

西南大学 家蚕基因组生物学国家重点实验室, 重庆 400716
收稿日期:2017-12-15;接收日期:2018-03-14; 网络出版时间:2018-03-19 基金项目:国家自然科学基金(No.81672502), 重庆高校创新团队建设计划(No.CXTDX201601010)资助
摘要:神经导向因子Netrins作为分泌蛋白与膜结合蛋白, 在神经细胞迁移、分化及凋亡等生物学过程中发挥重要的作用。自发现以来, Netrins被认为负责指导中枢神经系统组织形态的发育, 随后发现它也广泛参与一些非神经组织的黏附、迁移和分化等生理过程, 如血管生成、淋巴管形成和炎症等。最近的研究表明, Netrins也参与调控肿瘤的发生与发展, 在结直肠癌、胰腺导管腺癌等多种肿瘤组织中发挥重要的调控作用。由于Netrins的功能多样性且通过结合不同受体在肿瘤组织中发挥不同的生物学效应, 其在肿瘤中的具体作用机制尚不清楚。文中结合课题组目前的实验研究进展, 阐述了近年来关于Netrins的研究, 特别是在肿瘤领域取得的最新研究结果, 总结了其作用机制, 并对其研究及应用前景进行了展望。
关键词:Netrin-1 Netrin-4 肿瘤 血管生成 凋亡 靶向治疗
Roles and mechanisms of netrins in cancer development
Jie Yang, Zhen Dong, Hongjuan Cui


State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400716, China
Received: December 15, 2017; Accepted: March 14, 2018; Published: March 19, 2018
Supported by: National Natural Science Foundation of China (No.81672502), Chongqing University Innovation Team Building Program Funded Projects (No.CXTDX201601010)
Corresponding author:Hongjuan Cui.Tel:+86-23-68251713; Fax:+86-23-68251128; E-mail:hongjuan.cui@gmail.com, hcui@swu.edu.cn
Abstract: As secreted protein and membrane-bound proteins, netrins play important roles in biological processes such as neural cell migration, differentiation and apoptosis.Netrins were thought to guide axon outgrowth and central nervous system morphology since they were discovered.Besides, they also participate in physiological processes such as cell adhesion, migration, differentiation, angiogenesis, lymph angiogenesis, and inflammation in non-neural tissues.Recent studies showed that netrins also involved in the regulation of initiation and development of various tumors including colorectal cancer, pancreatic ductal adenocarcinoma.Because of the functional diversity of netrins and the different biological effects of different receptors in tumor tissue, the specific mechanism of their action in tumors remains unclear.Based on current research progress of our group, this review summed up recent research findings on netrins in relation to cancer biology, suggested possible mechanisms, and discussed the implications in cancer research and intervention.
