
 , 张学礼2,3
, 张学礼2,3 1. 河南科技学院 园艺园林学院,河南 新乡 453000;
2. 中国科学院天津工业生物技术研究所,天津 300308;
3. 中国科学院系统微生物工程重点实验室,天津 300308
收稿日期:2019-05-13;接收日期:2019-08-30;网络出版时间:2019-10-10
基金项目:国家自然科学基金(Nos.31770059, 31770105),中国科学院STS项目(No.KFJ-SW-STS-164),河南省高等学校重点项目(No.18A210015)资助
摘要:甲羟戊酸途径(MVA途径)被引入重组大肠杆菌中,能够提高重组大肠杆菌中萜类化合物的合成能力。但因重组大肠杆菌中萜类化合物合成途径中间产物积累,导致细胞生长和萜类化合物合成受到限制。本研究在稳定表达MVA途径以及优化2-甲基-D-赤藻糖醇-4-磷酸途径(MEP途径)、番茄红素合成途径关键基因表达的重组大肠杆菌LYC103中,用质粒高表达MVA途径和番茄红素合成途径关键基因,挖掘该途径的限速步骤。结果表明,ispA、crtE、mvaK1、idi和mvaD基因过表达后,细胞生长没有明显变化,番茄红素产量依次提高了13.5%、16.5%、17.95%、33.7%和61.1%,说明这几个基因可能是合成番茄红素的限速步骤。mvaK1、mvaK2、mvaD三个基因在同一操纵子上,用mRNA稳定区(RNA stabilizing region)进行启动子文库(mRSL)调控mvaK1,相当于对3个基因同时调控。用高效基因组编辑技术(CAGO)对mvaK1基因的mRNA稳定区进行启动子文库的调控,得到菌株LYC104。番茄红素产量与对照菌株LYC103相比增加了2倍,细胞生长提高了32%。然后,利用CRISPR-Cas9技术在染色体lacZ位点整合idi基因,得到LYC105菌株。与出发菌株LYC103相比,细胞生长提高了147%,番茄红素产量增加了2.28倍。本研究在染色体上具有完整MVA途径的基础上,利用质粒高表达单个基因挖掘限速步骤,用同源重组方法整合限速基因、解除限速,为代谢工程构建高产菌株提供新策略。
关键词:番茄红素甲羟戊酸途径CRISPR-Cas9系统大肠杆菌
Role of rate-limiting step of mevalonate pathway in improving lycopene production in Escherichia coli
Zhenxia Li1, Qianqian Chen1,2, Jinlei Tang2,3, Qingyan Li2,3

 , Xueli Zhang2,3
, Xueli Zhang2,3 1. School of Horticulture and Garden, Henan Institute of Science and Technology, Xinxiang 453000, Henan, China;
2. Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China;
3. Key Laboratory of Systems Microbial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China
Received: May 13, 2019; Accepted: August 30, 2019; Published: October 10, 2019
Supported by: National Natural Science Foundation of China (Nos. 31770059, 31770105), the STS Project of the Chinese Academy of Sciences (No. KFJ-SW-STS-164), Key Projects of Henan Provincial Universities (No. 18A210015)
Corresponding author: Qingyan Li. Tel/Fax: +86-22-84861946; E-mail: li_qy@tib.cas.cn.
Abstract: The introduction of the mevalonate pathway (MVA pathway) in recombinant Escherichia coli can improve the synthesis of terpenoids. But the imbalance expression of MVA pathway genes and accumulation of intermediates inhibit cell growth and terpenoids production. In this study, each gene of MVA pathway and key genes of lycopene synthesis pathway were cloned in plasmid to express in the recombinant E. coli LYC103 with optimizing the expression of the key genes of the 2-methyl-D-erythritol-4-phosphate pathway (MEP pathway), chromosome recombinant MVA pathway and the lycopene synthesis pathway. The results showed that the overexpression of ispA, crtE, mvaK1, idi and mvaD genes did not affect the cell growth, while lycopene production increased by 13.5%, 16.5%, 17.95%, 33.7% and 61.1% respectively, indicating that these genes may be the rate-limiting steps for the synthesis of lycopene. mvaK1, mvaK2, mvaD of MVA pathway were the rate-limiting steps and were in an operon. The mvaK1, mvaK2, mvaD operon was regulated by mRS (mRNA stabilizing region) library in front of mvaK1, obtaining strain LYC104. Lycopene yield of LYC104 was doubled and cell growth was increased by 32% compared with the control strain LYC103. CRISPR-cas9 technology was used to integrate idi into chromosome at lacZ site to obtain LYC105 strain. Cell growth of LYC105 was increased by 147% and lycopene yield was increased by 2.28 times compared with that of LYC103. In this study, each gene of lycopene synthesis pathway was expressed in plasmid to certify the rate-limiting gene based on the complete MVA pathway on the chromosome. Then the rate-limiting gene was integrated in chromosome with homologous recombination to release the rate-limiting, which providing a new strategy for the construction of high-yield strains for metabolic engineering.
