
 , 郑平1,2,3, 孙际宾1,2,3
, 郑平1,2,3, 孙际宾1,2,3 1. 中国科学技术大学 生命科学学院,安徽 合肥 230026;
2. 中国科学院 系统微生物工程重点实验室,天津 300308;
3. 中国科学院 天津工业生物技术研究所,天津 300308
收稿日期:2020-06-09;接收日期:2020-08-19;网络出版时间:2020-09-10
基金项目:国家重点研发计划(No. 2018YFA0901500),国家自然科学基金(No. 31700044) 资助
摘要:甲醇和甲烷等一碳原料来源广泛,价格低廉,是生物制造的理想原料。甲醇脱氢酶(Methanol dehydrogenase,MDH) 催化甲醇生成甲醛是一碳代谢的关键反应。目前已从天然甲基营养菌中发现了多种利用不同辅因子,具有不同酶学性质的MDH。其中,烟酰胺腺嘌呤双核苷酸(NAD) 依赖型MDH被广泛应用于构建人工甲基营养菌。但是,NAD依赖型MDH的甲醇氧化活性较低,对甲醇的亲和力较差,导致甲醇氧化成为人工甲基营养菌代谢甲醇的限速步骤。为了提高甲醇氧化速率,进而提高人工甲基营养菌的甲醇利用效率,近年来大量研究集中于MDH的挖掘与改造研究。文中系统综述了不同类型MDH的发现、表征、改造以及在人工甲基营养菌中的应用进展,详细阐述了MDH的定向进化和多酶复合体的构建,并展望了通过细胞生长偶联的蛋白质进化和蛋白质理性设计获得高活性MDH的潜在策略。
关键词:甲醇脱氢酶甲醇定向进化人工甲基营养菌
Methanol dehydrogenase, a key enzyme of one-carbon metabolism: a review
Liwen Fan1,2,3, Yu Wang2,3

 , Ping Zheng1,2,3, Jibin Sun1,2,3
, Ping Zheng1,2,3, Jibin Sun1,2,3 1. School of Life Sciences, University of Science and Technology of China, Hefei 230026, Anhui, China;
2. Key Laboratory of Systems Microbial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China;
3. Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China
Received: June 9, 2020; Accepted: August 19, 2020; Published: September 10, 2020
Supported by: National Key Research and Development Program of China (No. 2018YFA0901500), National Natural Science Foundation of China (No. 31700044)
Corresponding author: Yu Wang. Tel/Fax: +86-22-84861943; E-mail: wang_y@tib.cas.cn.
Abstract: One-carbon compounds such as methanol and methane are cheap and readily available feedstocks for biomanufacturing. Oxidation of methanol to formaldehyde catalyzed by methanol dehydrogenase (MDH) is a key step of microbial one-carbon metabolism. A variety of MDHs that depend on different co-factors and possess different enzymatic properties have been discovered from native methylotrophs. Nicotinamide adenine dinucleotide (NAD)-dependent MDHs are widely used in constructing synthetic methylotrophs, whereas this type of MDH usually suffers from low methanol oxidation activity and low affinity to methanol. Consequently, methanol oxidation is considered as a rate-limiting step of methanol metabolism in synthetic methylotrophs. To accelerate methanol oxidation, thereby improving the methanol utilization efficiency of synthetic methylotrophs, massive researches have focused on discovery and engineering of MDHs. In this review, we summarize the ongoing efforts to discover, characterize, and engineer various types of MDHs as well as the applications of MDHs in synthetic methylotrophs. Directed evolution of MDH and construction of multi-enzyme complexes are described in detail. In the future prospective part, we discuss the potential strategies of growth-coupled protein evolution and rational protein design for acquisition of superior MDHs.
Keywords: methanol dehydrogenasemethanoldirected evolutionsynthetic methylotroph
随着世界人口的快速增长和工业的迅速发展,资源短缺和环境污染是21世纪所面临的巨大挑战[1]。因此,急需开发可再生和环境友好的燃料和化学品生产工艺。由于生物制造具有产品多样、产品选择性高和过程绿色环保等特点,越来越多的目光投向生物制造。但传统的生物制造原料主要是糖类和其他农产品,易受季节变化和天气状况的影响,且存在与食品供应链竞争的问题[2]。因而寻找糖质原料的替代品至关重要。甲烷和甲醇等一碳化合物,生产原料来源广泛,可以以煤炭、天然气、沼气和二氧化碳为原料生产;与糖类相比,其价格较低且平稳,而且还具有平均碳原子还原程度高等优势,可为生物合成过程提供更多的还原力[3-4]。因此,甲烷和甲醇等一碳化合物是生物制造大宗化学品的理想原料。