Key words: Netrin-1 Netrin-4 tumor angiogenesis apoptosis targeted therapy
随着近几年分子生物学、细胞生物学及表观遗传学等研究技术的不断发展,各种生命活动和病理进程的分子机制逐渐被揭开。Netrins作为一类自分泌蛋白或者膜结合蛋白,可以通过结合不同类型受体发挥不同的作用。它们最初被鉴定为参与中枢神经系统的轴索指导,负责神经细胞的迁移、分化和凋亡,可降低促炎介质的血清水平,并稳定血脑屏障,限制免疫细胞进入中枢神经系统[1]。除此之外,Netrins[2]也参与介导非神经组织的黏附、迁移和分化等生理过程。目前的研究表明Netrins信号通路与重度抑郁症[3]、阿尔兹海默症[4]、心脏肥大和心力衰竭[5]、肥胖症[6]等疾病均有密切联系。特别是,Netrins家族成员促进了多种肿瘤的进展,因此,进一步探索Netrins及其受体在肿瘤中的作用机制及防治意义成为在预防、治疗肿瘤相关机制研究方面的热点,并受到广大科研****的青睐。文中就Netrins在肿瘤中的最新研究成果,对Netrins在肿瘤中的研究进展进行总结,希望对其在肿瘤临床、靶向治疗的研发提供一定帮助。
1 Netrins及其受体简介神经导向因子Netrins家族是一类与神经轴突指导相关的蛋白,其结构类似于层粘连蛋白。1990年,Netrins家族UNC6第一次在秀丽隐杆线虫Caenorhabditis elegans中被发现,证明其与神经轴突导向及细胞迁移相关[7]。目前已识别出5个自分泌蛋白Netrin-1?5[8]和两个膜结合蛋白NetrinG1、G2[9]。1994年,在脊椎动物中,UNC6的同源物Netrin-1被发现[10]。人类的Netrin-1基因定位于17p13.1,编码604个氨基酸大约67.7 kDa;Netrin-2目前发现在鸟类和斑马鱼中表达,而Netrin-3则是Netrin-2在哺乳动物的同源基因,定位于16p13.3,编码580个氨基酸大约61 kDa;人类Netrin-4[11]是中枢神经系统中参与突起生长和迁移的自分泌蛋白,基因定位于12q22-q23,编码629个氨基酸蛋白大约70-84 kDa。如图 1所示,它们大多是具有N-末端层粘连蛋白结构域(包含3个EGF重复序列)、中枢层粘连蛋白结构域和C末端结构域(NTR)的层粘连相关蛋白[12]。而新发现的家庭成员Netrin-5[13]则缺乏N末端结构域和上皮生长因子(Epidermal growth factor,EGF)重复序列。另外Netrin-GS[14]在其C末端具有糖基磷脂酰基醇锚(Glycosylphosphatidylinositol,GPI),并且通过结合糖基磷脂酰基醇实现细胞膜的结合,从而发挥其功能。
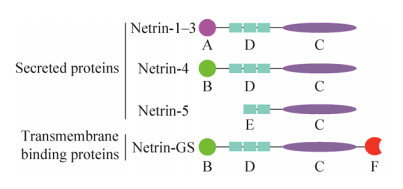 |
| 图 1 Netrins的结构示意图(改自Ylivinkka等[15]) Figure 1 Schematic illustration of the structure of netrins (adapted from Ylivinkka et al[15]). Different netrin domains are marked as follows: A: N-terminal domain related to laminin γ-chain; B: N-terminal domain related to laminin-chain; C: NTR domain; D: 3 EGF repeats; E: 2 EGF repeats; F: GPI-anchor protein. |
| 图选项 |
Netrins的功能与两类受体相关,一类是结直肠癌缺失蛋白(Deleted in colon cancer,DCC)[16]家族及其同系物再生蛋白(Neogenin)[17];另一类是UNC5S (Uncoordinated phenotype-5S)[18]家族和最新发现的受体唐氏综合症细胞黏附分子(Down syndrome cell adhesion molecule,DSCAM)[19]、腺苷A2b (Adenosine receptor A2b,A2b)[20]受体和整合素(Integrin)[21]。DCC和Neogenin介导Netrin-1诱导轴突化学吸引和排斥[22],在DCC受体缺乏的情况下,UNC5家族的4个成员被证明介导Netrin-1诱导的轴突化学排斥[12, 23]。
