

1. 南京农业大学 生命科学学院 农业部农业环境微生物重点实验室,江苏 南京 210095;
2. Institute for Ecopreneurship, University of Applied Sciences and Arts Northwestern Switzerland (FHNW), School of Life Sciences, Hofackerstrasse 30, Muttenz 4132, Switzerland;
3. 南京大学环境学院 污染控制与资源化利用国家重点实验室,江苏 南京 210023;
4. 中国科学院微生物研究所 微生物资源前期开发国家重点实验室,北京 100101
收稿日期:2021-06-04;接收日期:2021-07-26
基金项目:国家自然科学基金委员会-欧盟合作项目(No. 3181101464),欧盟H2020框架计划(No. 826244) 资助
作者简介:蒋建东?? 南京农业大学生命科学学院院长。2012年国家自然科学基金“优秀青年科学基金”获得者,教育部新世纪优秀人才,2013年江苏省****基金获得者,2016年入选江苏省第五期“333高层次人才培养工程”第二层次培养对象人选。主要研究领域为环境与土壤微生物学,研究方向为农药类持久性有机污染物的微生物降解与修复。主持国家重点研发专项课题、国家自然科学基金、江苏省****基金、教育部新世纪优秀人才项目、国家863高科技计划项目、江苏省科技支撑计划社会发展项目等,以通讯作者在ISME J、Molecular Microbiology、Environmental Microbiology、Journal of Bacteriology、Applied and Environmental Microbiology等SCI期刊上发表论文80余篇,获国家科技进步二等奖(第9完成人)、教育部技术发明二等奖(第5完成人)和农业部农牧渔业丰收三等奖(第8完成人) 各1项。任中国微生物学会环境微生物学专业委员会副主任委员,中国微生物学会科学普及工作委员会委委员,International Biodeterioration & Biodegradation杂志副主编,Applied and Environmental Microbiology编委(Editorial Board),Frontiers in Microbiotechnology,Ecotoxicology and Bioremediation编委(Review editor).
摘要:排放到环境中的各种农药、多环芳烃、卤代芳烃等有机污染物以及阻燃剂等新兴污染物,对环境污染、农产品质量和环境安全造成了沉重负担。因此,有效去除环境中的有机污染物已成为迫在眉睫的挑战。3D生物打印技术已经在医学材料、制药等行业中发挥着重要作用。现在,越来越多的微生物被确定适合通过3D生物打印生产具有复杂结构和功能的生物材料。微生物的3D生物打印越来越受到环境微生物学家和生物技术专家的关注。本文综述了用于污染物微生物去除的不同3D生物打印技术的原理和优缺点,及用于微生物生物修复技术的可行性,并指出了可能遇到的限制和挑战。
关键词:有机污染物微生物修复技术3D生物打印技术多细胞3D生物打印
Insights into the applications of 3D bioprinting for bioremediation technologies
Zhuang Ke1,2, Osagie Obamwonyi2, Boris Kolvenbach2, Rong Ji3, Shuangjiang Liu4, Jiandong Jiang1

 , Philippe F.-X. Corvini2,3
, Philippe F.-X. Corvini2,3

1. Department of Microbiology, Key Laboratory of Environmental Microbiology for Agriculture, Ministry of Agriculture, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, Jiangsu, China;
2. Institute for Ecopreneurship, University of Applied Sciences and Arts Northwestern Switzerland (FHNW), School of Life Sciences, Hofackerstrasse 30, Muttenz 4132, Switzerland;
3. State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023, Jiangsu, China;
4. State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
Received: June 4, 2021; Accepted: July 26, 2021
Supported by: National Natural Science Foundation of China-European Union Joint Program (No. 3181101464), the H2020 Framework Program of the European Union (No. 826244)
Author: Philippe Fran?ois-Xavier Corvini??is currently the director of the Institute for Ecopreneurship at the University of Applied Sciences and Arts Northwestern Switzerland (FHNW). In 2000, he obtained a Ph.D in biotechnology from the Institut National Polytechnique de Lorraine (INPL) in France. He is currently serving as the Vice Chairman of the European Association of Biotechnology (EFB), Chairman of the Environmental Biotechnology Division, member of the IUPAC Biotechnology Committee, member of the EU-USA Environmental Biotechnology Working Group, board member of the International Society of Environmental Biotechnology, member of the advisory group of the Swiss Federal Environment Agency, Chairman of the Swiss Bioresources Technology Platform and other positions. He has been engaged in environmental biotechnology research for almost twenty years, especially in the microbial removal technology of organic pollutants in water and soil environment. His group deciphered the microbial degradation pathways of pollutants and antibiotics. In the past five years, he has led several EU FP7 and Horizon 2020 projects as well as plenty of national research projects; 2 granted patents; publications in Nature Communications, Environ Sci Technol, Water Res, Appl Environ Microbiol and other journals.
