KG, Luna RA. Autism spectrum disorder as a brain-gut-microbiome axis disorder. Dig Dis Sci, 2020, 65(3): 818-828. DOI:10.1007/s10620-020-06133-5 |
| [51] | Hartman RE, Patel D. Dietary approaches to the management of autism spectrum disorders. Adv Neurobiol, 2020, 24: 547-571. |
| [52] | Bhandari R, Paliwal JK, Kuhad A. Dietary phytochemicals as neurotherapeutics for autism spectrum disorder: plausible mechanism and evidence. Adv Neurobiol, 2020, 24: 615-646. |
| [53] | Fan YY, Zhang JM. Dietary modulation of intestinal microbiota: future opportunities in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Microbiol, 2019, 10: 740. DOI:10.3389/fmicb.2019.00740 |
| [54] | Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol, 2017, 13(1): 25-36. |
| [55] | Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet, 2018, 391(10130): 1622-1636. DOI:10.1016/S0140-6736(18)30481-1 |
| [56] | Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA, 2017, 114(40): 10713-10718. DOI:10.1073/pnas.1711235114 |
| [57] | Kozhieva M, Naumova N, Alikina T, et al. Primary progressive multiple sclerosis in a Russian cohort: relationship with gut bacterial diversity. BMC Microbiol, 2019, 19(1): 309. DOI:10.1186/s12866-019-1685-2 |
| [58] | Takewaki D, Suda W, Sato W, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci USA, 2020, 117(36): 22402-22412. DOI:10.1073/pnas.2011703117 |
| [59] | Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm, 2018, 5(4): e459. DOI:10.1212/NXI.0000000000000459 |
| [60] | Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers, 2017, 3: 17013. DOI:10.1038/nrdp.2017.13 |
| [61] | Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna), 2017, 124(8): 901-905. DOI:10.1007/s00702-017-1686-y |
| [62] | Li G, Ma J, Cui S, et al. Parkinson's disease in China: a forty-year growing track of bedside work. Transl Neurodegener, 2019, 8: 22. DOI:10.1186/s40035-019-0162-z |
| [63] | Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol, 2020, 27(1): 27-42. DOI:10.1111/ene.14108 |
| [64] | Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease. Neuron, 2019, 103(4): 627-641.e7. DOI:10.1016/j.neuron.2019.05.035 |
| [65] | Lubomski M, Tan AH, Lim SY, et al. Parkinson's disease and the gastrointestinal microbiome. J Neurol, 2020, 267(9): 2507-2523. DOI:10.1007/s00415-019-09320-1 |
| [66] | Gazerani P. Probiotics for Parkinson's disease. Int J Mol Sci, 2019, 20(17): 4121. DOI:10.3390/ijms20174121 |
| [67] | Barichella M, Severgnini M, Cilia R, et al. Unraveling gut microbiota in Parkinson's disease and atypical Parkinsonism. Mov Disord, 2019, 34(3): 396-405. DOI:10.1002/mds.27581 |
| [68] | Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord, 2017, 32(5): 739-749. DOI:10.1002/mds.26942 |
| [69] | Romano S, Savva GM, Bedarf JR. Meta-analysis of the Parkinson's disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis, 2021, 7(1): 27. DOI:10.1038/s41531-021-00156-z |
| [70] | Sun MF, Zhu YL, Zhou ZL, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun, 2018, 70: 48-60. DOI:10.1016/j.bbi.2018.02.005 |
| [71] | Zhou ZL, Jia XB, Sun MF, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson's disease mice via gut microbiota and metabolites. Neurotherapeutics, 2019, 16(3): 741-760. DOI:10.1007/s13311-019-00719-2 |
| [72] | Kuai XY, Yao XH, Xu LJ, et al. Evaluation of fecal microbiota transplantation in Parkinson's disease patients with constipation. Microb Cell Fact, 2021, 20(1): 98-101. DOI:10.1186/s12934-021-01589-0 |