2 Netrins与肿瘤近年来,越来越多的研究表明,Netrins在肿瘤的发生发展中也起着至关重要的作用,其作用形式也是一个复杂的生物学过程,这一过程中受到众多肿瘤明星分子及多种信号通路的调节,如表 1所示。Netrins家族成员在肿瘤中的作用也不尽相同,绝大多数证据表明其具有促癌作用。比如,Netrin-1在结肠直肠癌、神经母细胞瘤、胶质母细胞瘤和转移性乳腺癌等肿瘤中均表达上调,并且发现Netrin-1是癌细胞生存侵袭的促进因子[15]。这一角色是Netrins通过结合一系列受体介导的,包括UNC5S和DCC家族。比如,Netrin-1可以通过结合受体DCC家族和UNC5家族直接与肿瘤细胞凋亡和血管再生相关,而且在某些肿瘤组织中促进了癌基因的表达[24]。
表 1 Netrins在不同肿瘤中的表达情况、功能及其介导的下游信号通路Table 1 Summary of the netrins expression, suggested functions and novel pathways in different cancers
| Cancer type | Members | Expression | Dependence receptors | Signal pathways | Effect | References |
| Colorectal cancer | NTN1 NTN4 | ↑ ↓ | DCC/UNC5C Neogenin | Hippo VEGF/cyclooxygenase-2 | Proliferation Migration Metastasis Angiogenesis | [33] [36] [37] |
| Pancreatic cancer | NTN1 | ↑↓ | UNC5S | -p53/MDM2 FAK Mek/Erk/c-Jun | Proliferation Migration | [42] [43] [44] |
| Gastric cancer | NTN1 NTN4 | ↑ ↑ | Neogenin | Jak/Stat PI3K/Akt Erk/MAPK DR’s aberrant methylation | Proliferation Migration | [47] [48] |
| Breast cancer | NTN1 NTN4 | ↑ ↑↓ | DCC/UNC5S α6β1 | DAPK Rho GTPases Src/FAK | Migration Metastasis Angiogenesis Lymphangiogenesis | [59] [64] [66] |
| Ovarian cancer | NTN1 | ↑ | DCC/UNC5S | Proliferation Migration Angiogenesis | ||
| Glioblastoma | NTN1 NTN4 | ↑ ↑ | UNC5A ITGB4 | Notch NF-κB Akt-mToR Erk/MAPK | Proliferation Migration Angiogenesis | [72] [75] [77] [78] |
| Neuroblastoma | NTN1 NTN4 | ↑ ↑ | DCC/UNC5S Neogenin | Rho GTPases Hedgehog | Migration Metastasis | [81-82] [86-90] |
| “↑” stand for increased in this tumor; “↓” stand for reduced in this tumor. | ||||||
表选项
DCC和UNC5S属于依赖性受体,都具有所谓的死亡结构域[25]。当受体不与Netrins结合时,死亡结构域暴露和Caspase-9结合,导致Caspase-3被激活促进细胞凋亡[26]。这类受体通过细胞内信号诱导肿瘤细胞凋亡,包括结直肠癌、乳腺癌、卵巢癌、宫颈癌、胃癌、肺癌或肾癌等多种癌症,这种特征赋予这些受体肿瘤抑制活性,所以这一类受体也被视为抑癌因子,在肿瘤的发生发展中扮演了重要的角色。而当Netrins存在时,受体指导细胞存活、迁移和增殖,这可能与Netrins引发的受体DCC和UNC5S多聚化相关[27]。并且,UNC5B的表达由p53直接调节,在没有Netrins的情况下,UNC5B介导p53依赖性途径诱导细胞凋亡。相反,在Netrin-1的存在下,Netrin-1和DCC/UNC5S之间相互作用激活的信号通路抑制p53诱导的细胞凋亡,同时防止Caspase-3级联激活,导致抗凋亡能力增强[28],如图 2所示。另一方面,有研究显示Netrin-4在结直肠癌[29]、肝癌[30]中也扮演着抑癌因子的角色,但目前还不是很清楚Netrins及其受体在不同肿瘤中引起差异的原因,Netrins及其受体在肿瘤的发展过程中扮演的精确角色还有待进一步研究。
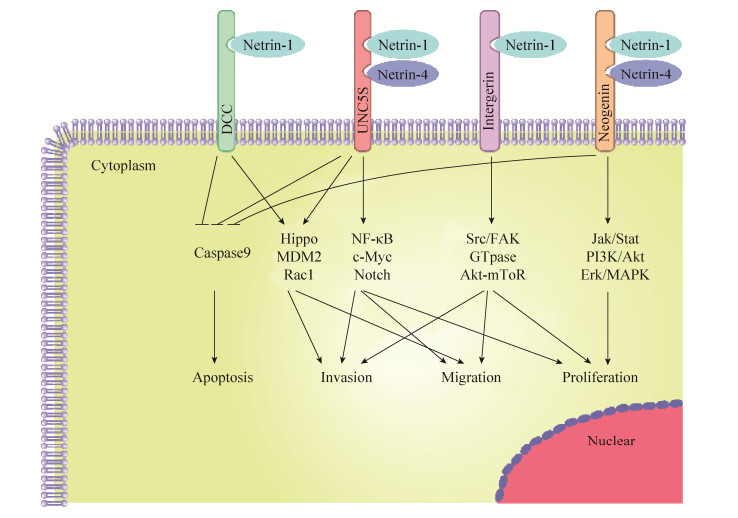 |