Corresponding author: Jiang JD. E-mail: jiang_jjd@njau.edu.cn;
Philippe F.-X. Corvini. E-mail: philippe.corvini@fhnw.ch.
Abstract: A plethora of organic pollutants such as pesticides, polycyclic and halogenated aromatic hydrocarbons, and emerging pollutants, such as flame retardants, is continuously being released into the environment. This poses a huge threat to the society in terms of environmental pollution, agricultural product quality, and general safety. Therefore, effective removal of organic pollutants from the environment has become an important challenge to be addressed. As a consequence of the recent and rapid developments in additive manufacturing, 3D bioprinting technology is playing an important role in the pharmaceutical industry. At the same time, an increasing number of microorganisms suitable for the production of biomaterials with complex structures and functions using 3D bioprinting technology, have been identified. This article briefly discusses the principles, advantages, and disadvantages of different 3D bioprinting technologies for pollutant removal. Furthermore, the feasibility and challenges of developing bioremediation technologies based on 3D bioprinting have also been discussed.
Keywords: organic pollutantsmicrobial bioremediation3D bioprintingmulticellular 3D bioprinting
1 IntroductionDue to the rapid societal development, the manufacturing and use of organic chemicals in industry and agriculture are increasing. Many organic chemicals, such as pesticides, polycyclic aromatic hydrocarbons, halogenated aromatic hydrocarbons and flame retardants[1-2]are released into the environment, causing serious environmental pollution and threatening the ecosystems and human health. Therefore, the effective removal of organic pollutants from the environment is an urgent and yet unsolved problem. The technologies, used for the removal of organic pollutants from the environment, mainly include the physical, chemical and biological treatments or the combinations of any of these methods. Bioremediation is generally considered as an environment-friendly in-situ remediation technology[3-4]. This technology uses microorganisms and presents many advantages, such as high efficiency, low cost and in some cases ultimate degradation. Ideally, the microorganisms mineralize or transform the toxic pollutants into less toxic or non-toxic forms[5-6]. However, some persistent organic pollutants are partially catabolized by one microorganism, forming intermediate products, which require other microorganisms or consortia for further degradation and mineralization[7-8]. Microbial remediation, which is based on the synergistic cleaning action of several members of a microbial consortium, can be limited by the specific physiological requirements of some individuals, such as nutritional and redox potential conditions, which cannot satisfy the growth conditions of each key microorganism in the matrix. Other technologies such as the genetic modification of microorganisms to broaden their pollutant degrading capacities[9-10] and to achieve increased stability of microbial consortia, where individual members can act synergistically via metabolic modelling[11] are needed to harness the natural cleaning function of microorganisms.
Since its invention in the 1980s[12], the 3D (three-dimensional) printing technology has been playing an important role in various applications, such as automotive, aircraft, medical, food and robotics industries[13-17]. The 3D printing technology has the potential to create complex parts and structures that could not have been easily manufactured in the past. With the precise deposition of materials and mixtures, it is now possible to generate complex inorganic and organic 3D structures, which are of great interest for various advanced, multi-functional and sustainable applications[18]. The continuous development of novel manufacturing methods and printable materials, precise control of cellular distribution and deposition, scalability, and cost-effectiveness have greatly increased the development and applications of 3D bioprinting technology in the past decade[19]. An exciting prospect is the combination of various microorganisms, bearing different functionalities within spatially well-defined systems through 3D bioprinting technology. Therefore, it is envisaged to establish a functional platform, enabling the spatial connection between "incompatible" microorganisms[20]. Such a platform can also allow building the systems, having complex biological functions. Recently, microorganisms, including bacteria, are increasingly being used for the formulation of various biological inks to produce complex biological materials using 3D bioprinting technology[21-24]. However, the manipulation of microorganisms to produce "biological materials" with perfectly controlled 3D shapes, microstructures, and metabolic activities remain a challenge in terms of the reliability and reproducibility of the methods. This short perspective article summarizes the main 3D bioprinting technologies, as well as their advantages and disadvantages. The combination of different 3D bioprinting systems to print multicellular biomaterials, current progresses in the applications of 3D bioprinting materials to remove pollutants, and the feasibility of developing microbial remediation techniques based on the 3D bioprinting technology is also discussed (Fig. 1). Finally, the limits and challenges, encountered in the 3D bioprinting of multi-bacterial biomaterials, are also briefly reported.
 |
| Fig. 1 Summary of the topics discussed in this review. |
| 图选项 |
2 3D bioprinting technologiesBioprinting technology enables the accurate deposition of cells and biological materials with micron or even sub-micron precision, provided the printing technology is compatible with the living cells[25]. Due to the diversities in the type of cells or microorganisms and their specific physiological properties, the development of bioprinting technology, compatible with multiple cellular systems that can fulfill complex functions, is challenging. Any progress in this direction will be a first step towards the printing of biomaterials with increasing levels of complexity. The methodologies currently used in bioprinting technology include inkjet, mechanical extrusion and laser-assisted bioprinting technologies[26]. The advantages and disadvantages of these three major bioprinting technology methods are briefly summarized in Table 1.