自然界中存在一类天然甲基营养菌,能够利用甲烷和甲醇等一碳化合物作为唯一碳源和能源生长,并合成蛋白质、氨基酸、聚羟基丁酸酯等产物[5]。在微生物一碳代谢过程中,甲烷首先通过甲烷单加氧酶氧化生成甲醇,甲醇随后经甲醇脱氢酶(MDH) 氧化生成甲醛,甲醛通过不同的同化途径合成细胞生长代谢所需的能量和碳骨架,如核酮糖单磷酸途径(Ribulose monophosphate pathway,RuMP)、木酮糖单磷酸途径(Xylulose monophosphate pathway,XuMP) 和丝氨酸循环(Serine pathway) 等,或者通过异化途径氧化成CO2[5-8]。随着合成生物学和系统代谢工程的快速发展,在遗传背景清晰、基因组编辑工具成熟、工业应用广泛的平台菌株中引入异源的一碳代谢途径,构建可利用甲烷或甲醇为碳源的人工甲基营养菌,为一碳原料的生物转化利用提供了一条新的路径[9]。无论在天然还是人工的甲基营养菌中,MDH催化的甲醇氧化都是不可或缺的一步。亦有研究表明MDH的酶学性质欠佳,导致甲醇氧化成为一碳代谢的限速步骤之一[10]。因此,MDH的挖掘、表征和改造一直是一碳代谢研究领域的热点。本文将综述近年来发现的不同类型的MDH,总结通过定向进化等策略提高MDH酶学性质和人工甲基营养菌的甲醇利用效率的研究进展,最后展望挖掘与改造高活性MDH的潜在策略。
1 甲醇脱氢酶的分类根据电子受体的不同,甲基营养菌中的MDH可分为3类:吡咯喹啉醌(PQQ) 依赖型MDH、氧依赖型醇氧化酶及烟酰胺腺嘌呤双核苷酸(NAD)依赖型MDH (图 1)。
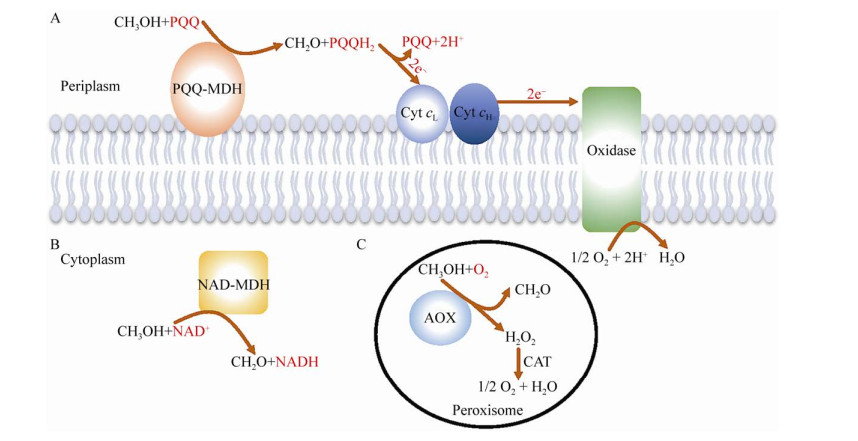 |
| 图 1 甲醇经不同种类的MDH氧化为甲醛 Fig. 1 Oxidation of methanol to formaldehyde by different types of MDHs. (A) Methanol oxidation by PQQ-dependent MDH occurs in the periplasm of Gram-negative bacteria. Methanol is oxidized to formaldehyde and reduced PQQ (PQQH2) is produced by PQQ-dependent MDH. Then, PQQH2 is reoxidized to PQQ by cytochrome cL. Electrons reach a terminal oxidase with the electron transport. (B) NAD-dependent MDH catalyzes methanol oxidation in the cytoplasm of Gram-positive bacteria, which utilizes NAD as the cofactor and generates NADH, providing reducing power for metabolite production. (C) AOX locates in the peroxisome of yeast. O2 serves as the electron acceptor. Methanol is oxidized to formaldehyde with production of hydrogen peroxide, which is subsequently decomposed to H2O and O2. |
| 图选项 |
1.1 PQQ依赖型MDHPQQ依赖型MDH位于革兰氏阴性甲基营养菌的周质部位,是研究较为透彻的一类MDH,主要分为MxaFI和XoxF两种类型[11]。其中MxaFI类型的MDH的结构和功能研究较为广泛,其结构是由两个大亚基(α,MxaF) 和两个小亚基(β,MxaI) 组成的四聚体(α2β2),且每个大亚基分别含有一个对酶催化功能必不可少的Ca2+和PQQ辅基[12]。XoxF是从革兰氏阴性甲基营养菌中发现的另一类催化活性较高MDH,其结构为同型二聚体[11]。与MxaFI不同,XoxF主要是通过稀土元素(如镧和铈) 来代替Ca2+实现甲醇氧化的催化功能。PQQ依赖型MDH通过PQQ辅基捕获电子,然后将电子传递给电子受体细胞色素c,实现底物氧化,对甲醇的亲和力以及催化效率较高[9, 13-14]。然而微生物中以PQQ作为辅因子的生物合成反应较少,因此PQQ依赖型MDH催化甲醇氧化产生的还原力,难以直接用于化学品和燃料的生物合成。此外,PQQ的合成需要氧气,在无氧条件下,PQQ合成受阻,这使得PQQ依赖型MDH的应用范围受限[15-16]。
1.2 氧依赖型醇氧化酶(AOX)AOX是甲基营养型酵母特有的一类催化甲醇氧化成甲醛的酶。在不同的甲基营养型酵母中,AOX编码基因的数目不同,如在博伊丁假丝酵母Candida boidinii和汉逊酵母Hansenula polymorpha中,分别只有一个AOD1和MOX基因编码AOX,而在巴斯德毕赤酵母Pichia pastoris和甲醇毕赤酵母P. methanolica中,均具有两个AOX编码基因(分别为AOX1和AOX2,以及MOD1和MOD2)[17]。与PQQ依赖型MDH相似,该类氧化酶也需要在有氧条件下发挥作用,其利用氧气作为电子受体,将甲醇氧化为甲醛和过氧化氢,具有毒性的过氧化氢随即通过过氧化氢酶分解为O2和H2O[4]。与其他类型的MDH相比,AOX催化甲醇氧化反应的吉布斯自由能最低,甲醇氧化效率最高[9]。由于AOX存在于过氧化物酶体中,很好地阻碍了高毒性中间产物甲醛的扩散,避免了高反应活性的甲醛与DNA和蛋白质等的游离氨基发生反应对细胞造成的损伤,但同时也限制了该酶在细菌中的应用[17]。
1.3 NAD依赖型MDHNAD依赖型MDH常见于嗜热型革兰氏阳性细菌中。该类酶位于胞质中,利用NAD作为辅因子,与PQQ依赖型MDH相比,结构简单,催化整个氧化反应只需要一个基因的参与[18]。目前对甲醇芽孢杆菌Bacillus methanolicus来源的NAD依赖型MDH研究报道较多。B. methanolicus MGA3和PB1中分别存在3个NAD依赖型MDH。以B. methanolicus MGA3为例,位于染色体上的MDH2BmMGA3和MDH3BmMGA3之间的同源性高达96%,与位于质粒pBM19上的MDHBmMGA3同源性约为60%。Krog等[18]对B. methanolicus MGA3和PB1中NAD依赖型MDH的酶学性质进行了分析比较,表明该类脱氢酶最适温度为45 ℃左右,在pH 9.5–10.0条件下酶活力最高,且底物谱较广泛,对甲醇的亲和性较差,催化甲醇氧化的活性较低,但对丙醇、丁醇等长链醇有较高的底物亲和性和催化活性,且对甲醛的亲和性以及甲醛还原反应的催化速率大幅高于甲醇氧化(表 1)。此外,激活蛋白(ACT) 可显著提高NAD依赖型MDH对甲醇的亲和力及氧化速率。虽然NAD依赖型MDH在动力学上的有一定的缺陷,但与另外两种MDH相比,NAD依赖型MDH在有氧和无氧条件下均可发挥功能,其电子受体NAD作为辅因子普遍存在于生物体中,且经过还原后可形成NADH,直接为生物合成提供还原力,因此NAD依赖型MDH在人工甲基营养菌中具有较大的应用潜力。
表 1 不同来源的NAD依赖型MDH的动力学参数Table 1 Kinetic constants of NAD-dependent MDHs from different sources
| Enzyme | Specific activity (U/mg) | Km (mmol/L) | Km/kcat L/(mol·s) | Assay condition | References |