| [73] | Sampson T. The impact of indigenous microbes on Parkinson's disease. Neurobiol Dis, 2020, 135: 104426. DOI:10.1016/j.nbd.2019.03.014 |
| [74] | Goldberg EM, Coulter DA. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci, 2013, 14(5): 337-349. DOI:10.1038/nrn3482 |
| [75] | Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia, 2014, 55(4): 475-482. DOI:10.1111/epi.12550 |
| [76] | Epilepsy[EB/OL]. [2021-03-25]. https://www.who.int/news-room/fact-sheets/detail/epilepsy |
| [77] | Fan YY, Wang H, Liu XY, et al. Crosstalk between the ketogenic diet and epilepsy: from the perspective of gut microbiota. Mediat Inflamm, 2019, 2019: 8373060. |
| [78] | Kossoff EH, Zupec-Kania BA, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the international ketogenic diet study group. Epilepsia Open, 2018, 3(2): 175-192. DOI:10.1002/epi4.12225 |
| [79] | Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell, 2018, 173(7): 1728-1741.e13. DOI:10.1016/j.cell.2018.04.027 |
| [80] | Lindefeldt M, Eng A, Darban H, et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes, 2019, 5(1): 5. DOI:10.1038/s41522-018-0073-2 |
| [81] | Peng A, Qiu X, Lai W, et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res, 2018, 147: 102-107. DOI:10.1016/j.eplepsyres.2018.09.013 |
| [82] | ?afak B, Altunan B, Top?u B, et al. The gut microbiome in epilepsy. Microb Pathog, 2020, 139: 103853. DOI:10.1016/j.micpath.2019.103853 |
| [83] | Gong X, Liu X, Chen C, et al. Alteration of gut microbiota in patients with epilepsy and the potential index as a biomarker. Front Microbiol, 2020, 11: 517797. DOI:10.3389/fmicb.2020.517797 |
| [84] | Yeom JS, Park JS, Kim YS, et al. Neonatal seizures and white matter injury: role of rotavirus infection and probiotics. Brain Dev, 2019, 41(1): 19-28. DOI:10.1016/j.braindev.2018.07.001 |
| [85] | He Z, Cui BT, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol, 2017, 23(19): 3565-3568. DOI:10.3748/wjg.v23.i19.3565 |
| [86] | Long JM, Holtzman DM. Alzheimer' disease: an update on pathobiology and treatment strategies. Cell, 2019, 179(2): 312-339. DOI:10.1016/j.cell.2019.09.001 |
| [87] | Liu S, Gao J, Zhu M, et al. Gut microbiota and dysbiosis in Alzheimer's disease: implications for pathogenesis and treatment. Mol Neurobiol, 2020, 57(12): 5026-5043. DOI:10.1007/s12035-020-02073-3 |
| [88] | Askarova S, Umbayev B, Masoud AR, et al. The links between the gut microbiome, aging, modern lifestyle and Alzheimer's disease. Front Cell Infect Microbiol, 2020, 10: 104. DOI:10.3389/fcimb.2020.00104 |
| [89] | Grochowska M, Laskus T, Radkowski M. Gut microbiota in neurological disorders. Arch Immunol Ther Exp (Warsz), 2019, 67(6): 375-383. DOI:10.1007/s00005-019-00561-6 |
| [90] | Prince M, Albanece E, Guerchet MP, et al. World Alzheimer Report 2014[EB/OL]. 2021-03-25]. https://www.alzint.org/resource/world-alzheimer-report-2014/. |
| [91] | Angelucci F, Cechova K, Amlerova J, et al. Antibiotics, gut microbiota, and Alzheimer's disease. J Neuroinflammation, 2019, 16(1): 1-10. DOI:10.1186/s12974-018-1391-2 |
| [92] | Calsolaro V, Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement, 2016, 12(6): 719-732. DOI:10.1016/j.jalz.2016.02.010 |
| [93] | Borsom EM, Lee K, Cope EK. Do the bugs in your gut eat your memories? relationship between gut microbiota and Alzheimer's disease. Brain Sci, 2020, 10(11): 814. DOI:10.3390/brainsci10110814 |
| [94] | Tran TTT, Corsini S, Kellingray L, et al. APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer's disease pathophysiology. Faseb J, 2019, 33(7): 8221-8231. DOI:10.1096/fj.201900071R |