Table 1 Comparison of three common types of bioprinting technologies
| Bioprinting methods | Ink viscosity | Cell viability | Resolution | Printing speed | Related costs | References |
| Inkjet | Very Low (< 12 mPa/s) | > 85% | Moderate-high (10–100 μm droplets) | High (< 10 000 droplets/s) | Moderate | [26, 28-32] |
| Extrusion | Wide range (30–107 mPa/s) | < 80% | Low-moderate (100–300 μm filaments) | Low (~5 mm/s) | Moderate | [26, 30, 33-37] |
| Laser-assisted | Low (< 300 mPa/s) | > 95% | High (~10 μm droplets) | Moderate (~100 droplets/s) | High | [26, 30, 38-40] |
表选项
The inkjet bioprinting is a bioprinting technology which has been developed relatively earlier[27]. In this technology, the bioink is mixed with cells and loaded into the ink cartridge; the droplets of controllable size are dropped according to the requirement at a very high frequency through the connected print-head based on the thermal transmission or piezoelectric transmission principle (Fig. 2)[28]. The use of inkjet bioprinting enablesthe deposition of a small amount of bioink (1–100 pL) with extremely high resolution and precision, and guarantee cell viability[29]. The inkjet bioprinting is limited to print the inks with very low viscosities only (< 12 mPa/s)[26, 30] because the highly viscous bioinks obstruct the print-head, leading to the failure of the printing process[31]. Another disadvantage of inkjet bioprinting is the so-called "deposition effect"[32]. Although, the ink is carefully mixed in order to obtain a homogeneous cellular suspension before loading into the cartridge, the cells or other biological materials settle in the printing nozzle during the printing process. This increases the viscosity of the bioink locally and finally blocks the printing nozzle.
 |
| Fig. 2 Schematic description of the three main 3D bioprinting technologies. |
| 图选项 |
Extrusion bioprinting is currently the most widely used bioprinting technology and is regarded as the evolved version of the inkjet bioprinting technology[19]. The printing system uses compressed air or a mechanical screw plunger to extrude the bioink from the cartridge in a controlled and homogeneous manner, forming a continuous uniform filament, which is deposited in order to form the complex 3D structures (Fig. 2)[33]. The bioinks of various viscosities and aggregates with high cell density can be printed using extrusion bioprinters[30, 34]. Such a system is perfectly compatible with the use of many optical and chemical cross-linking systems for biomaterial hardening[34]. Although these systems are compatible with bioinks of different viscosities, they submit the cells contained in a high-viscosity bioinks to high mechanical stress. This is especially the case when the cells pass through the printing nozzle[35-36]. In addition, due to the use of the high viscosity of ink for extrusion printing, its printing speed and printing resolution (100–300 μm) are relatively low[26, 37]. However, such disadvantages can be overcame by upgrading the extrusion system of 3D bioprinters and improving the ink materials used for bioprinting.
Laser-assisted bioprinting is a type of bioprinting technology that uses lasers without a printhead. The working principle of this system is to stimulate the vaporization of a thin absorbing layer of metal, such as gold or titanium, using laser in order to produce a liquid jet of the bioink situated below the absorbing layer and eject it onto the corresponding receiving substrate (Fig. 2), thereby completing the printing process after cross-linking[38]. As compared to other bioprinting technologies, the laser-assisted bioprinting technology avoids the contact of bioink with ink cartridges and printing nozzles; therefore, the higher-viscosity inks can be printed without clogging in the printhead[39]. This non-contact bioprinting ensures the survival of printed cells (usually higher than 95% survival rate) since the cells are not exposed to shear stress. This bioprinting technology is compatible with bioinks of different viscosities. However, the high cost and the heat generated by the laser might cause cellular stress and aggregation problems[40], thereby limiting the application of this technology.