| MDHBmMGA3 | 0.06 | 170.00±20.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH2BmMGA3 | 0.09 | 360.00±30.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH3BmMGA3 | 0.07 | 200.00±70.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDHBmMGA3+ACT | 0.40 | 26.00±7.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH2BmMGA3+ACT | 0.20 | 200.00±20.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH3BmMGA3+ACT | 0.40 | 150.00±10.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDHBmPB1 | 0.03 | 220.00±30.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH1BmPB1 | 0.02 | 170.00±60.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH2BmPB1 | 0.08 | 330.00±0.05 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDHBmPB1+ACT | 0.20 | 10.00±1.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH1BmPB1+ACT | 0.05 | 5.00±1.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDH2BmPB1+ACT | 0.38 | 110.00±50.00 | ND | 45 ℃, pH 9.5 glycine-KOH buffer | [18] |
| MDHBs2334 | 0.02 | 26.00±5.50 | 0.54 | 30 ℃, pH 7.4 potassium phosphate buffer | [22] |
| MDHCn | 0.32 | 132.00±15.40 | 1.60 | 30 ℃, pH 9.5 sodium bicarbonate buffer | [23] |
| MDHCn CT4-1 | 0.29 | 21.60±1.50 | 9.30 | 30 ℃, pH 9.5 sodium bicarbonate buffer | [23] |
| AdhACg | 0.29 | 97.00±9.80 | 2.10 | 30 ℃, pH 9.5 sodium bicarbonate buffer | [23] |
| MDH2BmMGA3 (Q5L, A363L) | 0.13 | 432.00±32.00 | 1.18 | 37 ℃, pH 9.5 glycine-KOH buffer | [24] |
| MDHLx | 0.30 | 3.23±1.05 | 66.80 | 55 ℃, pH 9.5 2-cyclohexylaminoethanesulfonic acid buffer | [25] |
| BmMGA3: B. methanolicus MGA3, BmPB1: B. methanolicusPB1, Bs2334: B. stearothermophilus DSM 2334, Cn: C. necator N-1, Cg: C. glutamicum, Lx: L. xylanilyticus, ND: Not detected. | |||||
表选项
此外,在革兰氏阳性甲基营养菌嗜甲基拟无枝酸菌Amycolatopsis methanolica和胃分支杆菌Mycobacterium gastri MB19中还存在另一类可氧化甲醇的N, N’-二甲基-4-亚硝基苯胺氧化还原酶(MDO),MDO与NAD依赖型的MDH在四级结构、辅因子组成和酶学性质方面类似,但MDO除展现出N, N’-二甲基-4-亚硝基苯胺依赖的甲醇氧化活性和NADH依赖的甲醛还原活性外,还具有甲醛岐化酶活性,将甲醛氧化为甲酸的同时,将另一分子的甲醛还原为甲醇[19-21]。
1.4 非甲基营养菌中的MDH除了已报道的在天然甲基营养菌中发现的MDH,在非甲基营养菌中,也存在一些NAD依赖型的可氧化甲醇的醇脱氢酶。Sheehan等[26]报道了嗜热脂肪芽孢杆菌B. stearothermophilus DSM 2334来源的NAD依赖型MDH (MDHBs2334),对甲醇Km值约为20 mmol/L,37 ℃时Vmax约为2.1 U/mg,最适pH为6.0,且在pH 6.0–10.0条件下始终保持较高酶活。由于MDHBs2334比来自B. methanolicus的MDH具有更高的底物亲和性和催化活性,已被应用于人工甲基营养菌中。Witthoff等[27]鉴定了谷氨酸棒杆菌Corynebacterium glutamicum中的醇脱氢酶AdhA可催化甲醇氧化,该细菌具有完整的甲醇至CO2的异化途径。Wu等[23]首次报道了一个存在于革兰氏阴性、嗜温的非甲基营养菌钩虫贪铜菌Cupriavidus necator N-1中的NAD依赖型MDH (MDHCn)。MDHCn与先前报道的MDHBmMGA3类似,底物谱广,对甲醇的亲和力较低,Km值约为132 mmol/L,30 ℃时Vmax为0.32 U/mg,对丙醇的催化活性最高。但是,MDHCn对ACT不敏感。有无ACT时,MDHCn表现出相当的酶活力。Lee等[25]表征了革兰氏阳性细菌解木糖赖氨酸芽孢杆菌Lysinibacillus xylanilyticus来源的MDH (MDHLx),该酶也无需ACT激活,其最适反应条件为pH 9.5和55 ℃。与其他MDH相比,MDHLx对甲醇的亲和性较高,Km值约为3 mmol/L,且对甲醇具有相对较高的底物特异性。虽然这些MDH来自非甲基营养菌,但仍具有较好的酶学性质,因此也可用于构建人工甲基营养菌。
2 MDH在人工甲基营养菌中的应用2.1 人工甲基营养细菌目前,人工甲基营养菌的构建主要是通过在C. glutamicum和大肠杆菌Escherichia coli等平台菌株中组装MDH和甲醛同化途径实现的,基于上述分析,NAD依赖型MDH是首选的MDH。Witthoff等[28]首次在C. glutamicum中构建了甲醇利用途径,比较了不同启动子对B. methanolicus MGA3来源的MDH的表达效果,发现使用组成型启动子Ptuf控制MDHBmMGA3和ACT的效果较好,使得重组C. glutamicum的甲醇消耗速率可达(1.7±0.3) mmol/(L·h)。Le?meier等[29]类似地比较了B. methanolicus MGA3和PB1来源的MDH,与Witthoff等的研究结果一致,MDHBmMGA3在C. glutamicum中酶活力最高。当同时表达ACT时,粗酶液中MDHBmMGA3的酶活力提高了2.6倍左右,但全细胞催化结果显示,ACT在体内对MDH的酶活力无显著的提升。由于先前报道的来自B. methanolicus的NAD依赖型MDH为嗜热酶,其最适反应温度在常用平台菌株最佳生长温度范围之外,这极大地限制了人工甲基营养菌的甲醇利用速度。随后,Tuyishime等[30]比较发现在C. glutamicum中,MDHBs2334比B. methanolicus MGA3和C. necator N-1来源的MDH具有更高的甲醇氧化活性,在此基础上构建和进化获得的甲醇依赖型C. glutamicum具有较快的甲醇利用速度,甲醇和共底物木糖的共利用比例超过7︰1[31]。