| 图 2 Netrins及其受体在肿瘤中发生发展中的作用机制(改自Kefeli等[24],Arakawa[28]) Figure 2 Diagram of the mechanisms of netrins and its receptors in tumor initiation and progression (adapted from Kefeli et al[24], Arakawa[28]). |
| 图选项 |
因此,研究Netrins及其受体在肿瘤发生发展中的作用机制及其调控意义对人类肿瘤的治疗具有重要意义,关注Netrins在癌症、肿瘤细胞发生发展过程中的生物学作用已成为焦点。由于肿瘤是一类疾病的总称,不同肿瘤的遗传背景差异很大。因此,下面将从Netrins在不同肿瘤中的研究现状进行总结。
2.1 Netrins与结直肠癌结直肠癌(Colorectal cancer,CRC)是严重影响人类健康的消化系统恶性肿瘤之一,具有较高的发病率、死亡率[31]。
在对结直肠癌生物学研究过程中发现Netrin-1的受体DCC、UNC5C在结直肠癌及其他肿瘤组织中高频缺失,暗示Netrin-1受体缺失途径在癌症中的重要性。而Netrin-1通过抑制细胞凋亡,在结直肠癌发生过程中发挥着促癌作用[32],通过其跨膜受体DCC和UNC5S参与Hippo信号通路的调节[33],使细胞核中的YAP水平升高,增强YAP的信号积累,从而促进癌细胞增殖和迁移。此外,在肿瘤发生期间,Netrin-1受体分子改变的时间并不是随机的,UNC5C的失活发生在肿瘤早期,而DCC缺失发生在多级结肠直肠癌的后期阶段[34]。但与野生型UNC5C相比,突变体UNC5C- A628K却能减少细胞凋亡,促进肿瘤恶化[35],因此,探究Netrin-1和受体之间的作用对治疗结直肠癌具有潜在意义。
除了Netrin-1外,Netrin-4与结直肠癌的关系也被逐步确定。Xu等研究发现,在结直肠癌肝转移中发现血管内皮生长因子(Vascular endothelial cell growth factor,VEGF)和选择素(E-selectin)上调,而Netrin-4和LK-68则被抑制[36],表明Netrin-4与肿瘤血管生成有着密切联系。Eveno等进一步研究发现,Netrin-4可以通过与其受体Neogenin结合而表现出抗血管生成活性,但Netrin-4不影响体外VEGF诱导的血管通透性急性增加[37]。此外,通过比对原发性结直肠癌和肝、肺部转移的肿瘤,发现过表达Netrin-4既能抑制异种移植的原发肿瘤生长,也能显著降低结直肠癌肝转移模型肿瘤数量和肝转移体积[29],表明Netrin-4能降低结直肠癌肺转移。这些结果表明Netrin-4具有抑制结直肠癌生长和主要转移部位的潜力。
2.2 Netrins与胰腺导管腺癌胰腺导管腺癌(Pancreatic ductal adenocarcinoma,PDAC)具有发病隐匿、进展迅速、预后极差等特点,素有癌中之王之称[38]。近年来,研究人员发现Netrin-1也在胰腺导管腺癌的进展中发挥着重要的作用。
Ramesh[39]和Yildirim[40]等发现Netrin-1在胰腺癌、乳腺癌、前列腺癌、肝癌等肿瘤组织中高表达,而且,在胰腺癌患者中,没有表达或者很少表达Netrin-1的中位生存期为10个月,而中等强度或者高表达Netrin-1的患者中位生存期仅仅为4个月[41]。除此之外,Netrin-1及其受体UNC5H3表达对患有分化不良的肿瘤患者的总生存率有显著影响[41]。进一步研究发现,Netrin-1在体外赋予肿瘤和内皮细胞凋亡抗性[42],为肿瘤细胞提供粘附底物,诱导其侵袭,其可能机制是Netrin-1通过MDM2 (Murine double minute 2)下调p53的表达,促进肿瘤细胞体外增殖[43]。但也有报道显示,Netrin-1在体内可以抑制胰腺导管腺癌细胞的生长,其与受体UNC5B相互作用激活黏着斑激酶(Focal adhesion kinase,FAK),刺激一氧化氮(Nitric oxide,NO)的产生,增强蛋白磷酸酶(Protein phosphatase 2A,PP2A)的表达,抑制Mek/Erk/c-Jun信号通路进而下调ITGB4的表达,从而抑制胰腺导管腺癌的生长[44]。这可能是不同遗传背景下的胰腺癌中Netrin-1介导的不同受体有关,其具体的效用和机制还需要进一步评价。
2.3 Netrins和胃癌胃癌是一种严重威胁人类健康的恶性肿瘤之一,其发病率位居第四,死亡率位居第二[45]。研究发现Netrins在人类胃癌细胞组织及病人血清中表达上调,暗示Netrins可能与胃癌相关[46]。进一步的研究发现,Netrin-1[47]和Netrin-4[48]通过其受体Neogenin引起致瘤途径(Jak/Stat、PI3K/Akt和Erk/MAPK)的激活,从而促进胃癌细胞的增殖和侵袭能力;当敲低Netrins及其受体能显著抑制胃癌细胞增殖和侵袭,同时引起Stat3、Erk、Akt和p38的磷酸化水平降低。除此之外,同结直肠癌类似,Netrins受体缺陷也是胃癌的常见特征,并且在不同时期,受体表达量具有明显差异。在胃癌发生的早期进程中,DCC和UNC5C基因异常甲基化[49-50],但在晚期胃癌中这种现象又逐渐消失,并且Netrin-1浓度升高[51],表明Netrin-1受体的累积变化是胃癌进展的晚期事件。在晚期的胃癌中,这种动态的变化也许有另外的因子参与了重要调节,导致受体的失活,具体的机制还需要进一步研究。