Keywords: lycopenemevalonate pathwayCRISPR-Cas9 systemEscherichia coli
在自然界中,类胡萝卜素是一类重要的萜类化合物,在许多光合生物(包括细菌、藻类和高等植物)和一些非光合生物中都可以合成[1]。番茄红素是一种C40的类胡萝卜素,作为天然萜类色素,可以从西瓜、番茄等植物的成熟果实中获得。由于番茄红素含有特殊的分子结构,具有很强的消除抗氧化能力和自由基能力,其抗氧化能力分别是胡萝卜素和维生素E的3.2倍和100倍[2]。番茄红素可以预防皮肤癌、乳腺癌、肺癌和肝癌[3-4]等疾病。因此,番茄红素能够被广泛应用于保健食品、制药和化妆品行业。
异戊烯焦磷酸(IPP)和二甲丙烯焦磷酸酯(DMAPP)是合成番茄红素等萜类化合物的前体物质[5]。在自然界中存在2-甲基-D-赤藻糖醇-4-磷酸(MEP)和甲羟戊酸(MVA)两条途径(图 1)合成IPP和DMAPP[6-7]。大肠杆菌自身具有MEP途径,可以合成萜类化合物的前体物质IPP与DMAPP,还可以在大肠杆菌中引入外源甲羟戊酸途径(MVA途径)来提高萜类化合物的前体物质供给。将合成番茄红素的外源基因导入大肠杆菌中,分别是GGPP合成酶CrtE (Geranylgeranyl pyrophosphate synthase)、八氢番茄红素合成酶CrtB (Phytoene synthase)、八氢番茄红素脱氢酶CrtI (Phytoene desaturase),经缩合、脱氢、环化等反应生成番茄红素[8],使不能直接生产类胡萝卜素的大肠杆菌具有合成类胡萝卜素的能力。
 |
图 1 重组大肠杆菌中番茄红素合成途径 Fig. 1 Lycopene synthetic pathway in recombinant E.coli. 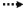 |
| 图选项 |
近年来,多个研究组对提高重组大肠杆菌中类胡萝卜素产量进行了研究,主要包括MEP途径研究[9-13]、中央代谢途径改造[14-18]、引入MVA途径增加前体物质供应[19-23]等几个方面。通过MVA途径增加萜类化合物前体物质供应,提高重组大肠杆菌类胡萝卜素产量已经有较为详细研究。Vadali等将MVA途径基因引入大肠杆菌,增加番茄红素合成前体物质IPP和DMAPP供应,番茄红素产量提高一倍[19]。Yoon等将MVA途径的后半部分几个基因引入大肠杆菌中,并通过增加MVA途径中间产物甲羟戊酸,成功提高番茄红素产量[20-21]。Anthony等发现MVA途径中甲羟戊酸激酶(MK)是一种限速酶[24],通过改变限速基因启动子和质粒拷贝数,目标产物产量提高了7倍。但是,有研究表明甲羟戊酸途径酶表达不平衡可以抑制细胞生长[5, 19]。Pitera等过表达MVA途径上游的几个基因,MVA途径中间体羟甲基戊二酸单酰辅酶A (HMGCoA)积累,抑制了细胞生长[5, 25]。冯凡等将MVA途径分成3个代谢元件模块,并通过调控元件的表达来增强模块功能,从而使异戊二烯的产量得到提高[26]。Ye等将MVA途径分成两个模块在染色体上进行整合,并对两个模块建库调控,β-胡萝卜素产量提高了51%[23]。Wei等将MVA途径分成上下游两个模块基于染色体多位点整合策略,提高大肠杆菌合成番茄红素的产量[27]。Ye等构建MVA途径的乙酰辅酶A乙酰转移酶/HMG-CoA还原酶基因(mvaE)和mvaS基因等5个基因的RBS文库,根据菌株颜色,筛选到使β-胡萝卜素产量提高的质粒[22]。然后将得到协调表达的MVA途径基因整合到产番茄红素的重组大肠杆菌的染色体上,使番茄红素产量提高了1.19倍[5]。
本研究对所得的LYC102菌株进行调控得到LYC103菌株[5, 28]。LYC103菌株中MEP途径经过精确调控[13, 18, 29],并且在染色体上含有完整的MVA途径基因[5, 28]。MVA途径基因虽然经过RBS建库协调表达,但是可能因为本菌株是单拷贝的染色体表达,而Ye文献[22]中是多拷贝的质粒表达,并且本菌株MVA途径操纵子又经过了更换启动子的改造,所以MVA途径可能重新出现限速步骤。另外,在LYC103中添加了MVA途径,进一步增加了IPP/DMAPP的供应,下游也可能成为限速步骤,因此,确保整合MVA途径的菌株中前体物质的平衡供给,同时寻找并解除下游合成途径中的限速步骤。本研究首次在染色体整合完整MVA途径基因的基础上,将MVA途径中的5个基因进行研究,本方法优于对MVA途径5个基因同时进行研究。另外对番茄红素合成途径下游的异戊烯基焦磷酸异构酶(idi)、香叶醇转移酶(ispA)、GGPP合成酶基因(crtE)以单基因、多基因组合等方法在重组大肠杆菌中过量表达,从而寻找新的限速步骤,研究细胞生长和番茄红素产量的变化,并进一步提高番茄红素产量。
1 材料与方法1.1 材料质粒小量快速提取试剂盒购自美国Axygen公司;氨苄青霉素、氯霉素和SanPrep柱式PCR产物纯化试剂盒,购自生工生物工程(上海)股份有限公司;DNA Marker、EasyTaq PCR SuperMix DNA聚合酶购自北京全式金生物技术有限公司;Gold View I型核酸染色剂,购自北京索莱宝科技有限公司;PhusionTM超保真DNA聚合酶购自NEB公司[28];其他试剂均为分析纯。
1.1.2 菌株和质粒本研究所用菌株和质粒见表 1。
表 1 本研究所用的菌株和质粒Table 1 Strains and plasmids used in this study
| Strains | Relative characteristics | Sources |
| E. coli MG1655-ΔpoxB | E. coli MG1655, poxB was replaced by cam marker and N20PAM | [30] |
| LYC001 | ATCC 8739, M1-37::dxs, M1-46::idi, M1-93::crtEIB | [5, 29] |
| LYC103 | LYC102, ldhA::trc::crtEIB | [28] |
| LYC104 | LYC103, mRSL-7::mvaK1 | [28] |
| LYC105 | LYC104, LacZ::trc::idi | [28] |
| Plasmids | ||
| pRed_Cas9 | Kan, derived from pKD46, exo, bet, gam, arabinose operon, Cas9 | [5, 31] |
| pCAGO | Amp, derived from pKD46, exo, bet, gam, arabinose operon, Cas9, gRNA | [30] |
| pLacZ-N20 | Cat, derived from pACYC184-gRNA, gRNA with N20 and homologous arms of LacZ | Lab collection, [28] |