类似的工作也在E. coli中开展,Müller等[6]为了找到最适于在E. coli中表达的MDH,扩大了筛选范围,测试了来自B. methanolicus、凝结芽孢杆菌B. coagulans、库氏脱硫肠状菌Desulfotomaculum kuznetsovii、四氯乙烯降解菌Desulfitobacterium hafniense、纺锤形赖氨酸芽孢杆菌L. fusiformis和球形赖氨酸芽孢杆菌L. sphaericus的具有甲醇氧化潜力的醇脱氢酶。体外测试表明表达MDH2BmMGA3和ACT最有效,体内测试表明表达MDH2BmMGA3效果最佳,但ACT对MDH在体内的酶活力无正向影响。Whitaker等[32]在E. coli中验证了B. stearothermophilus DSM 2334来源的MDHBs2334在体外和体内的酶活力均高于MDH2BmMGA3,更有利于甲醇利用。Kim等[33]依据产乙酸菌等细菌代谢甲醇和甲酸的厌氧还原型乙酰辅酶A途径(Anaerobic reductive acetyl-CoA,rAcCoAP),在E. coli中整合内源和外源的酶,构建了线性一碳利用途径——还原型甘氨酸途径(Reductive glycine pathway,rGlyP),再结合短期适应性进化,重组E. coli每消耗1 mol甲酸的细胞干重可达2.3 g。之后,Kim等在rGlyP途径基础上,异源表达不同菌株来源的MDH,包括MDHBs2334、MDHCg、MDHCn、MDH2BmMGA3、MDH3BmMGA3和MDH2BmMGA3 (Q5L、A363L),而只有含MDHBs2334的重组菌株可代谢甲醇,同时13C甲醇标记实验进一步表明甲醇通过rGlyP途径被用于蛋白氨基酸的合成。MDH催化甲醇氧化为可逆反应,因此提高甲醛同化效率,可驱动甲醇氧化。Woolston等[34]通过过表达E. coli的景天庚酮糖二磷酸酶,激活了景天庚酮糖-1, 7-二磷酸合成景天庚酮糖-7-磷酸的反应,推动甲醛受体Ru5P的再生,加快了甲醛的固定,进一步提高了甲醇的同化效率。
2.2 人工甲基营养酵母真核生物酿酒酵母Saccharomyces cerevisiae对高浓度甲醇有较强的耐受性,是构建人工甲基营养菌的理想宿主[35]。Dai等[36]在S. cerevisiae中测试了来源于B. methanolicus MGA3的MDH和B. subtilis 168的RuMP途径的组装效果,但可能由于密码子偏好性,酶未成功表达,重组S. cerevisiae不具备甲醇利用能力。随后,甲基营养酵母P. pastoris来源的甲醇利用相关酶(AOX、过氧化氢酶、二羟基丙酮合成酶和二羟丙酮激酶)被用于构建的人工甲基营养型酵母,最终成功实现了甲醇到丙酮酸的转化。Espinosa等[37]在S. cerevisiae中分别组装了AOX-XuMP (P. pastoris来源)、MDHBs2334-XuMP和MDHBs2334-RuMP (B. methanolicus来源) 甲醇利用途径,结果表明异源表达MDHBs2334-XuMP和MDHBs2334-RuMP途径的重组S. cerevisiae均可利用甲醇,而含有AOX-XuMP途径的重组S. cerevisiae不能代谢甲醇。在甲基营养型酵母利用甲醇生长过程中,过氧化物酶体的增殖起重要作用[38]。然而,S. cerevisiae中过氧物酶体的增殖受限于脂肪酸氧化等特定的应激反应且需要多种基因的调控[39]。因此Espinosa等[37]推测未进化的S. cerevisiae中过氧化物酶体的增殖不受甲醇的诱导,进而限制了细胞通过AOX-XuMP途径代谢甲醇。此外,Espinosa等还通过13C甲醇标记实验首次证明野生型S. cerevisiae CEN. PK 113-5D具有内源的甲醇同化途径,为进一步研究S. cerevisiae中的天然或者人工一碳利用途径提供了研究基础。
以上研究表明MDH和甲醛同化途径在不同宿主中的应用效果有一定差异,在构建人工甲基营养细菌时,有必要针对宿主进行MDH和甲醛同化途径的选择和优化。
3 MDHs的定向进化和理性设计目前利用NAD依赖型MDH成功构建了多种人工甲基营养菌,但已报道的NAD依赖型MDH对甲醇的Km值较高,催化甲醇氧化反应的活性较低,且最适反应条件多为高温和碱性环境,在常用平台菌株中的工作效果不理想[18]。因此,基于蛋白质工程,对MDH进行改造和进化,提高甲醇氧化活性逐渐成为研究热点(图 2)。Wu等[23]基于传统的Nash试剂显色反应对MDHCn突变体库进行高通量筛选。其中最优突变体CT4-1 (A26V、A31V、A169V) 对C2-C4长链醇的活性有不同程度的降低,而对甲醇的亲和性大幅度提高,催化甲醇氧化的kcat/Km值提高了6倍,这为定向进化提高MDH催化活性提供了参考。但使用Nash试剂检测甲醛浓度的方法灵敏度较低,且测定前需将上清和细胞分离,操作繁琐,亟需开发更加高效的高通量筛选方法。
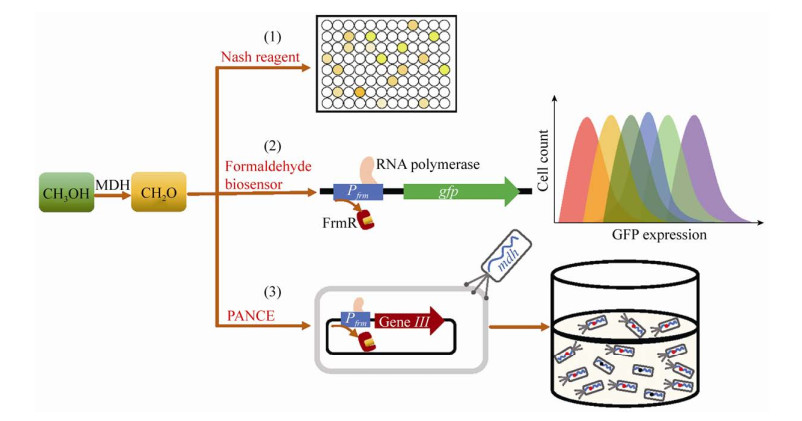 |
| 图 2 MDH突变体的高通量筛选方法 Fig. 2 High-throughput screening methods for MDH variants. (1) Nash reagent. Nash reaction can detect extracellular formaldehyde produced by methanol oxidation through the reaction of formaldehyde, acetylacetone, and ammonium acetate. Diacetyldihydrolutidine, the Nash reaction product, exhibits yellow color and can be quantified by absorbance at 412 nm. This method can correlate the MDH activity with the absorbance of Nash reaction product. (2) Formaldehyde biosensor. This method is based on the formaldehyde-inducible promoter Pfrm, its regulator FrmR, and a gfp reporter gene. In the absence of formaldehyde, the FrmR binds to the regulatory region of Pfrm, preventing the transcription of downstream gfp. In the presence of formaldehyde, the nucleophilic Cys36 of FrmR reacts with formaldehyde and causes a change in conformational. Consequently, FrmR dissociates from Pfrm and the transcription of gfp is activated. This method can correlate the MDH activity with the GFP fluorescence. The MDH library can be partitioned into bins via flow cytometry on the basis