2.4 Netrins与乳腺癌乳腺癌是世界各地妇女癌症死亡的主要原因,在我国,乳腺癌是女性中发病率第一、致死率第六的恶性肿瘤,并且近年来发病率逐渐上升[52]。而肿瘤转移是乳腺癌患者死亡的主要原因,通常在多个部位发生[53],常见于肺[54]、肝[55]、脑[56]和骨[57]等。
在研究乳腺癌转移的机制过程中,发现与人类非转移性乳腺癌不同的是,大部分转移性乳腺癌高表达Netrin-1[58]。在Netrin-1高表达的乳腺转移性肿瘤细胞中,降低Netrin-1表达或者加入其可溶性受体会发生凋亡[32]。因此大部分人转移性乳腺癌中,高表达的Netrin-1具有肿瘤细胞存活的选择性优势。在针对乳腺癌的治疗研究中,对于Netrin-1靶点治疗研究表明,Netrin-1的沉默、干扰Netrin-1/DRs (Dependence receptors)的相互作用、地西他滨和抗Netrin-1中和抗体的组合可以有效地减少不同的肿瘤生长和转移[25, 32, 59-62]。而在非转移性的乳腺癌中,显示Netrin-1和DAPK1依赖DNA甲基化表达的缺失[59],DAPK1是一种丝氨酸苏氨酸激酶,能够转导Netrin-1依赖性受体促细胞凋亡,DAPK1的缺失导致了肿瘤细胞的进一步恶化。
另一个Netrins家族成员Netrin-4与侵袭性的乳腺癌预后相关[63],Oncomine数据也显示,与正常乳腺组织相比,乳腺癌中Netrin-4降低。Netrin-4过表达能诱导黏着蛋白(N-cadherin)和波形蛋白(Vimentin)下调,从而减弱细胞迁移和侵袭[64]。但也有报道显示,Netrin-4在乳腺癌或乳腺癌渗出液中表达很高[65],并且Netrin-4和α6β1共同整合在特定的血管床上,促进了肿瘤血管的生成作用[20]。此外,Netrin-4也可以激活小GTP酶和Src家族激酶/FAK来刺激体外和体内淋巴通透性,诱导转基因小鼠皮肤和乳腺肿瘤中淋巴管和血管的生长、迁移,导致转移增强[66]。这些研究表明,Netrin-4与乳腺癌的转移密切相关;然而,究竟Netrin-4是促进乳腺癌转移还是抑制其转移,还需要进一步评价。
2.5 Netrins与卵巢癌卵巢癌是死亡率最高的妇科恶性肿瘤之一,5年生存率不到45%[67]。据报道,在卵巢癌中,Netrin-1随卵巢癌的恶化而表达升高[68],并且与SOX6 (Sex-determining region Y box 6)表达也有相关性[69]。已经证实,SOX6[70]是肿瘤抑制因子,在细胞增殖、分化中起重要作用,包括卵巢癌、食管鳞状细胞癌、肝癌和慢性骨髓性白血病。而SOX6与Netrin-1在卵巢肿瘤中表达成反比关系[69],SOX6对卵巢癌细胞体内增殖、细胞侵袭和异种移植生长具有抑制作用,而Netrin-1的过表达会显著地消除这一现象。总之,这些研究表明Netrin-1在调节肿瘤生长中具有重要的作用,对于开发针对卵巢癌的治疗药物具有潜在意义。
2.6 Netrins与胶质母细胞瘤胶质母细胞瘤(Glioblastoma,GBM)是颅内最常见的原发性恶性肿瘤,中位生存期只有一年多,也没有标准的二线治疗[71]。
目前,在临床GBM病理标本中发现Netrin-1高表达,并且Netrin-1的表达与干细胞标记物巢蛋白(Nestin)相关,导致干细胞球体的形成增加[72]。研究表明,Netrin-1[73]通过激活Notch信号,与干细胞结合,改变非侵袭性GBM细胞表型,促使扩散入侵并增加GBM干细胞标记物巢蛋白的表达。并且,Netrin-1与细胞增殖、肿瘤恶性分级呈正相关[74],可在体外和体内介导UNC5A诱导NF-κB p65ser536磷酸化和c-Myc表达,促进GBM细胞增殖[75]。并且研究发现靶向抑制Netrin-1的表达,特别是干细胞样细胞浸润生长受到显著抑制[72],肿瘤细胞增殖、存活率和侵袭能力也显著减弱[76]。Netrin-1作为GBM干细胞干性和运动的重要调节因子,为探索治疗胶质母细胞瘤的药物靶点提供有力支持。
而另一个成员Netrin-4可能作为致癌基因,与ITGB4共同作用激活Akt-mToR信号通路促进成胶质母细胞瘤增殖;而且发现抑制Netrin-4或者ITGB4都会增强胶质母细胞瘤对药物替莫唑胺(Temozolomide,TMZ)的敏感性,外源性添入Netrin-4和ITGB4会减弱TMZ诱导的细胞凋亡[77]。但也有研究显示,Netrin-4也能在胶质母细胞瘤内激活Erk-MAPK信号通路,从而抑制GBM细胞的增殖[78]。总之,研究显示,Netrin-4在胶质母细胞瘤的研究中有重要的作用,但为何形成这种差异还需要进一步探究。