| pLacZ-N20-trc-idi | Cat; trac promoter followed by pSC103 amplified from pSC103-idi cloned into pLacZ-N20 | [28] |
| pSC103 | Low copy plasmid, ori and rep A from p SC102, bla; trc promoter and rrn terminator from pTRC99A, cat from p ACYC184 | Lab collection |
| pSC103-idi | idi, from Bacillus subtilis idi in pSC103 | [28] |
| pSC103-crtE | crtE, from Pantoea agglomerans in pSC103 | [28] |
| pSC103-mvaK1 | mvaK1, from Streptococcus pneumoniae, mvaK1 in pSC103 | [28] |
| pSC103-ispA | ispA, from Pantoea agglomerans, ispA in pSC103 | [28] |
| pSC103-mvaE | mvaE, from Enterococcus faecalis, mvaS in pSC103 | [28] |
| pSC103-mvaS | mvaS, from Enterococcus faecalis, mvaS in pSC103 | [28] |
| pSC103-mvaK2 | mvaK2, from Streptococcus pneumoniae, mvaK2 in pSC103 | [28] |
| pSC103-mvaD | mvaD, from Streptococcus pneumoniae, mvaD in pSC103 | [28] |
表选项
1.2 方法1.2.1 培养基及培养方法LB培养基、LB固体培养基和发酵用的LB+2%甘油培养基配制见参考文献[5, 32-33]。氨苄青霉素(Amp)、硫酸卡那霉素(Kan)、氯霉素(Cam)终浓度分别为100、50和34 μg/mL。
保存于-80 ℃的菌种在LB平板上划线活化,按照参考文献方法培养菌体,用于番茄红素产量测定[5]。
1.2.2 番茄红素产量测定方法测定番茄红素产量时,先取500 μL待测菌液,参考文献所述方法处理样品,并用液相色谱测定番茄红素产量[5, 29]。
1.2.3 构建单基因质粒菌株采用环形聚合酶延伸克隆(Circular polymerase extension cloning,CPEC)[34]的方法构建质粒(图 2),以质粒pSC103为模板,通过引物99A-cpec-F/ 99A-cpec-R,扩增质粒骨架,得到正确的片段大小后,回收PCR所得骨架。提取LYC103菌株DNA基因组作为模板,用MvaE-cpec-F/ MvaE-cpec-R、Ef-MvaS-cpec-F/Ef-MvaS-cpec-R、Sp-MK1-cpec-F/Sp-MK1-cpec-R、Sp-MvaK2- cpec-F/Sp-MvaK2-cpec-R和Sp-MvaD-cpec- F/Sp-MvaD-cpec-R为引物,扩增MVA途径中的5个基因,分别是mvaE、mvaS、mvaK1、mvaK2和mvaD基因条带;用99A-F/99A-R为引物扩增合成番茄红素下游的3个基因,分别是idi、crtE和ispA基因条带,得到正确大小的片段后,回收片段,然后分别和骨架用摩尔比1︰1的连接体系,使用CPEC的PCR程序进行扩增,电转化将连接产物转入LYC103菌株,涂cam板(氯霉素平板),挑单克隆用引物99A-F和相应下游引物验证,验证正确的克隆接菌送测序,将测序正确的质粒分别命名为质粒pSC103-mvaE、pSC103-mvaS、pSC103-mvaK1、pSC103-mvaK2、pSC103-mvaD、pSC103-idi、pSC103-crtE和pSC103-ispA。构建菌株及所用质粒见表 1。
 |
| 图 2 CPEC构建质粒 Fig. 2 Construction of plasmid using CPEC. |
| 图选项 |
1.2.4 大肠杆菌转化方法根据文献制备电转化感受态细胞,转化质粒和条带,完成转化[35]。
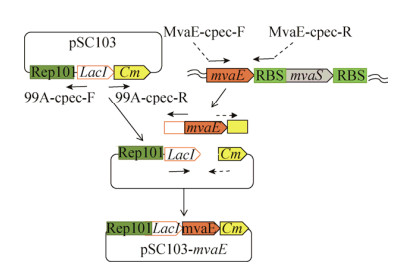
1.2.5 mvaK1启动子文库建立CRISPR/Cas9主要是利用靶点特异性的RNA (Guide RNA,gRNA)和Cas9核酸酶对特定基因位点进行切割导致突变。随着该技术的发展,已经成功在多个物种染色体上实现基因敲除、整合、突变等[5, 30, 31, 36]。Zhao等构建了一步法CRISPR/Cas9高效基因组编辑技术(CAGO),该方法可以在基因组任意位点实现非脱靶编辑。整个过程仅需要一个片段和一个通用pCAGO质粒,无需构建新的gRNA质粒[30]。本研究用CAGO技术,对mvaK1构建mRS(mRNA稳定区)启动子文库[37]。MvaST-594-I-F/MvaST-BsaI-CGCT-R引物,以LYC103菌株基因组为模板,扩增得500 bp左右条带,作为同源重组的上同源臂。设计简并引物MvaK1-mRSL-down (NNNNNNNNNNNNN NNNNN),本引物包括50 bp下同源臂片段、18个兼并碱基(mRS文库区)和扩增M1-P的20 bp同源区。以MvaK1-mRSL-down/Refull_BsaI_F为引物,以E. coli MG1655-?poxB基因组为模板,扩增约1.2 kb条带,该片段包括氯霉素抗性基因、Cas9识别序列、启动子文库和下游50 bp同源臂。两片段以等摩尔混合进行Golden Gate连接[38],连接后用于一步同源重组。将得到的重组条带,转化含带有pCAGO质粒的LYC103菌株,30 ℃、75 r/min复苏4 h,涂含有34 μg/mL氯霉素的LB平板,30 ℃培养过夜。随机挑选14个单克隆进行验证,验证引物为Mvas-1000-I-F/Mvak1-316-I-r,得到1.6 kb条带即为正确。挑选正确的菌株于37℃进行摇瓶发酵,测定番茄红素产量。将产量最高菌株按照Zhao的方法[30],去除cam抗性基因及多余序列,得到除启动子之外没有多余序列的菌株LYC104。LYC104菌株构建流程如图 3所示,其中L-short和mRS文库区属于M1-P的一部分。敲除marker后,在mvaK1基因前仅留M1-P启动子序列。
 |
| 图 3 LYC104构建流程图 Fig. 3 Construction of LYC104. |
| 图选项 |