| [95] | Zhan G, Yang N, Li S, et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging, 2018, 10(6): 1257-1267. DOI:10.18632/aging.101464 |
| [96] | Xu HM, Huang HL, Zhou YL, et al. Fecal microbiota transplantation: a new therapeutic attempt from the gut to the brain. Gastroenterol Res Pract, 2021, 6699268. |
| [97] | Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol, 2007, 6(9): 805-815. DOI:10.1016/S1474-4422(07)70216-8 |
| [98] | Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain, 2002, 125(Pt 7): 1450-1461. |
| [99] | Kountouras J, Deretzi G, Gavalas E, et al. Aquaporin 4, Helicobacter pylori and potential implications for neuromyelitis optica. J Neuroimmunol, 2013, 263(1/2): 162-163. |
| [100] | Cui C, Ruan Y, Qiu W. Potential role of the gut microbiota in neuromyelitis optica spectrum disorder: implication for intervention. J Clin Neurosci, 2020, 82(Pt B): 193-199. |
| [101] | Shi ZY, Qiu YH, Wang JC, et al. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: a cross sectional study. J Neuroimmunol, 2020, 339: 577126. DOI:10.1016/j.jneuroim.2019.577126 |
| [102] | Zamvil SS, Spencer CM, Baranzini SE, et al. The gut microbiome in neuromyelitis optica. Neurotherapeutics, 2018, 15(1): 92-101. DOI:10.1007/s13311-017-0594-z |
| [103] | Willison HJ, Jacobs BC, Van Doorn PA. Guillain-Barré syndrome. Lancet, 2016, 388(10045): 717-727. DOI:10.1016/S0140-6736(16)00339-1 |
| [104] | Brooks PT, Brakel KA, Bell JA, et al. Transplanted human fecal microbiota enhanced Guillain Barré syndrome autoantibody responses after Campylobacter jejuni infection in C57BL/6 mice. Microbiome, 2017, 5(1): 92. DOI:10.1186/s40168-017-0284-4 |
| [105] | Brooks PT, Bell JA, Bejcek CE, et al. An antibiotic depleted microbiome drives severe Campylobacter jejuni-mediated type 1/17 colitis, type 2 autoimmunity and neurologic sequelae in a mouse model. J Neuroimmunol, 2019, 337: 577048. DOI:10.1016/j.jneuroim.2019.577048 |
| [106] | Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the european association for the study of the liver. Hepatology, 2014, 60(2): 715-735. DOI:10.1002/hep.27210 |
| [107] | Campion D, Giovo I, Ponzo P, et al. Dietary approach and gut microbiota modulation for chronic hepatic encephalopathy in cirrhosis. World J Hepatol, 2019, 11(6): 489-512. DOI:10.4254/wjh.v11.i6.489 |
| [108] | Amodio P. Hepatic encephalopathy: diagnosis and management. Liver Int, 2018, 38(6): 966-975. DOI:10.1111/liv.13752 |
| [109] | Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther, 2007, 25(Suppl 1): 3-9. |
| [110] | Mancini A, Campagna F, Amodio P, et al. Gut: liver: brain axis: the microbial challenge in the hepatic encephalopathy. Food Funct, 2018, 9(3): 1373-1388. DOI:10.1039/C7FO01528C |
| [111] | Bajaj JS, Hylemon PB, Ridlon JM, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol, 2012, 303(6): G675-G685. DOI:10.1152/ajpgi.00152.2012 |
| [112] | Bajaj JS, Fagan A, White MB, et al. Specific gut and salivary microbiota patterns are linked with different cognitive testing strategies in minimal hepatic encephalopathy. Am J Gastroenterol, 2019, 114(7): 1080-1090. DOI:10.14309/ajg.0000000000000102 |
| [113] | Kaji K, Takaya H, Saikawa S, et al. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol, 2017, 23(47): 8355-8366. DOI:10.3748/wjg.v23.i47.8355 |
| [114] | Wang JY, Bajaj JS, Wang JB, et al. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: a multicenter, randomized controlled trial. J Dig Dis, 2019, 20(10): 547-556. DOI:10.1111/1751-2980.12816 |