2.7 Netrins与神经母细胞瘤神经母细胞瘤(Neuroblastoma,NB)是儿童早期最常见的实体瘤之一,在新生儿中的发病率为1/10 000-1/8 000[79]。Netrin-1通过阻断UNC5S的促凋亡活性,在侵袭性NB的生长和传播中传递了选择性优势[80]。神经母细胞瘤之所以侵袭性强,是因为小GTP酶Rac1活性是神经轴突生长所必需的,UNC5A和DCC通过激活Rac1引发细胞皮层肌动蛋白重组,而肌动蛋白的装配会引起肿瘤细胞的侵袭转移,恰巧Netrin-1能加强刺激UNC5A和DCC导致Rac1激活[81-82]。而另外一个受体UNC5D是p53家族成员直接靶向的依赖性受体[83]。在NB中,神经生长因子(Nerve growth factor,NGF)大大加强了UNC5D、E2F1和p53的调控,诱导的UNC5D被天冬氨酸蛋白水解酶2/3 (Caspase2/3)裂解,释放的细胞内片段转移到细胞核中并与E2F1相互作用,选择性地反式激活前凋亡靶基因,UNC5D与p53和E2F1形成正反馈回路,以促进神经母细胞瘤回归过程中NGF依赖介导的程序性细胞死亡[84],但Netrin-1的表达强烈抑制UNC5D的裂解及其诱导的凋亡[85],以此来抑制神经母细胞瘤的程序性死亡。
Netrins的另外一个受体Neogenin-1也是一种在胚胎发育和成体内稳态期间,参与轴突引导、血管生成、神经元细胞迁移和细胞死亡的跨膜受体[86-89]。Villanueva[90]等发现在NB中,在其配体不存在的情况下,Neogenin-1也能促进细胞死亡,但敲低Neogenin-1会减少细胞迁移。Neogenin-1及其配体Netrin-1和Netrin-4在原代神经母细胞瘤中的表达较高,预示着患者存活率差[90]。在神经母细胞瘤中,DCC、UNC5S和Neogenin-1的表达预示着肿瘤的迁移侵袭能力增强,而它们却都能促进细胞的凋亡,一方面发挥着肿瘤抑制的角色,一方面又促进了肿瘤的发展。这些数据表明Netrins及其受体可能作为治疗神经母细胞瘤特定的靶标因子,具有极其重要的作用。
2.8 Netrins与其他肿瘤除了上述肿瘤外,Netrins及其受体在其他肿瘤中目前研究还较少,但是也发挥着重要的作用。例如,阻断中枢系统成神经管细胞瘤中表达的内源性Netrin-1、Neogenin或UNC5B能够抑制其侵袭[91]。在多发性骨髓瘤[92]、前列腺癌细胞[93]中Netrin-1水平显著升高,而其受体UNC5B表达受到抑制[93]。在黑色素瘤中,Netrin-1通过结合受体UNC5B促进黑色素瘤细胞侵袭和迁移,在恶性黑色素瘤的进展中起重要作用[94],随着近年来抗体疗法的出现,2C9作为UNC5B的单克隆特异性抗体,经过2C9处理后的黑色素瘤细胞的迁移侵袭作用受到显著抑制[95-96]。在晚期非小细胞肺癌组织中发现Netrin-1浓度随化疗而降低[40],以及在弥漫性大B细胞淋巴瘤和套细胞淋巴瘤中[97],也发现Netrin-1高表达,Netrin-1的功能干扰与DCC、UNC5H介导的细胞死亡有关,得益于受体作为抑癌因子的作用。在宫颈癌或晚期子宫内膜异位症(Ⅲ-Ⅳ期)中,miR-196和miR-20a高表达,并通过靶向结合Netrin-4 mRNA 3′-UTR中的一个结合位点来抑制Netrin-4的表达,在这里,Netrin-4扮演着肿瘤抑制因子的角色[98-99]。在成纤维肉瘤HT1080细胞中,敲低Netrin-4能抑制该肿瘤细胞的增殖,但促进了迁移、侵袭能力[100]。这些研究表明,Netrins家族成员,尤其是Netrin-1和Netrin-4,与许多肿瘤的发生发展密切相关。然而,它们在肿瘤中的研究还比较局限,很多作用机制还不够清楚,需要更多数据来支持。
3 总结与展望文中总结了近年来Netrins及其受体在结直肠癌、胰腺癌、胃癌、肺癌、乳腺癌、卵巢癌、胶质母细胞瘤、神经母细胞瘤及其他肿瘤中的最新研究进展,显示了Netrin-1、Netrin-4在这些肿瘤中的重要意义。Kefeli等也总结了大量研究资料得出Netrin-1具有促癌作用,被认为是一个非常重要的治疗靶标[24]。
大多数Netrins受体如DCC和UNC5S家族往往扮演着抑癌的角色。它们激活p53等凋亡途径的凋亡活性,从而降低肿瘤细胞的存活能力。通过缺失受体、过表达配体或抑制下游信号传导能够抑制Netrins受体的抑癌作用。例如,依赖性受体DNA的异常甲基化、突变都会使受体的凋亡活性丧失。
而Netrins,尤其是Netin-1和Netrin-4,可以通过结合这些受体,竞争性地抑制其活性,封闭其抑癌作用,而促进了肿瘤的发生发展。Netrins作为促癌基因对于肿瘤细胞的存活是有利的,Netrins的表达激活了血管内皮生长因子的表达,以及Mek/Erk、Akt/Stat3、Notch、mToR和NF-κB等信号途径,并可以促进原癌基因c-Myc的表达,从而促进肿瘤的发展。因此,进一步研究Netrins在肿瘤中的作用及其机制,有利于针对Netrins及其受体,开发出新型的靶向治疗抗体或者其特异性抑制。