1.2.6 CRISPR-Cas9技术辅助整合idi基因采用CRISPR-Cas9系统辅助整合idi基因[31]。首先构建质粒pLacZ-N20-trc-idi,以pLacZ-N20质粒为模板,用lacZ-B-BsaI-F2/lacZ-B-BsaI-R2为引物,PCR扩增骨架,以pSC103-idi质粒为模板,用Ptrc-BsaI-AGCT-F/BS-idi-BsaI-R为引物,扩增trc-idi条带,两片段以等摩尔混合进行Golden Gate连接,并加入DpnⅠ酶消化模板,然后将连接产物化转商品感受态Trans-T1,涂于含有34 μg/mL cam的LB平板,37 ℃培养过夜。用P15A-yz-up/99A-CPEC-R验证得到2 kb条带即为正确。在LYC104菌株中转化pRed_Cas9和pLacZ-N20-trc-idi质粒,阿拉伯糖来诱导cas9表达,切割染色体上LacZ的N20区域;pLacZ-N20-trc-idi提供上下同源臂、trc启动子和idi基因进行染色体整合。整合后用99A-F/ LacZ-yz-down引物进行验证,测序正确菌株命名为LYC105。验证引物见表 2。
表 2 本研究所用的引物[28]Table 2 Primers used in this work[28]
| Primers | Sequences (5′–3′) |
| 99A-cpec-F | TGTTTTGGCGGATGAGAGAA |
| 99A-cpec-R | GGTCTGTTTC CTGTGTGAAA TTG |
| MvaE-cpec-F | CAATTTCACA CAGGAAACAG ACC ATGAAAACAGTAGTTATTATTGATGCATT |
| MvaE-cpec-R | TTCTCTCATCCGCCAAAACATTATTGTTTTCTTAAATCAT TTAAAATAGC C |
| Ef-MvaS-cpec-F | CAATTTCACACAGGAAACAGACCATGACAATTGGGATTGATAAAATTAGT |
| Ef-MvaS-cpec-R | TTCTCTCATCCGCCAAAACATTAGTTTCGATAAGAGCGAACG |
| Sp-MK1-cpec-F | CAATTTCACA CAGGAAACAG ACC ATGACAAAAAAAGTTGGTGTCG |
| Sp-MK1-cpec-R | TTCTCTCATC CGCCAAAACA TTACAGGCTCTCTATCCATGTCTG |
| Sp-MvaK2-cpec-F | CAATTTCACACAGGAAACAGACCATGATTGCTGTTAAAACTTGCG |
| Sp-MvaK2-cpec-R | TTCTCTCATCCGCCAAAACATTACGATTTGTCGTCATGTCCTAT |
| Sp-MvaD-cpec-F | CAATTTCACACAGGAAACAGACCATGGATAGAGAGCCTGTAACAGTACG |
| Sp-MvaD-cpec-R | TTCTCTCATCCGCCAAAACATTAACAGCAATCATCTTGACTCAAAT |
| 99A-F | TTGCGCCGACATCATAAC |
| 99A-R | CTGCGTTCTGATTTAATCTG |
| MvaST-594-I-F | CCGTATCCTATGGTCGATGGT |
| MvaST-BsaI-AGCG-R | CCAGGTCTCAAGCGTTAGTTTCGATAAGAGCGAACGGTAT |
| MvaK1-mRSL-down | TCTTACTATGTGCCTGACCGACACCAACTTTTTTTGTCATAGCTGTTTCCTGTGTGAAAT (N18) GGCTCAATTATATCAACG |
| Refull_BsaI_F | CCAGGTCTCACGCTTTATCTCTGGCGGTGTTGACACTGGAGCAC |
| Mvak1-316-I-r | GAACGCAGGCTTCTGTGATA |
| lacZ-B-BsaI-F2 | CCAGGTCTCACCAG AATAACCGGGCAGGCCAT |
| lacZ-B-BsaI-R2 | CCAGGTCTCA AGCTGTTTCCTGTGTGAAATTGT |
| Ptrc-BsaI-AGCT-F | CCAGGTCTCA AGCT GGCATGCATTTACGTTGACA |
| BS-idi-BsaI-R | CCAGGTCTCACTGG TTATCGCACA CTATAGCTTG ATGTATT |
| P15A-yz-up | TTTATCTCTTCAAATGTAGCACCT |
| LacZ-yz-down | ATGGTGAACATGATGCCGACA |
表选项
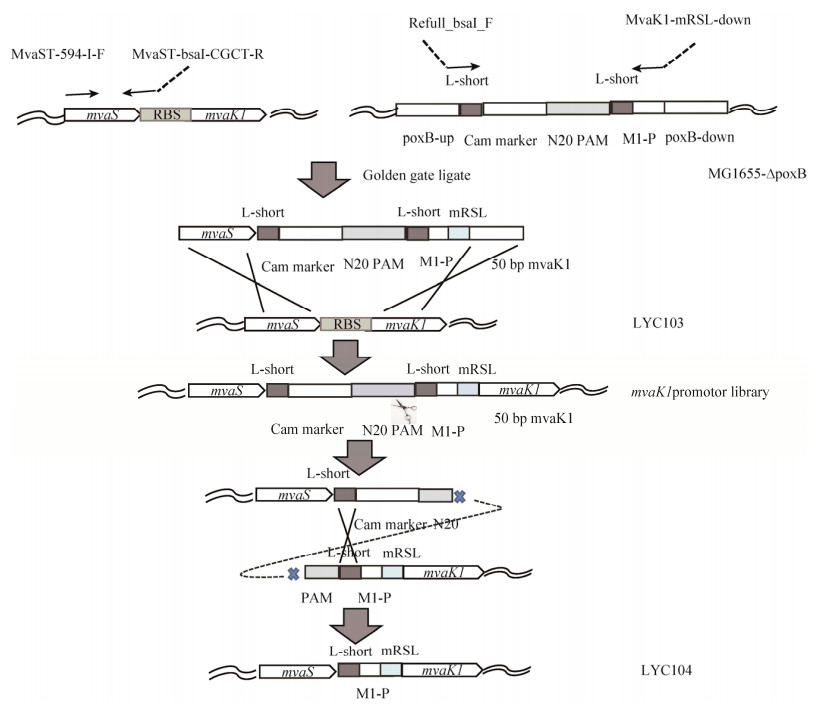
2 结果与分析2.1 单基因质粒构建通过将番茄红素合成基因过表达的形式来寻找番茄红素合成途径中的限速步骤。以pSC103质粒为模板扩增大小为5 kb的骨架,以LYC103菌株DNA基因组为模板,将mvaE、mvaS、mvaK1、mvaK2和mvaD基因以及idi、ispA和crtE基因进行PCR扩增,依次得到片段大小为2 412 bp、1 152 bp、879 bp、954 bp、1 011 bp、1 049 bp、900 bp和919 bp。核酸电泳图如图 4所示。采用CPEC方法将单个基因连接到中pSC103质粒上,然后电转化菌株LYC103,取阳性单克隆用99A-F和扩增相应基因的下游引物进行菌落PCR,验证正确连接质粒。将PCR验证正确质粒送样测序,测序正确的质粒分别依照表 1中的质粒名称进行命名。
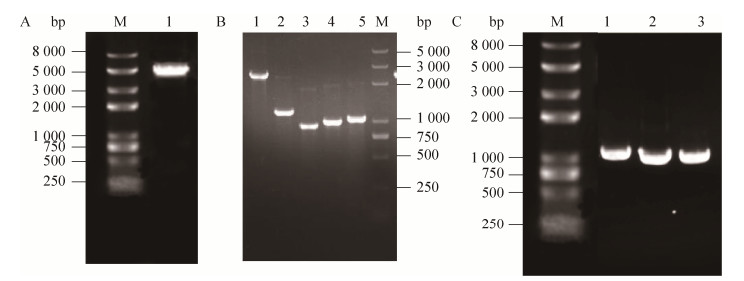 |
| 图 4 PCR验证单基因质粒构建 Fig. 4 Certification of single gene plasmid construction by PCR. (A) M: Trans 2000 plus Ⅱ; 1: Backbone. (B) M: Trans 2000 plus; 1: mvaE; 2: mvaS; 3: mvaK1; 4: mvaK2; 5: mvaD. (C) M: Trans 2000 plus Ⅱ; 1: idi; 2: ispA; 3: crtE. |
| 图选项 |
2.2 单基因过表达对番茄红素产量的影响将所构建的单基因质粒转入含有MVA途径的LYC103番茄红素菌株,发酵测定细胞生长和番茄红素产量,发酵结果如图 5所示;过表达mvaS番茄红素产量和单位细胞产率明显降低,分别是LYC103的51%和62%;过表达mvaE番茄红素产量和单位细胞产率有一定降低,说明当前条件下再表达MVA途径的前两个基因,可能会使MVA途径中间体物质积累,进一步影响类胡萝卜素的合成和细胞生长。
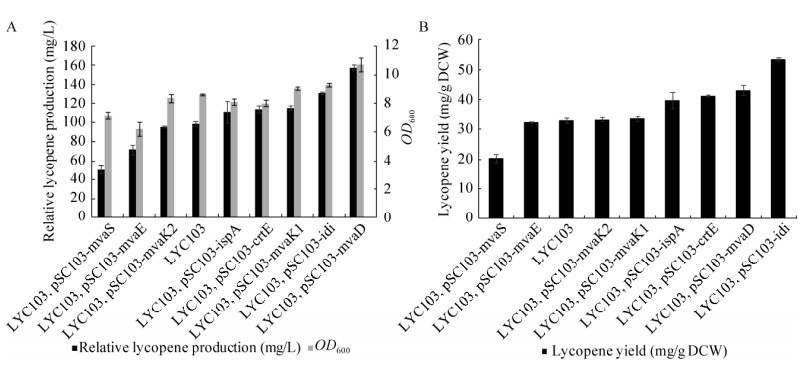 |
| 图 5 过表达单基因菌株的番茄红素产量 Fig. 5 Lycopene production by strains with single gene plasmids. |
| 图选项 |