of GFP fluorescence. (3) PANCE (phage-assisted noncontinuous evolution). PANCE is also based on Pfrm and FrmR but correlates MDH activity with the phage propagation rate. The essential phage gene Ⅲ is cloned to the downstream of Pfrm. For E. coli cells infected by a phage harboring a superior MDH variant, formaldehyde is produced by methanol oxidation at a high rate and gene Ⅲ is expressed at a high level. Consequently, the phage propagation is accelerated and superior MDH variants are enriched in the system. Red dots represent permissive and beneficial mutations, and black dots represent detrimental mutations. |
| 图选项 |
在E. coli中存在一种天然的甲醛诱导型启动子(Pfrm),该启动子受阻遏蛋白FrmR的负反馈调控。在甲醛存在时,FrmR蛋白与甲醛结合,构型发生改变从而解除对启动子Pfrm的抑制作用,启动下游基因表达[40]。将该启动子Pfrm与报告基因相结合,该系统可被改造为体内响应甲醛的生物传感器[41]。通过报告系统的信号强度,可灵敏反应胞内的甲醛浓度。Woolston等[41]通过优化阻遏蛋白FrmR与启动子的结合区,显著提高了甲醛生物传感器的灵敏性,最低检测阈值达到1 μmol/L甲醛。Roth等[24]利用该甲醛传感器,将噬菌体增殖速度与甲醇氧化生成甲醛的量相偶联,开发了噬菌体辅助非连续进化(PANCE) 方法,对MDH2BmMGA3进行进化,筛选获得的最优突变体(Q5L、A363L) 的Vmax较野生型MDH2BmMGA3提高3.5倍。为更好地理解这些突变位点提高MDH催化活性的机制,Roth等还通过蛋白质同源建模对突变位点对结构的影响进行分析,结果显示所有的关键突变都定位于NAD辅因子结合口袋附近的区域,为MDH的理性设计进一步改造提供了理论基础。来源于B. stearothermophilus DSM 2334的MDHBs2334具有相对较高的甲醇氧化活性并广泛应用于人工甲基营养菌[22, 30, 42]。Guo等[43]解析了MDHBs2334的晶体结构,并对酶学性质进行分析,发现异丙醇是其最适底物,分子对接实验表明这种底物偏好性主要归因于蛋白质-底物复合物的构象能;该研究表明,对底物结合口袋进行定向进化,有望提高MDHBs2334对甲醇的特异性和催化活性。Lee等[25]通过蛋白质同源建模和分子对接分析,对鉴定的16个MDHLx的活性口袋和NAD结合区域相关的氨基酸残基进行理性改造,获得的突变体Vmax提高了13%–60%。以上研究表明,定向进化和理性设计为提高MDH的催化活性提供了更多可行性。
4 MDH多酶复合体的构建NAD依赖型MDH催化的甲醇-甲醛转换反应为可逆反应,MDH3BmMGA3催化甲醇到甲醛的kcat/Km值仅为催化甲醛到甲醇的1/1 000[18],因此甲醇氧化生成的甲醛极易被重新被还原为甲醇,从而影响甲醇的利用速度。此外,甲醛是一种非常活泼的高毒性化合物,可以和细胞内的蛋白质及核酸发生非特异性反应,严重抑制细胞的生长代谢[44]。为拉动甲醇氧化反应,避免甲醛积累,Price等[45]利用SH3配体间的相互作用,将Mdh3BmMGA3和来源于M. gastri的3-己酮糖-6-磷酸合成酶(HPS) 和3-己酮糖-6-磷酸合成酶(PHI) 构建为三酶复合体MDH-sSH3lig-SH3-HPS-PHI,并引入乳酸脱氢酶解除NADH对MDH的反馈抑制,使得体外转化甲醇合成果糖-6-磷酸(F6P) 的产量提高了97倍。全细胞催化条件下甲醇消耗率可达1.7 mmol/(L·h)。Fan等[22]理性设计并构建了MDH-HPS-PHI的融合蛋白,同样可提高甲醇至F6P的转化效率。通过比较不同来源的RuMP途径关键酶HPS和PHI的酶活力,筛选最优的酶进行融合蛋白构建,随后利用柔性连接肽(GGGGS)×3和(GGGGS)×6与MDHBs2334构建多种融合蛋白。最优融合蛋白催化甲醇生成F6P的速率提高了30%。同时,该研究还发现当MDHBs2334与HPS或HPS-PHI融合表达时,具有了更高的甲醇氧化活性,表明多酶复合体的构建是提高MDH催化活性和甲醇生物转化效率的有效策略。
5 总结与展望甲醇氧化是一碳利用的关键步骤,但由于NAD依赖型MDH对甲醇的亲和性较差,催化活性较低,甲醇氧化也是人工甲基营养菌甲醇利用的限速步骤[46]。为解决NAD依赖型MDH在动力学上的不足,不少****尝试通过蛋白质定向进化结合基于甲醛传感器的高通量筛选策略筛选酶活提高的突变体,但目前所获得的突变体仍难以满足高效甲醇生物转化的需求。目前已有多个研究团队构建了甲醇依赖型的C. glutamicum和E. coli[30-31, 47-49],将甲醇的利用速度与细菌生长速度直接偶联,这些甲醇依赖型菌株可用于基于生长的MDH文库筛选。通过将MDH突变体文库转入甲醇依赖型菌株中,具有较高甲醇氧化活性的MDH突变体将为细菌带来生长优势,在连续培养过程中将被富集,该方法比基于甲醛传感器的筛选方法更加简便,也较容易获得体内应用效果更佳的MDH突变体。另一方面,基于已有的MDH蛋白晶体结构,可对其底物和辅因子结合口袋进行理性设计,改善MDH的底物结合特异性,提高MDH对甲醇的亲和性和催化活性。此外,随着生物信息学大数据的快速发展,可以利用基因挖掘策略,例如从基因组数据中基于探针酶序列和结构信息挖掘新的酶基因,发现新的酶学性质优良的MDH[50]。de Simone等[51]以B. methanolicus PB1的MDH2序列为模板在UniRef50数据库中根据序列一致性进行比对分析,成功筛选到新的MDH。生物信息学、结构生物学和蛋白质工程的快速发展,将为筛选和改造性能优秀的MDH,突破甲醇氧化速率慢的瓶颈提供技术支撑,结合系统生物学和合成生物学的手段解析并解决一碳利用的其他限制因素,有望构建出高效利用甲醇和甲烷为唯一碳源和能源进行生物制造的人工甲基营养菌。
参考文献
| [1] | R?uchle K, Plass L, Wernicke HJ, et al. Methanol for renewable energy storage and utilization. Energy Technol, 2016, 4(1): 193-200. DOI:10.1002/ente.201500322 |
| [2] | 王凯, 贺明丽, 王梦, 等. 以CO2为原料的绿色生物制造. 化工进展, 2019, 38(1): 538-544. Wang K, He ML, Wang M, et al. Green biological manufacture with CO2 as raw material. Chem Ind Eng Prog, 2019, 38(1): 538-544 (in Chinese). |