另外,Netrins作用机制研究的不断深入不仅有利于开发新型的抗肿瘤疗法,也会对进一步理解Netrins在神经系统及免疫过程中的作用机制提供参考。比如Netrins调控的mToR信号通路也广泛参与了神经细胞的调节和免疫细胞的活性[101-102]。
但是,目前对Netrin家族其余成员Netrin-2、Netrin-3、Netrin-5及Netrin-GS在肿瘤发生发展中的研究尚少。因此,对Netrin家族其余成员在肿瘤细胞中是否起着关键作用也是值得探究的。这些研究对于实现肿瘤的靶向治疗具有重要的意义,希望能对临床用药及其机制研究提供帮助。
参考文献
| [1] | Mulero P, Córdova C, Hernández M, et al. Netrin-1 and multiple sclerosis:A new biomarker for neuroinflammation?.Eur J Neurol, 2017, 24(9): 1108–1115.DOI: 10.1111/ene.2017.24.issue-9 |
| [2] | Yebra M, Diaferia GR, Montgomery AM, et al. Endothelium-derived netrin-4 supports pancreatic epithelial cell adhesion and differentiation through integrins α2β1 and α3β1.PLoS ONE, 2011, 6(7): e22750.DOI: 10.1371/journal.pone.0022750 |
| [3] | Zeng Y, Navarro P, Fernandez-Pujals AM, et al. A combined pathway and regional heritability analysis indicates netrin1 pathway is associated with major depressive disorder.Biol Psychiatry, 2017, 81(4): 336–346.DOI: 10.1016/j.biopsych.2016.04.017 |
| [4] | Hashimoto Y, Toyama Y, Kusakari S, et al. An alzheimer disease-linked rare mutation potentiates netrin receptor uncoordinated-5c-induced signaling that merges with amyloid β precursor protein signaling.J Biol Chem, 2016, 291(23): 12282–12293.DOI: 10.1074/jbc.M115.698092 |
| [5] | Wang N, Cao YS, Zhu Y. Netrin-1 prevents the development of cardiac hypertrophy and heart failure.Mol Med Rep, 2016, 13(3): 2175–2181.DOI: 10.3892/mmr.2016.4755 |
| [6] | Ramkhelawon B, Hennessy EJ, Ménager M, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity.Nat Med, 2014, 20(4): 377–384.DOI: 10.1038/nm.3467 |
| [7] | Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C.Elegans.Neuron, 1990, 4(1): 61–85.DOI: 10.1016/0896-6273(90)90444-K |
| [8] | Garrett AM, Jucius TJ, Sigaud LP, et al. Analysis of expression pattern and genetic deletion of netrin5 in the developing mouse.Front Mol Neurosci, 2016, 9: 3. |
| [9] | Larrieu-Lahargue F, Thomas KR, Li DY. Netrin ligands and receptors:Lessons from neurons to the endothelium.Trends Cardiovasc Med, 2012, 22(2): 44–47.DOI: 10.1016/j.tcm.2012.06.010 |