质粒过表达ispA、crtE、mvaK1、idi和mvaD基因后,番茄红素产量相对于LYC103依次提高了13.5%、16.5%、17.95%、33.7%和61.1%。其中含有pSC103-idi质粒的番茄红素单细胞产量最高,从32.95 mg/g增加到53.52 mg/g DCW,是对照菌株的1.63倍。文献报道大肠杆菌引入MVA途径后,会因为MVA途径各基因表达不协调引起中间代谢物积累,包括甲羟戊酸及其多种衍生物的生成会产生细胞毒性,从而影响细胞生长[24-25]。过表达mvaK1、mvaK2、mvaD可以减少MVA等中间代谢物积累,促进细胞生长,因此本研究中质粒表达mvaK1、mvaK2、mvaD基因,菌株番茄红素单位细胞产量都有一定提高(图 5B);另外,MVA途径的引入同时也提高了前体物质IPP的积累,idi基因过量的表达来平衡IPP和DMAPP的量,ispA基因过量表达可以减少IPP积累和平衡外源MVA途径。促使下游产物的顺利合成,使番茄红素产量提高。
2.3 mvaK1-mRSL文库的构建基因以质粒形式在大肠杆菌中表达时会因为质粒不稳定影响番茄红素的产量,还会造成代谢负荷等弊端[39-40]。产番茄红素的重组大肠杆菌LYC103中,mvaK1、mvaK2、mvaD三个基因在同一操纵子上,用启动子的mRNA稳定区(mRS)文库调控mvaK1,相当于对3个基因同时调控。本研究采用CAGO技术一步同源重组方法在染色体上直接对mvaK1基因进行调控,在mRNA稳定区设计简并引物构建mRS文库。
首先准备重组片段。图 6A中1、2泳道为重组片段的上同源臂的PCR验证条带,3、4泳道为重组片段中下同源臂、cam抗性基因、gRNA-PAM的PCR验证条带。用Golden Gate方法将两片段以等摩尔混合,用T4 DNA连接酶和BsaⅠ酶切连接。得到约1.7 kb片段(见图 6B),直接转化带有pCAGO质粒的LYC103感受态,在含有amp和cam抗生素的LB固体平板上筛选。然后随机选单克隆用cat-up/ Mvak1-316-I-r进行PCR验证,得到约1.6 kb的条带为正确克隆,部分PCR验证电泳结果见图 6C,挑正确的单克隆接菌发酵。
 |
| 图 6 mvaK1-mRSL文库的构建及验证 Fig. 6 Construction and certification of mvak1-mRSL library. (A) Certification of the fragments for preparing recombinant sequence. (B) Certification of recombinant sequence. (C) Certification of the stains after recombination. |
| 图选项 |
2.4 mvaK1启动子文库建立对番茄红素产量的影响随机选取14株在mRNA稳定区文库调控mvaK1基因正确的菌株进行发酵,结果见图 7。调控mvaK1基因后,番茄红素产量为56.08– 196.07 mg/L,单位细胞产量为25.35–73.69 mg/g。其中番茄红素产量最高的菌株是LYC103- mvaK1-7,番茄红素产量是对照的2倍;OD600从8.65提高到11.38,提高了32%,mvaK1基因的调控使细胞生长变快,减少了中间产物的积累和细胞毒性。
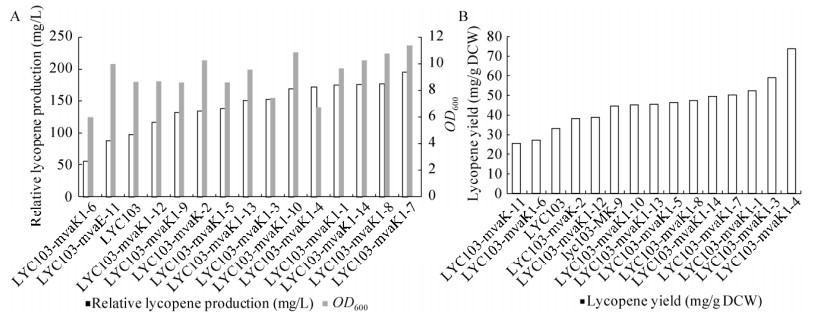 |
| 图 7 mvaK1启动子文库对番茄红素产量的影响 Fig. 7 Effect of mvaK1 promoter library on lycopene yield. |
| 图选项 |
mvaK1、mvaK2、mvaD三个基因的协调表达提高了前体物质IPP和DMAPP的供应,使番茄红素产量得到进一步的提高。
本研究采用文献所述方法消除LYC103-mvaK1-7中cam抗性基因,得到不含任何抗生素的标记的菌株,命名为LYC104。
2.5 单基因质粒转化LYC104菌株对番茄红素产量的影响LYC104中mvaK1、mvaK2、mvaD经过了文库调控后,番茄红素产量得到提高,验证了mvaK1、mvaK2、mvaD为当前菌株的限速步骤并消除限速。为了研究在LYC104中crtE、idi和ispA基因是否同样为限速步骤,本研究在LYC104中用单基因质粒过表达crtE、idi和ispA基因,结果如图 8所示。添加pSC103-crtE和pSC103-ispA后,番茄红素产量和LYC104相比没有明显差别(P > 0.05),而添加pSC103-idi质粒后番茄红素菌株细胞生长最好、番茄红素产量最高。细胞OD600是LYC104的1.15倍,番茄红素产量提高了34.4%,说明在建库调控的番茄红素高产菌株LYC104中,idi依然是限速步骤。因为LYC104中MVA途径得到进一步优化表达,而使MVA途径催化乙酰辅酶A转化为IPP增加,需要继续高表达idi基因来平衡两者之间的转化。
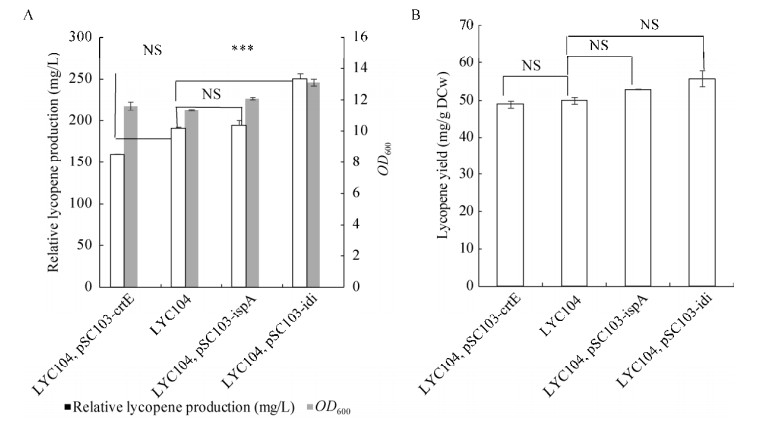 |
| 图 8 单基因质粒转化LYC104菌株对番茄红素产量的影响 Fig. 8 Effect of single gene plasmid transformed LYC104 strain on lycopene production. NS: not significant; *** significant difference. |
| 图选项 |
2.6 调控idi基因对番茄红素产量的影响为了稳定表达idi基因,本实验CRISPR-Cas9基因编辑技术在LYC104菌株的LacZ位点对idi基因进行染色体整合,验证测序正确后,将该菌株命名为LYC105。发酵结果见表 3,与出发菌株LYC103相比,LYC105的细胞生长提高了147%,番茄红素产量增加了2.28倍;与对照菌株LYC104相比,LYC105的细胞生长提高了90.1%,番茄红素产量增加了1.95倍。结果见表 3。
表 3 调控idi基因对番茄红素产量的影响Table 3 Effect of regulating idi gene on lycopene yield
| Strains | OD600 | Dry cell weight (g/L) | Relative lycopene production (mg/L) | Increase of lycopene yield |
| LYC103 | 8.66±0.07 | 3.25±0.06 | 97.93±2.61 | 1.00 |
| LYC104 | 11.37±0.05 | 4.26±0.17 | 191.74±1.15 | 1.95 |
| LYC105 | 21.40±0.08 | 5.65±0.03 | 223.12±4.75 | 2.28 |
表选项