| [115] | Stavropoulou E, Bezirtzoglou E. Probiotics in medicine: a long debate. Front Immunol, 2020, 11: 2192. DOI:10.3389/fimmu.2020.02192 |
| [116] | Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers, 2017, 3: 17071. DOI:10.1038/nrdp.2017.71 |
| [117] | Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol, 2013, 9(11): 617-628. DOI:10.1038/nrneurol.2013.203 |
| [118] | Di Gioia D, Bozzi Cionci N, Baffoni L, et al. A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med, 2020, 18(1): 153. DOI:10.1186/s12916-020-01607-9 |
| [119] | Beers DR, Appel SH. Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. Lancet Neurol, 2019, 18(2): 211-220. DOI:10.1016/S1474-4422(18)30394-6 |
| [120] | Nicholson K, Bjornevik K, Abu-Ali G, et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener, 2021, 22(3/4): 186-194. |
| [121] | Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature, 2019, 572(7770): 474-480. DOI:10.1038/s41586-019-1443-5 |
| [122] | Zhai CD, Zheng JJ, An BC, et al. Intestinal microbiota composition in patients with amyotrophic lateral sclerosis: establishment of bacterial and archaeal communities analyses. Chin Med J (Engl), 2019, 132(15): 1815-1822. DOI:10.1097/CM9.0000000000000351 |
| [123] | Sun J, Zhan Y, Mariosa D, et al. Antibiotics use and risk of amyotrophic lateral sclerosis in Sweden. Eur J Neurol, 2019, 26(11): 1355-1361. DOI:10.1111/ene.13986 |
| [124] | Nimgaonkar VL, Prasad KM, Chowdari KV, et al. The complement system: a gateway to gene-environment interactions in schizophrenia pathogenesis. Mol Psychiatry, 2017, 22(11): 1554-1561. DOI:10.1038/mp.2017.151 |
| [125] | Hjorth P, Medici CR, Juel A, et al. Improving quality of life and physical health in patients with schizophrenia: a 30-month program carried out in a real-life setting. Int J Soc Psychiatry, 2017, 63(4): 287-296. DOI:10.1177/0020764017702172 |
| [126] | Harrison P, Cowen P, Burns T, et al. Global psychiatry. Shorter Oxford Textbook of Psychiatry. Oxford: Oxford University Press, 2017: 674-680. |
| [127] | Zhu F, Ju YM, Wang W, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun, 2020, 11(1): 1-10. DOI:10.1038/s41467-019-13993-7 |
| [128] | Kesby JP, Eyles DW, McGrath JJ, et al. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry, 2018, 8(1): 30. DOI:10.1038/s41398-017-0071-9 |
| [129] | Hoftman GD, Dienel SJ, Bazmi HH, et al. Altered gradients of glutamate and gamma-aminobutyric acid transcripts in the cortical visuospatial working memory network in schizophrenia. Biol Psychiatry, 2018, 83(8): 670-679. DOI:10.1016/j.biopsych.2017.11.029 |
| [130] | Severance EG, Yolken RH. From infection to the microbiome: an evolving role of microbes in schizophrenia. Curr Top Behav Neurosci, 2020, 44: 67-84. |
| [131] | Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res, 2019, 204: 23-29. DOI:10.1016/j.schres.2018.09.014 |
| [132] | Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord, 2014, 16(1): PCC.13m01579. |
| [133] | Severance EG, Gressitt KL, Stallings CR, et al. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun, 2017, 62: 41-45. DOI:10.1016/j.bbi.2016.11.019 |
| [134] | Szeligowski T, Yun AL, Lennox BR, et al. The gut microbiome and schizophrenia: the current state of the field and clinical applications. Front Psychiatry, 2020, 11: 156. |
| [135] | Goldstein MC, Goldstein MA. Vitamins and minerals: fact versus fiction. ABC-CLIO, 2018. |