| [3] | Cotton CAR, Claassens NJ, Benito-Vaquerizo S, et al. Renewable methanol and formate as microbial feedstocks. Curr Opin Biotechnol, 2020, 62: 168-180. DOI:10.1016/j.copbio.2019.10.002 |
| [4] | Zhang WM, Zhang T, Wu SH, et al. Guidance for engineering of synthetic methylotrophy based on methanol metabolism in methylotrophy. RSC Adv, 2017, 7(7): 4083-4091. DOI:10.1039/C6RA27038G |
| [5] | Zhang WM, Song M, Yang Q, et al. Current advance in bioconversion of methanol to chemicals. Biotechnol Biofuels, 2018, 11: 260. DOI:10.1186/s13068-018-1265-y |
| [6] | Müller JEN, Meyer F, Litsanov B, et al. Engineering Escherichia coli for methanol conversion. Metab Eng, 2015, 28: 190-201. DOI:10.1016/j.ymben.2014.12.008 |
| [7] | Hakemian AS, Rosenzweig AC. The biochemistry of methane oxidation. Annu Rev Biochem, 2007, 76: 223-241. DOI:10.1146/annurev.biochem.76.061505.175355 |
| [8] | Nguyen AD, Hwang IY, Chan JY, et al. Reconstruction of methanol and formate metabolic pathway in non-native host for biosynthesis of chemicals and biofuels. Biotechnol Bioprocess Eng, 2016, 21(4): 477-482. DOI:10.1007/s12257-016-0301-7 |
| [9] | Whitaker WB, Sandoval NR, Bennett RK, et al. Synthetic methylotrophy: engineering the production of biofuels and chemicals based on the biology of aerobic methanol utilization. Curr Opin Biotechnol, 2015, 33: 165-175. DOI:10.1016/j.copbio.2015.01.007 |
| [10] | Zhang M, Yuan XJ, Zhang C, et al. Bioconversion of methanol into value-added chemicals in native and synthetic methylotrophs. Curr Issues Mol Biol, 2019, 33: 225-236. |
| [11] | Keltjens JT, Pol A, Reimann J, et al. PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol, 2014, 98(14): 6163-6183. DOI:10.1007/s00253-014-5766-8 |
| [12] | Anthony C, Ghosh M, Blake CCF. The structure and function of methanol dehydrogenase and related quinoproteins containing pyrrolo-quinoline quinone. Biochem J, 1994, 304(3): 665-674. DOI:10.1042/bj3040665 |
| [13] | Ochsner AM, Sonntag F, Buchhaupt M, et al. Methylobacterium extorquens: methylotrophy and biotechnological applications. Appl Microbiol Biotechnol, 2014, 99(2): 517-534. |
| [14] | Chistoserdova L, Chen SW, Lapidus A, et al. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J Bacteriol, 2003, 185(10): 2980-2987. DOI:10.1128/JB.185.10.2980-2987.2003 |
| [15] | Puehringer S, Metlitzky M, Schwarzenbacher R. The pyrroloquinoline quinone biosynthesis pathway revisited: a structural approach. BMC Biochem, 2008, 9: 8. DOI:10.1186/1471-2091-9-8 |
| [16] | Velterop JS, Sellink E, Meulenberg JJ, et al. Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J Bacteriol, 1995, 177(17): 5088-5098. DOI:10.1128/JB.177.17.5088-5098.1995 |
| [17] | Hartner FS, Glieder A. Regulation of methanol utilisation pathway genes in yeasts. Microb Cell Fact, 2006, 5: 39. DOI:10.1186/1475-2859-5-39 |
| [18] | Krog A, Heggeset TMB, Müller JEN, et al. Methylotrophic Bacillus methanolicus encodes two chromosomal and one plasmid born NAD+ dependent methanol dehydrogenase paralogs with different catalytic and biochemical properties. PLoS ONE, 2013, 8(3): e59188. DOI:10.1371/journal.pone.0059188 |
| [19] | Kato N, Yamagami T, Shimao M, et al. Formaldehyde dismutase, a novel NAD-binding oxidoreductase from Pseudomonas putida F61. Eur J Biochem, 1986, 156(1): 59-64. DOI:10.1111/j.1432-1033.1986.tb09548.x |