3 结论本研究在染色体含有MVA和MEP两个途径的重组番茄红素菌株LYC103基础上,通过以单质粒表达的形式将MVA途径中的mvaS、mvaE、mvaK1、mvaK2和mvaD五个基因以及番茄红素合成途径下游的idi、ispA和crtE三个基因进行过表达,系统研究了各基因表达对番茄红素产量和细胞生长的影响。结果表明,限制番茄红素产量和细胞生长的主要因素是前体供应不足和代谢过程中有毒中间体的过度积累,MVA途径后半部分基因和idi基因高表达,能明显提高番茄红素产量和细胞生长。与之前研究结果类似[41-42]。
本研究中mvaK1、mvaK2、mvaD三个基因在同一个操纵子中且位置相邻,对mvaK1基因进行调控,相当于对后面两个基因也进行了调控。本研究对mvaK1基因的mRNA稳定区进行了启动子文库的调控,其番茄红素产量相比对照菌株LYC103增加了2倍,细胞生长提高了32%。调控mvaK1、mvaK2、mvaD基因后,可以减少有毒中间物质HMG1-CoA的积累,从而增加细胞生长和番茄红素的产量。
添加MVA途径并协调表达,进一步增加了IPP的合成,文献报道大量积累IPP,会产生细胞毒性,从而影响细胞生长和萜类物质的合成[43-44],在重组番茄红素菌株LYC104基础上对idi基因进行染色体整合,来解决前体物质IPP和DMAPP供应不平衡等问题,番茄红素产量增加了127%。与以前文献报道完全用质粒表达MVA途径基因相比[19-23],本研究所得菌株LYC105性状更稳定,添加单个基因研究限速步骤的方法更简单,为重组大肠杆菌表达多个外源基因的研究提供新思路。
参考文献
| [1] | Wang F, Jiang JG, Chen Q. Progress on molecular breeding and metabolic engineering of biosynthesis pathways of C30, C35, C40, C45, C50 carotenoids. Biotechnol Adv, 2007, 25(3): 211-222. DOI:10.1016/j.biotechadv.2006.12.001 |
| [2] | Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys, 1989, 274(2): 532-538. DOI:10.1016/0003-9861(89)90467-0 |
| [3] | Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal, 2008, 10(3): 475-510. DOI:10.1089/ars.2007.1740 |
| [4] | Seren S, Lieberman R, Bayraktar UD, et al. Lycopene in cancer prevention and treatment. Am J Ther, 2008, 15(1): 66-81. DOI:10.1097/MJT.0b013e31804c7120 |
| [5] | Li ZX, Chen QQ, Tang JL, et al. Integrating balanced mevalonate pathway into chromosome for improving lycopene production in Escherichia coli. Chin J Biotech, 2019, 35(3): 404-414 (in Chinese). 李贞霞, 陈倩倩, 唐金磊, 等. 稳定表达MVA途径基因提高番茄红素产量. 生物工程学报, 2019, 35(3): 404-414. |
| [6] | Lv XM, Wang F, Zhou PP, et al. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat Commun, 2016, 7: 12851. DOI:10.1038/ncomms12851 |
| [7] | Zebec Z, Wilkes J, Jervis AJ, et al. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr Opin Chem Biol, 2016, 34: 37-43. DOI:10.1016/j.cbpa.2016.06.002 |
| [8] | Misawa N, Nakagawa M, Kobayashi K, et al. Elucidation of the erwinia-uredovora carotenoid biosynthetic-pathway by functional-analysis of gene-products expressed in Escherichia coli. J Bacteriol, 1990, 172(12): 6704-6712. DOI:10.1128/JB.172.12.6704-6712.1990 |
| [9] | Jin YS, Stephanopoulos G. Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab Eng, 2007, 9(4): 337-347. |
| [10] | Wang CL, Maratukulam PD, Lum AM, et al. Metabolic engineering of an aerobic sulfate reduction pathway and its application to precipitation of cadmium on the cell surface. Appl Environ Microbiol, 2000, 66(10): 4497-4502. DOI:10.1128/AEM.66.10.4497-4502.2000 |
| [11] | Farmer WR, Liao JC. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol, 2000, 18(5): 533-537. DOI:10.1038/75398 |
| [12] | Matthews PD, Wurtzel ET. Metabolic engineering of carotenoid accumulation in Escherichia coli by modulation of the isoprenoid precursor pool with expression of deoxyxylulose phosphate synthase. Appl Microbiol Biotechnol, 2000, 53(4): 396-400. DOI:10.1007/s002530051632 |
| [13] | Li QY, Fan FY, Gao X, et al. Balanced activation of IspG and IspH to eliminate MEP intermediate accumulation and improve isoprenoids production in Escherichia coli. Metab Eng, 2017, 44: 13-21. DOI:10.1016/j.ymben.2017.08.005 |