| [136] | American Psychiatric Association. Diagnostic and statistical manual of mental disorders[internet]. 5th ed. Washington, D.C.: American Psychiatric Association, 2013. |
| [137] | Boulkrane MS, Fedotova J, Kolodyaznaya V, et al. Vitamin D and depression in women: a mini-review. Curr Neuropharmacol, 2020, 18(4): 288-300. DOI:10.2174/1570159X17666191108111120 |
| [138] | Cyranowski JM, Frank E, Young E, et al. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry, 2000, 57(1): 21-27. DOI:10.1001/archpsyc.57.1.21 |
| [139] | Klimova B, Novotny M, Valis M. The impact of nutrition and intestinal microbiome on elderly depression—a systematic review. Nutrients, 2020, 12(3): 710. DOI:10.3390/nu12030710 |
| [140] | Capuco A, Urits I, Hasoon J, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther, 2020, 37(4): 1328-1346. DOI:10.1007/s12325-020-01272-7 |
| [141] | Barandouzi ZA, Starkweather AR, Henderson WA, et al. Altered composition of gut microbiota in depression: a systematic review. Front Psychiatry, 2020, 11: 541. |
| [142] | Skonieczna-?ydecka K, Grochans E, Maciejewska D, et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients, 2018, 10(12): 1939. DOI:10.3390/nu10121939 |
| [143] | Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology, 2019, 100: 213-222. DOI:10.1016/j.psyneuen.2018.10.010 |
| [144] | Lew LC, Hor YY, Yusoff NAA, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr, 2019, 38(5): 2053-2064. DOI:10.1016/j.clnu.2018.09.010 |
| [145] | Baos S, Brigden A, Anderson E, et al. Investigating the effectiveness and cost-effectiveness of FITNET-NHS (Fatigue in teenagers on the InterNET in the NHS) compared to activity management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): protocol for a randomised controlled trial. Trials, 2018, 19(1): 136. DOI:10.1186/s13063-018-2500-3 |
| [146] | Newberry F, Hsieh SY, Wileman T, et al. Does the microbiome and virome contribute to myalgic encephalomyelitis/chronic fatigue syndrome?. Clin Sci, 2018, 132(5): 523-542. DOI:10.1042/CS20171330 |
| [147] | Carruthers BM, Van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med, 2011, 270(4): 327-338. DOI:10.1111/j.1365-2796.2011.02428.x |
| [148] | Bj?rklund G, Dadar M, Pivina L, et al. Environmental, neuro-immune, and neuro-oxidative stress interactions in chronic fatigue syndrome. Mol Neurobiol, 2020, 57(11): 4598-4607. DOI:10.1007/s12035-020-01939-w |
| [149] | Iacob E, Light AR, Donaldson GW, et al. Gene expression factor analysis to differentiate pathways linked to fibromyalgia, chronic fatigue syndrome, and depression in a diverse patient sample. Arthritis Care Res (Hoboken), 2016, 68(1): 132-140. DOI:10.1002/acr.22639 |
| [150] | Smits LP, Bouter KE, De Vos WM, et al. Therapeutic potential of fecal microbiota transplantation. Gastroenterology, 2013, 145(5): 946-953. DOI:10.1053/j.gastro.2013.08.058 |
| [151] | Giloteaux L, Goodrich JK, Walters WA, et al. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome, 2016, 4(1): 30. DOI:10.1186/s40168-016-0171-4 |
| [152] | Wallis A, Ball M, Butt H, et al. Open-label pilot for treatment targeting gut dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: neuropsychological symptoms and sex comparisons. J Transl Med, 2018, 16(1): 24. DOI:10.1186/s12967-018-1392-z |
| [153] | Du Preez S, Corbitt M, Cabanas H, et al. A systematic review of enteric dysbiosis in chronic fatigue syndrome/myalgic encephalomyelitis. Syst Rev, 2018, 7(1): 241. DOI:10.1186/s13643-018-0909-0 |