3 Application of 3D bioprinting to produce multicellular biomaterialsThe structures of biological tissues are generally very complex due to the differences in the mechanical properties of different types of cells. It is impossible for a bioprinter operating with a single material to print biomaterials with different cell types and to achieve at the same time a desired spatial arrangement of the different cells in the printed biomaterial. This requires a bioprinting system with two or more ink cartridges loaded with different types of cells, cross-linking agents, and supporting materials to coordinate and complete bioprinting through automatized deposition. Zimmerman et al.[41] used a dual-head inkjet printer to dispense two different reactive hydrogel precursor solutions and printed a complex spatial structure. For example, the migration and morphogenesis of human bone marrow mesenchymal stem cells embedded in the bioink occurred along a concentration gradient of platelet derived growth factor that was printed in another layer of hydrogel. Merceron et al.[42]introduced an integrated organ printing system having four printheads to individually deposit four different components (two different cell types loaded in two different hydrogels and two different polymers without cells) to create a single integrated muscle-tendon unit structure. One side was printed using the thermoplastic polyurethane and hydrogel bioink loaded with C2C12 cells to simulate the elasticity of muscles. The other side was printed using the poly(ε-caprolactone) and NIH/3T3 cell hydrogel to simulate the stiffness of the tendon. These constructs displayed good cell viability within 7 days (more than 80% of the cells survived). Seol et al.[43] used a tissue-organ printing integrated system and successfully printed skin substitutes for repairing damaged skin. This system was used to create a "BioMask" for the repair of the damaged facial skin by separately depositing a porous polyurethane layer and two hydrogel layers loaded with keratinocytes and fibroblasts, respectively. Kang et al.[44] demonstrated the usefulness of an integrated tissue-organ printer based on multiple cartridges. The hydrogels and polymers loaded with the different types of cells were printed together and fixed on sacrificial hydrogels using an integrated tissue-organ printer to achieve the printing of human-scale tissue structures, such as jaws, skulls, cartilage and skeletal muscles. Horváth et al.[45]built a more sophisticated bioprinting platform by combining inkjet printing, extrusion printing and laser cross-linking units. This allowed them to successfully print an analogue of the human air-blood tissue barrier, which was composed of endothelial cells, basement membrane, and epithelial surface. As compared to the manual methods, this printing technology could achieve thinner, more uniform and reproducible cell layers.
With the steady development of multi-material bioprinting platforms, the engineering of 3D human organs and tissues by means of bioprinting shall become a reality. Bioprinting can be performed not only using cells but also the microorganisms in multi-materials. Liu et al.[23] reported a novel printing application of 3D bioprinting, which consisted in the manufacturing of a wearable living device (tattoo), which could respond to specific chemicals. The authors deposited bioinks containing different genetically engineered bacterial cells in specific areas of the printed structure. The embedded cells played the role of live sensors and responded to different chemicals by emitting fluorescence signals. The tattoos were printed on a thin layer of elastomer in a tree-like pattern and then attached to human skin. The hydrogels with different colors were used for the tattoo in order to materialize the different types of cells they contained. When rhamnose, β-D-1-thiogalactopyranoside or N-acyl homoserine lactone were smeared onto the skin area covered by the live tattoo, the chemicals diffused into the printed biological material and the engineered cells were shown to emit a signal of fluorescence. Johnston et al.[46]used a multi-material 3D printer to deposit F127-BUM hydrogel ink embedded with different microbial species (Saccharomyces cerevisiae, E. coli, and algae) into a single printing part and achieved cross-species multi-cell bioprinting. The good compatibility of F127-BUM hydrogel and the precise spatial arrangement of various microbial species allow to study the interactions among different cells, the behavior of microbial consortia, and the distributed metabolic processes in the bio-manufacturing of these consortia. Ceballos-González et al.[47] used the continuous chaotic bacterial bioprinting and printed a hydrogel structure with inserted bacteria in order to study how the spatial distribution of bacteria affects their social behavior (including competition and cooperation). Liu et al.[48] printed a hybrid biological photovoltaic device embedded with cyanobacteria and heterotrophic bacteria through 3D bioprinting technology. The organic biomass produced by the photosynthesis of cyanobacteria diffused through the highly porous hydrogel to the hydrogel layer embedded with heterotrophic bacteria, and then continuously generated bioelectricity using the respiration of heterotrophic bacteria. This device provided sustainable energy production and was used for a long time without any additional organic fuel. Johnston et al.[49]developed a microbial-loaded hydrogel platform, which could divide and spatially organize individual microbial populations and alliance members into a hydrogel structure for the production of small molecules and bioactive peptides. The hydrogel system could be reused and preserved through refrigeration or lyophilization to produce these molecules on demand in such a way that traditional liquid culture could not match.
The strategy of 3D bioprinting technology to deposit the different microorganisms sensing different compounds could be applied to place different microorganisms degrading different pollutants in the desired position in the biomaterials. The microorganisms embedded in the biomaterials will exert complementary catabolic functions, which might enable the degradation of recalcitrant molecules necessitating the degradation activity of various members of a microbial consortium. 3D bioprinting might be useful when striving at novel, efficient, and environment-friendly bioremediation technologies.
4 Application of 3D biomaterials to remove pollutantsIn recent years, many studies have reported the use of 3D printing for the removal of pollutants. Bergamonti et al.[50] prepared TiO2 chitosan scaffolds (TiO2/CS) with photocatalytic activity by 3D printing for photodegradation of amoxicillin under ultraviolet/visible light irradiation. Pei et al.[51] used a mixture of calcium alginate and gelatin (CA-GE) embedded with a copper trimellitate complex and printed it into various shapes of metal-organic frameworks (MOFs) by direct writing. It was found that the hexagonal MOF showed the best adsorption performance for a wide range of organic dyes (methylene blue, malachite green, methyl violet, rhodamine B, and auramine O). Wu et al.[52] prepared a new 3D MnO2 modified biochar-based porous hydrogel (MBCG) by the polymerization of the MnO2 modified biochar into a polyacrylamide gel network for the adsorption of Cd(Ⅱ) and Pb(Ⅱ) in aquatic environments.