| [20] | Park H, Lee H, Ro YT, et al. Identification and functional characterization of a gene for the methanol: N, N'-dimethyl-4-nitrosoaniline oxidoreductase from Mycobacterium sp. strain JC1 (DSM 3803). Microbiology, 2010, 156(2): 463-471. DOI:10.1099/mic.0.034124-0 |
| [21] | Hektor HJ, Kloosterman H, Dijkhuizen L. Nicotinoprotein methanol dehydrogenase enzymes in Gram-positive methylotrophic bacteria. J Mol Catal B Enzym, 2000, 8(1/3): 103-109. |
| [22] | Fan LW, Wang Y, Tuyishime P, et al. Engineering artificial fusion proteins for enhanced methanol bioconversion. Chem Bio Chem, 2018, 19(23): 2465-2471. DOI:10.1002/cbic.201800424 |
| [23] | Wu TY, Chen CT, Liu JTJ, et al. Characterization and evolution of an activator-independent methanol dehydrogenase from Cupriavidus necator N-1. Appl Microbiol Biotechnol, 2016, 100(11): 4969-4983. DOI:10.1007/s00253-016-7320-3 |
| [24] | Roth TB, Woolston BM, Stephanopoulos G, et al. Phage-assisted evolution of Bacillus methanolicus methanol dehydrogenase 2. ACS Synth Biol, 2019, 8(4): 796-806. DOI:10.1021/acssynbio.8b00481 |
| [25] | Lee JY, Park SH, Oh SH, et al. Discovery and biochemical characterization of a methanol dehydrogenase from Lysinibacillus xylanilyticus. Front Bioeng Biotechnol, 2020, 8: 67. DOI:10.3389/fbioe.2020.00067 |
| [26] | Sheehan MC, Bailey CJ, Dowds BCA, et al. A new alcohol dehydrogenase, reactive towards methanol, from Bacillus stearothermophilus. Biochem J, 1988, 252(3): 661-666. DOI:10.1042/bj2520661 |
| [27] | Witthoff S, Mühlroth A, Marienhagen J, et al. C1 metabolism in Corynebacterium glutamicum: an endogenous pathway for oxidation of methanol to carbon dioxide. Appl Environ Microbiol, 2013, 79(22): 6974-6983. DOI:10.1128/AEM.02705-13 |
| [28] | Witthoff S, Schmitz K, Niedenführ S, et al. Metabolic engineering of Corynebacterium glutamicum for methanol metabolism. Appl Environ Microbiol, 2015, 81(6): 2215-2225. DOI:10.1128/AEM.03110-14 |
| [29] | Le?meier L, Pfeifenschneider J, Carnicer M, et al. Production of carbon-13-labeled cadaverine by engineered Corynebacterium glutamicum using carbon-13-labeled methanol as co-substrate. Appl Microbiol Biotechnol, 2015, 99(23): 10163-10176. DOI:10.1007/s00253-015-6906-5 |
| [30] | Tuyishime P, Wang Y, Fan LW, et al. Engineering Corynebacterium glutamicum for methanol- dependent growth and glutamate production. Metab Eng, 2018, 49: 220-231. DOI:10.1016/j.ymben.2018.07.011 |
| [31] | Wang Y, Fan LW, Tuyishime P, et al. Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum. Commun Biol, 2020, 3: 217. DOI:10.1038/s42003-020-0954-9 |
| [32] | Whitaker WB, Jones JA, Bennett RK, et al. Engineering the biological conversion of methanol to specialty chemicals in Escherichia coli. Metab Eng, 2017, 39: 49-59. DOI:10.1016/j.ymben.2016.10.015 |
| [33] | Kim S, Lindner SN, Aslan S, et al. Growth of E. coli on formate and methanol via the reductive glycine pathway. Nat Chem Biol, 2020, 16: 538-545. DOI:10.1038/s41589-020-0473-5 |
| [34] | Woolston BM, King JR, Reiter M, et al. Improving formaldehyde consumption drives methanol assimilation in engineered E. coli. Nat Commun, 2018, 9: 2387. DOI:10.1038/s41467-018-04795-4 |
| [35] | Yasokawa D, Murata S, Iwahashi Y, et al. Toxicity of methanol and formaldehyde towards Saccharomyces cerevisiae as assessed by DNA microarray analysis. Appl Biochem Biotechnol, 2010, 160(6): 1685-1698. DOI:10.1007/s12010-009-8684-y |