| [154] | Newberry F, Hsieh SY, Wileman T, et al. Does the microbiome and virome contribute to myalgic encephalomyelitis/chronic fatigue syndrome?. Clin Sci, 2018, 132(5): 523-542. DOI:10.1042/CS20171330 |
| [155] | Ananbeh H, Vodicka P, Kupcova Skalnikova H. Emerging roles of exosomes in Huntington's disease. Int J Mol Sci, 2021, 22(8): 4085-4101. DOI:10.3390/ijms22084085 |
| [156] | Chang R, Liu X, Li S, et al. Transgenic animal models for study of the pathogenesis of Huntington's disease and therapy. Drug Des Devel Ther, 2015, 9: 2179-2188. |
| [157] | Gubert C, Kong G, Renoir T, et al. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiol Dis, 2020, 134: 104621. DOI:10.1016/j.nbd.2019.104621 |
| [158] | Kong G, Cao KL, Judd LM, et al. Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington's disease. Neurobiol Dis, 2020, 135: 104268. DOI:10.1016/j.nbd.2018.09.001 |
| [159] | Kong G, Ellul S, Narayana VK, et al. An integrated metagenomics and metabolomics approach implicates the microbiota-gut-brain axis in the pathogenesis of Huntington's disease. Neurobiol Dis, 2021, 148: 105199. DOI:10.1016/j.nbd.2020.105199 |
| [160] | Radulescu CI, Garcia-Miralles M, Sidik H, et al. Manipulation of microbiota reveals altered callosal myelination and white matter plasticity in a model of Huntington disease. Neurobiol Dis, 2019, 127: 65-75. DOI:10.1016/j.nbd.2019.02.011 |
| [161] | Wasser CI, Mercieca EC, Kong G, et al. Gut dysbiosis in Huntington's disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun, 2020, 2(2): fcaa110. DOI:10.1093/braincomms/fcaa110 |
| [162] | Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol, 2006, 26(7/8): 1057-1083. |
| [163] | Parr E, Ferdinand P, Roffe C. Management of acute stroke in the older person. Geriatrics, 2017, 2(3): 27. DOI:10.3390/geriatrics2030027 |
| [164] | Zhao LN, Yang LJ, Guo YY, et al. New insights into stroke prevention and treatment: gut microbiome. Cell Mol Neurobiol, 2021, 1-18. DOI:10.1007/s10571-021-01047-w |
| [165] | Roth WH, Cai AN, Zhang CN, et al. Gastrointestinal disorders and risk of first-ever ischemic stroke. Stroke, 2020, 51(12): 3577-3583. DOI:10.1161/STROKEAHA.120.030643 |
| [166] | Zeng X, Gao X, Peng Y, et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front Cell Infect Microbiol, 2019, 9: 4. DOI:10.3389/fcimb.2019.00004 |
| [167] | Xu KY, Gao XX, Xia GH, et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut, 2021: gutjnl-2020-323263. |
| [168] | Spychala MS, Venna VR, Jandzinski M, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol, 2018, 84(1): 23-36. DOI:10.1002/ana.25250 |
| [169] | Benakis C, Poon C, Lane D, et al. Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke, 2020, 51(6): 1844-1854. DOI:10.1161/STROKEAHA.120.029262 |
| [170] | Chen Y, Liang J, Ouyang F, et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front Neurol, 2019, 10: 661. DOI:10.3389/fneur.2019.00661 |
| [171] | Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health?. Neurosci Lett, 2016, 625: 56-63. DOI:10.1016/j.neulet.2016.02.009 |
| [172] | Li N, Wang XC, Sun CC, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol, 2019, 19(1): 191. DOI:10.1186/s12866-019-1552-1 |
| [173] | Koszewicz M, Jaroch J, Brzecka A, et al. Dysbiosis is one of the risk factor for stroke and cognitive impairment and potential target for treatment. Pharmacol Res, 2021, 164: 105277. DOI:10.1016/j.phrs.2020.105277 |