However, there are few studies, reporting the removal of pollutants by 3D bioprinting based on microorganisms. Schaffner et al.[21]printed Pseudomonas putida, which is capable of degrading phenol, in a 3D printed grid structure. The high surface area of the grid structure maximized the contact surface between the printed bacterial cells and the liquid medium. P. putida assimilates the phenolic contents from the medium for biomass production as well as for its own growth. The fluorescence intensity of the grid stained with ethidium bromide indicated that P. putida embedded in the grid could use phenol as a carbon source for cell proliferation. Huang et al.[53] produced the TasA amyloid fusion proteins using Bacillus subtilis to develop a highly flexible and engineered biofilm platform with a variety of practical applications. These fusion proteins could be secreted and self-assembled around living cells into a variety of extracellular nanostructures with adjustable physical and chemical properties. The 3D printing allowed their precise positioning in the printed structures (spatial control). The authors designed and constructed an engineered strain capable of producing extracellular TasA-MHETase nanofibers. The biofilm, containing the engineered strain, was able to convert 2-hydroxyethyl terephthalate (MHET) into less toxic terephthalic acid. Furthermore, a hybrid biofilm, which consisted of TasA-OPH biofilm and TasA-HisTag biofilm, degraded organophosphate pesticides through a two-step biocatalytic reaction. The organophosphate hydrolase (OPH) catalyzed the degradation of pesticide paraoxon into less harmful metabolite para-nitrophenol (PNP). Concomitant degradation of PNP into less harmful p-aminophenol was catalyzed by the gold nanoparticles that were immobilized through TasA-HisTag in the adjacent biofilm. This study showed that 3D bioprinting platform, in combination with engineered cells, such as the TasA amyloid fusion proteins- expressing system, could induce the complex cascades of biological reactions. Yu et al.[54] proposed and created a new water remediation method based on a self-propelled multifunctional 3D printed thumb-sized motor (TSM) for in-situ remediation of underwater contaminants. The TSM was propelled by the CO2 bubbles produced by the reaction of the green chemical reagents C4H6O3 and NaHCO3. After reaching the simulated pollutant area, the sealing layer of the TSM was slowly dissolved, so that the Bacillus subtilis loaded on the TSM was completely released for the degradation of rhodamine-B. At this stage, this technology is still in its infancy and has great potential for improvements, such as the cruising range, a practical and feasible magnetic permeability method, and dealing with the pollution of complex components.
5 Challenges and prospectsThere is a real need for the development of remediation technologies, necessitating low energy consumption, low treatment cost, and ideally without the addition of chemical reagents. Among the possible options to address this need, the remediation technologies based on microbes are generally recognized as a low-cost and environment friendly technologies. The efforts of scientific researchers to isolate numerous microorganisms, capable of degrading organic pollutants from the environment, have led to the identification of genes encoding for enzymes capable to degrade plethora of pollutants. The steadily expansion of the microbial resource library provides more options for the development of microbe-based remediation technologies. The progress in genomics and bioinformatics together with the development of mixed culture technology provides a deeper understanding of mechanisms, governing the degradation of pollutants. In case of multiple contaminations, i.e. the contamination of matrix by several pollutants, remediation processes based on the use of bacterial consortia can help in the degradation of a large spectrum of pollutants and particularly those, requiring the activities of several strains for their degradation. Multiple contamination being often a reality; these techniques might be suitable for real contaminated environments. With the continuous development of 3D bioprinting technology, different bioinks have also been developed. The progress in the formulation of bioinks and their tailoring to the different bioprinting devices will be useful to solve 3D bioprinting problems specifically related to the microorganisms that are used. These inks composed of two or more biocompatible materials should take into account the rheological properties required for 3D printing and the biocompatibility aspects required for embedding of living cells, e.g. permeability of oxygen. The multi-material direct ink writing techniques with unlimited complex shapes and material components in combination with the diversity of microorganisms' metabolic reactions have made it possible to manufacture bacteria-based biomaterials with unprecedented functions in a controlled way. This technology can enable the production of multiple strain-degradation systems in a well-defined space and solve herewith the needs for spatial vicinity and functional complementarity of the embedded microorganisms.