| [36] | Dai ZX, Gu HL, Zhang SJ, et al. Metabolic construction strategies for direct methanol utilization in Saccharomyces cerevisiae. Bioresour Technol, 2017, 245: 1407-1412. DOI:10.1016/j.biortech.2017.05.100 |
| [37] | Espinosa MI, Gonzalez-Garcia RA, Valgepea K, et al. Engineering and evolution of methanol assimilation in Saccharomyces cerevisiae. bioRxiv, 2020. DOI:10.1101/717942 |
| [38] | van der Klei IJ, Harder W, Veenhuis M. Methanol metabolism in a peroxisome-deficient mutant of Hansenula polymorpha: a physiological study. Arch Microbiol, 1991, 156(1): 15-23. DOI:10.1007/BF00418181 |
| [39] | Hiltunen JK, Mursula AM, Rottensteiner H, et al. The biochemistry of peroxisomal β-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev, 2003, 27(1): 35-64. DOI:10.1016/S0168-6445(03)00017-2 |
| [40] | Denby KJ, Iwig J, Bisson C, et al. The mechanism of a formaldehyde-sensing transcriptional regulator. Sci Rep, 2016, 6: 38879. DOI:10.1038/srep38879 |
| [41] | Woolston BM, Roth T, Kohale I, et al. Development of a formaldehyde biosensor with application to synthetic methylotrophy. Biotechnol Bioeng, 2018, 115(1): 206-215. DOI:10.1002/bit.26455 |
| [42] | Rohlhill J, Sandoval NR, Papoutsakis ET. Sort-seq approach to engineering a formaldehyde-inducible promoter for dynamically regulated Escherichia coli growth on methanol. ACS Synth Biol, 2017, 6(8): 1584-1595. DOI:10.1021/acssynbio.7b00114 |
| [43] | Guo XJ, Feng YB, Wang XY, et al. Characterization of the substrate scope of an alcohol dehydrogenase commonly used as methanol dehydrogenase. Bioorg Med Chem Lett, 2019, 29(12): 1446-1449. DOI:10.1016/j.bmcl.2019.04.025 |
| [44] | Feldman MY. Reactions of nucleic acids and nucleoproteins with formaldehyde. Prog Nucleic Acid Res Mol Biol, 1973, 13: 1-49. |
| [45] | Price JV, Chen L, Whitaker WB, et al. Scaffoldless engineered enzyme assembly for enhanced methanol utilization. Proc Natl Acad Sci USA, 2016, 113(45): 12691-12696. DOI:10.1073/pnas.1601797113 |
| [46] | Bogorad IW, Chen CT, Theisen MK, et al. Building carbon-carbon bonds using a biocatalytic methanol condensation cycle. Proc Natl Acad Sci USA, 2014, 111(45): 15928-15933. DOI:10.1073/pnas.1413470111 |
| [47] | Meyer F, Keller P, Hartl J, et al. Methanol-essential growth of Escherichia coli. Nat Commun, 2018, 9: 1508. DOI:10.1038/s41467-018-03937-y |
| [48] | Chen CT, Chen FYH, Bogorad IW, et al. Synthetic methanol auxotrophy of Escherichia coli for methanol-dependent growth and production. Metab Eng, 2018, 49: 257-266. DOI:10.1016/j.ymben.2018.08.010 |
| [49] | Hennig G, Haupka C, Brito LF, et al. Methanol- essential growth of Corynebacterium glutamicum: adaptive laboratory evolution overcomes limitation due to methanethiol assimilation pathway. Int J Mol Sci, 2020, 21(10): 3617. DOI:10.3390/ijms21103617 |
| [50] | Mak WS, Tran S, Marcheschi R, et al. Integrative genomic mining for enzyme function to enable engineering of a non-natural biosynthetic pathway. Nat Commun, 2015, 6: 10005. DOI:10.1038/ncomms10005 |
| [51] | de Simone A, Vicente CM, Peiro C, et al. Mixing and matching methylotrophic enzymes to design a novel methanol utilization pathway in E. coli. Metab Eng, 2020, 61: 315-325. DOI:10.1016/j.ymben.2020.07.005 |