| [174] | Yoshikata R, Myint KZ, Ohta H, et al. Inter-relationship between diet, lifestyle habits, gut microflora, and the equol-producer phenotype: baseline findings from a placebo-controlled intervention trial. Menopause, 2019, 26(3): 273-285. DOI:10.1097/GME.0000000000001202 |
| [175] | Laitinen K, Gueimonde M. Microbiota, Food, and health. Int J Mol Sci, 2019, 20(24): 6329. DOI:10.3390/ijms20246329 |
| [176] | Bezirtzoglou E, Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe, 2011, 17(6): 369-374. DOI:10.1016/j.anaerobe.2011.03.010 |
| [177] | Garcia-Mantrana I, Selma-Royo M, Alcantara C, et al. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol, 2018, 9: 890. DOI:10.3389/fmicb.2018.00890 |
| [178] | Aridi YS, Walker JL, Wright ORL. The association between the mediterranean dietary pattern and cognitive health: a systematic review. Nutrients, 2017, 9(7): E674. DOI:10.3390/nu9070674 |
| [179] | Cavaleri F, Bashar E. Potential synergies of β-hydroxybutyrate and butyrate on the modulation of metabolism, inflammation, cognition, and general health. J Nutr Metab, 2018, 2018: 1-13. |
| [180] | Dahlin M, Prast-Nielsen S. The gut microbiome and epilepsy. EBioMedicine, 2019, 44: 741-746. DOI:10.1016/j.ebiom.2019.05.024 |
| [181] | Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol, 2019, 10: 277. DOI:10.3389/fimmu.2019.00277 |
| [182] | Zhang L, Liu CD, Jiang QY, et al. Butyrate in energy metabolism: there is still more to learn. Trends Endocrinol Metab, 2021, 32(3): 159-169. DOI:10.1016/j.tem.2020.12.003 |
| [183] | Shaaban SY, El Gendy YG, Mehanna NS, et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci, 2018, 21(9): 676-681. DOI:10.1080/1028415X.2017.1347746 |
| [184] | Ka?u?na-Czaplińska J, B?aszczyk S. The level of arabinitol in autistic children after probiotic therapy. Nutrition, 2012, 28(2): 124-126. DOI:10.1016/j.nut.2011.08.002 |
| [185] | Khungar V, Poordad F. Hepatic encephalopathy. Clin Liver Dis, 2012, 16(2): 301-320. DOI:10.1016/j.cld.2012.03.009 |
| [186] | Cao Q, Yu CB, Yang SG, et al. Effect of probiotic treatment on cirrhotic patients with minimal hepatic encephalopathy: a meta-analysis. Hepatobiliary Pancreat Dis Int, 2018, 17(1): 9-16. DOI:10.1016/j.hbpd.2018.01.005 |
| [187] | Dalal R, McGee RG, Riordan SM, et al. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev, 2017, 2: CD008716. |
| [188] | Agrawal A, Sharma BC, Sharma P, et al. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol, 2012, 107(7): 1043-1050. DOI:10.1038/ajg.2012.113 |
| [189] | Kobayashi Y, Kinoshita T, Matsumoto A, et al. Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: an open-label, single-arm study. J Prev Alzheimers Dis, 2019, 6(1): 70-75. DOI:10.14283/jpad.2018.32 |
| [190] | Kobayashi Y, Kuhara T, Oki M, et al. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes, 2019, 10(5): 511-520. DOI:10.3920/BM2018.0170 |
| [191] | Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clin Nutr, 2019, 38(6): 2569-2575. DOI:10.1016/j.clnu.2018.11.034 |
| [192] | Zawistowska-Rojek A, Tyski S. Are probiotic really safe for humans?. Pol J Microbiol, 2018, 67(3): 251-258. DOI:10.21307/pjm-2018-044 |
| [193] | Markowiak P, ?li?ewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 2017, 9(9): 1021. DOI:10.3390/nu9091021 |
| [194] | Barraud D, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest, 2013, 143(3): 646-655. DOI:10.1378/chest.12-1745 |
| [195] | Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology, 2004, 39(5): 1441-1449. DOI:10.1002/hep.20194 |