However, the development of this novel bioremediation technology based on 3D bioprinting is affected by many aspects, and there are still yet unsolved problems. First of all, there is a huge spectrum of pollutants released into the environment, and for some of which, such as chlorothalonil, no bacteria have been yet isolated to be capable of mineralizing them. Secondly, many bacterial strains cannot mineralize some pollutants alone and need to be combined with other strains to achieve their ultimate degradation. Due to the needs for different conditions during the culturing of degrading microorganisms (e.g. use of different final electron acceptors), technical barriers are encountered during the printing and assembling of these strains in the same printed biofilm. The technological development and the research in the field of bioremediation technologies based on 3D bioprinting could be further progressed from the following aspects: 1) Further efforts in isolating from various environment matrices microorganisms that can degrade a broad-spectrum of micropollutants and elucidating the underlying degradation mechanisms. Advances in molecular biology can also be exploited to improve the degradation efficiency of microorganisms embedded in the 3D-bioprinted systems. For example, the integration of pollutant-degrading plasmids or genes into the genomes of indigenous microorganisms growing in the polluted environment shall enable microorganisms with good survival capacities to reach efficient bioremediation. 2) Further developments in the bioprinting systems and bioinks compatible with the different growth conditions of microorganisms could help in designing the printed biomaterials in which the concentration of chemical species can be controlled, and complementary functions of microorganisms can co-exist in a well-defined space of the printed systems (Fig. 3). For example, the chemical modifications of materials, the addition of important nutrients, the control of oxygen concentration and the establishment of micro-environmental conditions around the printed microorganisms might help in establishing a more suitable habitat for the microorganisms of technological interest in the near future (Fig. 4). Despite the current problems and challenges, there is a great potential in using the diverse degradation abilities of microorganisms and the customization of the micro-environment using 3D printing technology. The optimization of methods and technological updates might lead to a better integration of these two assets for the development of a set of environmental remediation technologies, which would be both affordable and highly efficient.
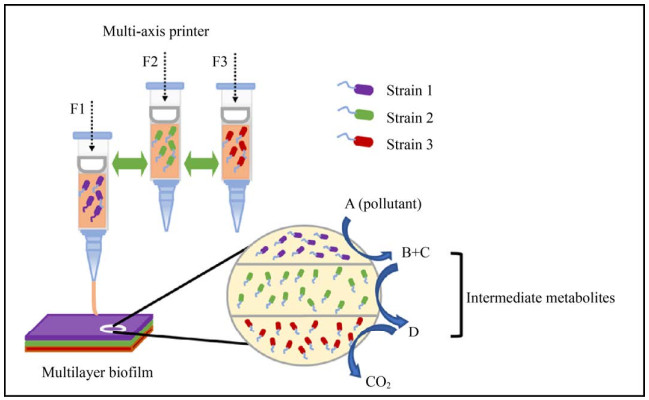 |
| Fig. 3 Schematic diagram of the synergistic degradation of pollutants by multiple strains in 3D printed biofilms. |
| 图选项 |
 |
| Fig. 4 Schematic diagram of coexistence of anaerobic bacteria and aerobic bacteria in the same printed biofilm. |
| 图选项 |
REFERENCES
| [1] | Hahladakis JN, Latsos A, Gidarakos E. Performance of electroremediation in real contaminated sediments using a big cell, periodic voltage and innovative surfactants. J Hazard Mater, 2016, 320: 376-385. DOI:10.1016/j.jhazmat.2016.08.003 |
| [2] | Goi A, Trapido M. Chlorophenols contaminated soil remediation by peroxidation. J Adv Oxid Technol, 2010, 13(1): 50-658. |
| [3] | Matsunaga A, Yasuhara A. Complete dechlorination of 1-chloronaphthalene by electrochemical reduction with naphthalene radical anion as mediator. Environ Sci Technol, 2003, 37(15): 3435-3441. DOI:10.1021/es026360z |
| [4] | Yang HC, Cho YJ, Eun HC, et al. Destruction of chlorinated organic solvents in a two-stage molten salt oxidation reactor system. Chem Eng Sci, 2007, 62(18/19/20): 5137-5143. |
| [5] | Yan X, Gu T, Yi Z, et al. Comparative genomic analysis of isoproturon-mineralizing sphingomonads reveals the isoproturon catabolic mechanism. Environ Microbiol, 2016, 18(12): 4888-4906. DOI:10.1111/1462-2920.13413 |
| [6] | Yang H, Hu S, Wang X, et al. Pigmentiphaga sp. strain D-2 uses a novel amidase to initiate the catabolism of the neonicotinoid insecticide acetamiprid. Appl Environ Microbiol, 2020, 86(6): e02425-19. |