| [14] | Alper H, Miyaoku K, Stephanopoulos G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat Biotechnol, 2005, 23(5): 612-616. DOI:10.1038/nbt1083 |
| [15] | Alper H, Jin YS, Moxley JF, et al. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng, 2005, 7(3): 155-164. |
| [16] | Choi HS, Lee SY, Kim TY, et al. In silico identification of gene amplification targets for improvement of lycopene production. Appl Environ Microbiol, 2010, 76(10): 3097-3105. DOI:10.1128/AEM.00115-10 |
| [17] | Farmer WR, Liao JC. Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog, 2001, 17(1): 57-61. |
| [18] | Zhao J, Li QY, Sun T, et al. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab Eng, 2013, 17: 42-50. DOI:10.1016/j.ymben.2013.02.002 |
| [19] | Vadali RV, Fu YC, Bennett GN, et al. Enhanced lycopene productivity by manipulation of carbon flow to isopentenyl diphosphate in Escherichia coli. Biotechnol Prog, 2005, 21(5): 1558-1561. DOI:10.1021/bp050124l |
| [20] | Yoon SH, Park HM, Kim JE, et al. Increased beta-carotene production in recombinant Escherichia coli harboring an engineered isoprenoid precursor pathway with mevalonate addition. Biotechnol Prog, 2007, 23(3): 599-605. |
| [21] | Yoon SH, Lee YM, Kim JE, et al. Enhanced lycopene production in Escherichia coli engineered to synthesize isopentenyl diphosphate and dimethylallyl diphosphate from mevalonate. Biotechnol Bioeng, 2006, 94(6): 1025-1032. DOI:10.1002/bit.20912 |
| [22] | Ye LJ, He P, Li QY, et al. Type Ⅱs restriction based combinatory modulation technique for metabolic pathway optimization. Microb Cell Fact, 2017, 16: 47. DOI:10.1186/s12934-017-0659-z |
| [23] | Ye LJ, Zhang CZ, Bi CH, et al. Combinatory optimization of chromosomal integrated mevalonate pathway for β-carotene production in Escherichia coli. Microb Cell Fact, 2016, 15: 202. DOI:10.1186/s12934-016-0607-3 |
| [24] | Anthony JR, Anthony LC, Nowroozi F, et al. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the anti-malarial drug precursor amorpha-4, 11-diene. Metab Eng, 2009, 11(1): 13-19. |
| [25] | Pitera DJ, Paddon CJ, Newman JD, et al. Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab Eng, 2007, 9(2): 193-207. |
| [26] | Feng F, Xu Y, Tao Y, et al. Improving isoprene production by engineered heterologous mevalonate pathway in Escherichia coli. Chin J Biotech, 2015, 31(7): 1073-1081 (in Chinese). 冯凡, 许杨, 陶勇, 等. 提高大肠杆菌通过MVA途径合成异戊二烯. 生物工程学报, 2015, 31(7): 1073-1081. |
| [27] | Wei YL, Mohsin A, Hong Q, et al. Enhanced production of biosynthesized lycopene via heterogenous MVA pathway based on chromosomal multiple position integration strategy plus plasmid systems in Escherichia coli. Bioresour Technol, 2018, 250: 382-389. DOI:10.1016/j.biortech.2017.11.035 |
| [28] | Chen QQ. Study and regulation of lycopene synthesis gene in Escherichia coli[D]. Xinxiang: Henan Institute of Science and Technology, 2019 (in Chinese). 陈倩倩.番茄红素合成基因在大肠杆菌中的调控研究[D].新乡: 河南科技学院, 2019. |