| [196] | Sandler RH, Finegold SM, Bolte ER, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol, 2000, 15(7): 429-435. DOI:10.1177/088307380001500701 |
| [197] | Lum GR, Olson CA, Hsiao EY. Emerging roles for the intestinal microbiome in epilepsy. Neurobiol Dis, 2020, 135: 104576. DOI:10.1016/j.nbd.2019.104576 |
| [198] | Angelucci F, Cechova K, Amlerova J, et al. Antibiotics, gut microbiota, and Alzheimer's disease. J Neuroinflammation, 2019, 16(1): 108. DOI:10.1186/s12974-019-1494-4 |
| [199] | Ulm L, Hoffmann S, Nabavi D, et al. The randomized controlled STRAWINSKI trial: procalcitonin-guided antibiotic therapy after stroke. Front Neurol, 2017, 8: 153. DOI:10.3389/fneur.2017.00153 |
| [200] | Kalra L, Irshad S, Hodsoll J, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet, 2015, 386(10006): 1835-1844. DOI:10.1016/S0140-6736(15)00126-9 |
| [201] | Westendorp WF, Vermeij JD, Zock E, et al. The preventive antibiotics in stroke study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet, 2015, 385(9977): 1519-1526. DOI:10.1016/S0140-6736(14)62456-9 |
| [202] | Kim KO, Gluck M. Fecal microbiota transplantation: an update on clinical practice. Clin Endosc, 2019, 52(2): 137-143. DOI:10.5946/ce.2019.009 |
| [203] | Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med, 2016, 165(9): 609-616. DOI:10.7326/M16-0271 |
| [204] | Evrensel A, Ceylan ME. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci, 2016, 14(3): 231-237. DOI:10.9758/cpn.2016.14.3.231 |
| [205] | Chen R, Xu Y, Wu P, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res, 2019, 148: 104403. DOI:10.1016/j.phrs.2019.104403 |
| [206] | Juul FE, Garborg K, Bretthauer M, et al. Fecal microbiota transplantation for primary Clostridium difficile infection. N Engl J Med, 2018, 378(26): 2535-2536. DOI:10.1056/NEJMc1803103 |
| [207] | ?ebrowska P, ?aczmańska I, ?aczmański ?. Future directions in reducing gastrointestinal disorders in children with ASD using fecal microbiota transplantation. Front Cell Infect Microbiol, 2021, 11: 630052-630062. DOI:10.3389/fcimb.2021.630052 |
| [208] | Kang DW, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome, 2017, 5(1): 10. DOI:10.1186/s40168-016-0225-7 |
| [209] | Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm, 2018, 5(4): e459. DOI:10.1212/NXI.0000000000000459 |
| [210] | He Z, Cui BT, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol, 2017, 23(19): 3565-3568. DOI:10.3748/wjg.v23.i19.3565 |
| [211] | Kim MS, Kim Y, Choi H, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model. Gut, 2020, 69(2): 283-294. DOI:10.1136/gutjnl-2018-317431 |
| [212] | Yu F, Han W, Zhan G, et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in streptozotocin-induced diabetic mice. Aging (Albany NY), 2019, 11(10): 3262-3279. |
| [213] | Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med, 2016, 22(5): 516-523. DOI:10.1038/nm.4068 |
| [214] | Singh V, Roth S, Llovera G, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci, 2016, 36(28): 7428-7440. DOI:10.1523/JNEUROSCI.1114-16.2016 |
| [215] | Spychala MS, Venna VR, Jandzinski M, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol, 2018, 84(1): 23-36. DOI:10.1002/ana.25250 |
| [216] | Martínez I, Stegen JC, Maldonado-Gómez MX, et al. The gut microbiota of rural Papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep, 2015, 11(4): 527-538. DOI:10.1016/j.celrep.2015.03.049 |
| [217] | Kendall MM, Sperandio V. Gut microbes regroup to aid defence after infection. Nature, 2021, 592(7852): 29-31. DOI:10.1038/d41586-021-00642-7 |