| [7] | Liu X, Chen K, Chuang S, et al. Shift in bacterial community structure drives different atrazine-degrading efficiencies. Front Microbiol, 2019, 10: 88. DOI:10.3389/fmicb.2019.00088 |
| [8] | Jia W, Ye Q, Shen D, et al. Enhanced mineralization of chlorpyrifos bound residues in soil through inoculation of two synergistic degrading strains. J Hazard Mater, 2021, 412: 125116. DOI:10.1016/j.jhazmat.2021.125116 |
| [9] | Pande V, Pandey SC, Sati D, et al. Bioremediation: an emerging effective approach towards environment restoration. Environ Sustain, 2020, 3(1): 91-103. DOI:10.1007/s42398-020-00099-w |
| [10] | Ofaim S, Zarecki R, Porob S, et al. Genome-scale reconstruction of Paenarthrobacter aurescens TC1 metabolic model towards the study of atrazine bioremediation. Sci Rep, 2020, 10(1): 13019. DOI:10.1038/s41598-020-69509-7 |
| [11] | Xu X, Zarecki R, Medina S, et al. Modeling microbial communities from atrazine contaminated soils promotes the development of biostimulation solutions. ISME J, 2019, 13(2): 494-508. DOI:10.1038/s41396-018-0288-5 |
| [12] | Savini A, Savini GG. A short history of 3D printing, a technological revolution just started. 2015 ICOHTEC/IEEE International History of High-Technologies and their Socio-Cultural Contexts Conference (HISTELCON). August 18-19, 2015, Tel-Aviv, Israel. IEEE, 2015: 1-8. |
| [13] | Zhu X, Xu Q, Li H, et al. Fabrication of high-performance silver mesh for transparent glass heaters via electric-field-driven microscale 3D printing and UV-assisted microtransfer. Adv Mater, 2019, 31(32): e1902479. DOI:10.1002/adma.201902479 |
| [14] | Wang YC, Chen T, Yeh YL. Advanced 3D printing technologies for the aircraft industry: a fuzzy systematic approach for assessing the critical factors. Int J Adv Manuf Technol, 2019, 105(10): 4059-4069. DOI:10.1007/s00170-018-1927-8 |
| [15] | Tarfaoui M, Nachtane M, Goda I, et al. 3D Printing to support the shortage in personal protective equipment caused by COVID-19 pandemic. Materials, 2020, 13(15): 3339. DOI:10.3390/ma13153339 |
| [16] | Hamidi A, Tadesse Y. 3D printing of very soft elastomer and sacrificial carbohydrate glass/elastomer structures for robotic applications. Mater Des, 2020, 187: 108324. DOI:10.1016/j.matdes.2019.108324 |
| [17] | Anukiruthika T, Moses JA, Anandharamakrishnan C. 3D printing of egg yolk and white with rice flour blends. J Food Eng, 2020, 265: 109691. DOI:10.1016/j.jfoodeng.2019.109691 |
| [18] | Kyle S. 3D Printing of Bacteria: the next frontier in biofabrication. Trends Biotechnol, 2018, 36(4): 340-341. DOI:10.1016/j.tibtech.2018.01.010 |
| [19] | Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv, 2016, 34(4): 422-434. DOI:10.1016/j.biotechadv.2015.12.011 |
| [20] | Ben Said S, Tecon R, Borer B, et al. The engineering of spatially linked microbial consortia-potential and perspectives. Curr Opin Biotechnol, 2020, 62: 137-145. DOI:10.1016/j.copbio.2019.09.015 |
| [21] | Schaffner M, Rühs PA, Coulter F, et al. 3D printing of bacteria into functional complex materials. Sci Adv, 2017, 3(12): eaao6804. DOI:10.1126/sciadv.aao6804 |
| [22] | Lehner BAE, Schmieden DT, Meyer AS. A straightforward approach for 3D bacterial printing. ACS Synth Biol, 2017, 6(7): 1124-1130. DOI:10.1021/acssynbio.6b00395 |
| [23] | Liu X, Yuk H, Lin S, et al. 3D printing of living responsive materials and devices. Adv Mater, 2018, 30(4). |
| [24] | Zhao S, Guo C, Kumarasena A, et al. 3D Printing of functional microalgal silk structures for environmental applications. ACS Biomater Sci Eng, 2019, 5(9): 4808-4816. DOI:10.1021/acsbiomaterials.9b00554 |
| [25] | Ji S, Guvendiren M. Complex 3D bioprinting methods. APL Bioeng, 2021, 5(1): 011508. DOI:10.1063/5.0034901 |
| [26] | Farhat W, Chatelain F, Marret A, et al. Trends in 3D bioprinting for esophageal tissue repair and reconstruction. Biomaterials, 2021, 267: 120465. DOI:10.1016/j.biomaterials.2020.120465 |
| [27] | Gu Z, Fu J, Lin H, et al. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharm Sci, 2020, 15(5): 529-557. DOI:10.1016/j.ajps.2019.11.003 |
| [28] | Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials, 2016, 102: 20-42. DOI:10.1016/j.biomaterials.2016.06.012 |
| [29] | Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res, 2010, 40(1): 395-414. DOI:10.1146/annurev-matsci-070909-104502 |