| [29] | Sun T, Miao LT, Li QY, et al. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol Lett, 2014, 36(7): 1515-1522. DOI:10.1007/s10529-014-1543-0 |
| [30] | Zhao DD, Feng X, Zhu XN, et al. CRISPR/Cas9-assisted gRNA-free one-step genome editing with no sequence limitations and improved targeting efficiency. Sci Rep, 2017, 7: 16624. DOI:10.1038/s41598-017-16998-8 |
| [31] | Zhao DD, Yuan SL, Xiong B, et al. Development of a fast and easy method for Escherichia coli genome editing with CRISPR/Cas9. Microb Cell Fact, 2016, 15: 205. DOI:10.1186/s12934-016-0605-5 |
| [32] | Dong Y, Hu KL, Li XL, et al. Improving β-carotene production in Escherichia coli by metabolic engineering of glycerol utilization pathway. Chin J Biotech, 2017, 33(2): 247-260 (in Chinese). 董悦, 胡坤乐, 李兴林, 等. 代谢工程改造甘油代谢途径提高β-胡萝卜素产量. 生物工程学报, 2017, 33(2): 247-260. |
| [33] | Zhao J, Liu Y, Li QY, et al. Modulation of isoprenoid gene expression with multiple regulatory parts for improved β-carotene production. Chin J Biotech, 2013, 29(1): 41-55 (in Chinese). 赵婧, 刘怡, 李清艳, 等. 多个调控元件调控萜类合成途径基因表达提高β-胡萝卜素的生产. 生物工程学报, 2013, 29(1): 41-55. |
| [34] | Quan JY, Tian JD. Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nat Protoc, 2011, 6(2): 242-251. DOI:10.1038/nprot.2010.181 |
| [35] | Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Huang PT, trans. 3rd ed. Beijing: Science Press, 2002: 99-102 (in Chinese). 萨姆布鲁克J, 拉塞尔DW.分子克隆实验指南.黄培堂, 译.第3版.北京: 科学出版社, 2002: 99-102. |
| [36] | Ma YW, Zhang LF, Huang XX. Genome modification by CRISPR/Cas9. FEBS J, 2014, 281(23): 5186-5193. DOI:10.1111/febs.13110 |
| [37] | Lu J, Tang JL, Liu Y, et al. Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol, 2012, 93(6): 2455-2462. DOI:10.1007/s00253-011-3752-y |
| [38] | Engler C, Marillonnet S. Generation of families of construct variants using golden gate shuffling//Lu C, Browse J, Wallis J, eds. cDNA Libraries. Totowa, NJ: Humana Press, 2011, 729: 167-181. |
| [39] | Yoon SH, Lee SH, Das A, et al. Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli. J Biotechnol, 2009, 140(3/4): 218-226. |
| [40] | Keasling JD. Synthetic biology for synthetic chemistry. ACS Chem Biol, 2008, 3(1): 64-76. |
| [41] | Sandmann G, Albrecht M, Schnurr G, et al. The biotechnological potential and design of novel carotenoids by gene combination in Escherichia coli. Trends Biotechnol, 1999, 17(6): 233-237. DOI:10.1016/S0167-7799(99)01307-4 |
| [42] | Klein-Marcuschamer D, Ajikumar PK, Stephanopoulos G. Engineering microbial cell factories for biosynthesis of isoprenoid molecules: beyond lycopene. Trends Biotechnol, 2007, 25(9): 417-424. DOI:10.1016/j.tibtech.2007.07.006 |
| [43] | Martin VJJ, Pitera DJ, Withers ST, et al. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol, 2003, 21(7): 796-802. DOI:10.1038/nbt833 |
| [44] | Sivy TL, Fall R, Rosenstiel TN. Evidence of isoprenoid precursor toxicity in Bacillus subtilis. Biosci Biotechnol Biochem, 2011, 75(12): 2376-2383. DOI:10.1271/bbb.110572 |