| [30] | Jana S, Lerman A. Bioprinting a cardiac valve. Biotechnol Adv, 2015, 33(8): 1503-1521. DOI:10.1016/j.biotechadv.2015.07.006 |
| [31] | Tekin E, Smith PJ, Schubert US. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter, 2008, 4(4): 703-713. DOI:10.1039/b711984d |
| [32] | Pepper ME, Seshadri V, Burg TC, et al. Characterizing the effects of cell settling on bioprinter output. Biofabrication, 2012, 4(1): 011001. DOI:10.1088/1758-5082/4/1/011001 |
| [33] | Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials, 2016, 76: 321-343. DOI:10.1016/j.biomaterials.2015.10.076 |
| [34] | Marga F, Jakab K, Khatiwala C, et al. Toward engineering functional organ modules by additive manufacturing. Biofabrication, 2012, 4(2): 022001. DOI:10.1088/1758-5082/4/2/022001 |
| [35] | Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol, 2014, 32(8): 773-785. DOI:10.1038/nbt.2958 |
| [36] | Laurent J, Blin G, Chatelain F, et al. Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat Biomed Eng, 2017, 1(12): 939-956. DOI:10.1038/s41551-017-0166-x |
| [37] | Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A, 2008, 14(1): 41-48. DOI:10.1089/ten.a.2007.0004 |
| [38] | Guillotin B, Souquet A, Catros S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials, 2010, 31(28): 7250-7256. DOI:10.1016/j.biomaterials.2010.05.055 |
| [39] | Sorkio A, Koch L, Koivusalo L, et al. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials, 2018, 171: 57-71. DOI:10.1016/j.biomaterials.2018.04.034 |
| [40] | Guillemot F, Souquet A, Catros S, et al. Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomed Lond Engl, 2010, 5(3): 507-515. DOI:10.2217/nnm.10.14 |
| [41] | Zimmermann R, Hentschel C, Schr?n F, et al. High resolution bioprinting of multi-component hydrogels. Biofabrication, 2019, 11(4): 045008. DOI:10.1088/1758-5090/ab2aa1 |
| [42] | Merceron TK, Burt M, Seol YJ, et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication, 2015, 7(3): 035003. DOI:10.1088/1758-5090/7/3/035003 |
| [43] | Seol YJ, Lee H, Copus JS, et al. 3D Bioprinted bioMask for facial skin reconstruction. Bioprinting, 2018, 10: e00028. DOI:10.1016/j.bprint.2018.e00028 |
| [44] | Kang HW, Lee SJ, Ko IK, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol, 2016, 34(3): 312-319. DOI:10.1038/nbt.3413 |
| [45] | Horváth L, Umehara Y, Jud C, et al. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep, 2015, 5: 7974. DOI:10.1038/srep07974 |
| [46] | Johnston TG, Fillman JP, Priks H, et al. Cell-laden hydrogels for multikingdom 3D printing. Macromol Biosci, 2020, 20(8): e2000121. DOI:10.1002/mabi.202000121 |
| [47] | Ceballos-González CF, Bolívar-Monsalve EJ, Quevedo-Moreno DA, et al. High-throughput and continuous chaotic bioprinting of spatially controlled bacterial microcosms. ACS Biomater Sci Eng, 2021, 7(6): 2408-2419. DOI:10.1021/acsbiomaterials.0c01646 |
| [48] | Liu L, Gao Y, Lee S, et al. 3D bioprinting of cyanobacteria for solar-driven bioelectricity generation in resource-limited environments. Annu Int Conf IEEE Eng Med Biol Soc, 2018, 2018: 5329-5332. |
| [49] | Johnston TG, Yuan SF, Wagner JM, et al. Compartmentalized microbes and co-cultures in hydrogels for on-demand bioproduction and preservation. Nat Commun, 2020, 11(1): 563. DOI:10.1038/s41467-020-14371-4 |
| [50] | Bergamonti L, Bergonzi C, Graiff C, et al. 3D printed chitosan scaffolds: a new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res, 2019, 163: 114841. DOI:10.1016/j.watres.2019.07.008 |
| [51] | Pei R, Fan L, Zhao F, et al. 3D-printed metal-organic frameworks within biocompatible polymers as excellent adsorbents for organic dyes removal. J Hazard Mater, 2020, 384: 121418. DOI:10.1016/j.jhazmat.2019.121418 |
| [52] | Wu Z, Chen X, Yuan B, et al. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(Ⅱ) and Pb(Ⅱ). Chemosphere, 2020, 239: 124745. DOI:10.1016/j.chemosphere.2019.124745 |
| [53] | Huang J, Liu S, Zhang C, et al. Programmable and printable Bacillus subtilis biofilms as engineered living materials. Nat Chem Biol, 2019, 15(1): 34-41. DOI:10.1038/s41589-018-0169-2 |
| [54] | Yu F, Hu Q, Dong L, et al. 3D printed self-driven thumb-sized motors for in-situ underwater pollutant remediation. Sci Rep, 2017, 7: 41169. DOI:10.1038/srep41169 |