| [10] | Serafini T, Kennedy TE, Galko MJ, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C.elegans unc-6.Cell, 1994, 78(3): 409–424.DOI: 10.1016/0092-8674(94)90420-0 |
| [11] | Koch M, Murrell JR, Hunter DD, et al. A novel member of the netrin family, β-netrin, shares homology with the β chain of laminin.J Cell Biol, 2000, 151(2): 221–234.DOI: 10.1083/jcb.151.2.221 |
| [12] | Cirulli V, Yebra M. Netrins:Beyond the brain.Nat Rev Mol Cell Biol, 2007, 8(4): 296–306.DOI: 10.1038/nrm2142 |
| [13] | Yamagishi S, Yamada K, Sawada M, et al. Netrin-5 is highly expressed in neurogenic regions of the adult brain.Front Cell Neurosci, 2015, 9: 146. |
| [14] | Nakashiba T, Nishimura S, Ikeda T, et al. Complementary expression and neurite outgrowth activity of netrin-g subfamily members.Mech Dev, 2002, 111(1/2): 47–60. |
| [15] | Ylivinkka I, Keski-Oja J, Hyytiainen M. Netrin-1:A regulator of cancer cell motility?.Eur J Cell Biol, 2016, 95(11): 513–520.DOI: 10.1016/j.ejcb.2016.10.002 |
| [16] | Keino-Masu K, Masu M, Hinck L, et al. Deleted in colorectal cancer (dcc) encodes a netrin receptor.Cell, 1996, 87(2): 175–185.DOI: 10.1016/S0092-8674(00)81336-7 |
| [17] | Kolodziej PA, Timpe LC, Mitchell KJ, et al. Frazzled encodes a drosophila member of the dcc immunoglobulin subfamily and is required for cns and motor axon guidance.Cell, 1996, 87(2): 197–204.DOI: 10.1016/S0092-8674(00)81338-0 |
| [18] | Leonardo ED, Hinck L, Masu M, et al. Vertebrate homologues of C.elegans unc-5 are candidate netrin receptors.Nature, 1997, 386(6627): 833–838.DOI: 10.1038/386833a0 |
| [19] | Ly A, Nikolaev A, Suresh G, et al. Dscam is a netrin receptor that collaborates with dcc in mediating turning responses to netrin-1.Cell, 2008, 133(7): 1241–1254.DOI: 10.1016/j.cell.2008.05.030 |
| [20] | Corset V, Nguyen-Ba-Charvet KT, Forcet C, et al. Netrin-1-mediated axon outgrowth and camp production requires interaction with adenosine a2b receptor.Nature, 2000, 407(6805): 747–750.DOI: 10.1038/35037600 |
| [21] | Li YN, Pinzón-Duarte G, Dattilo M, et al. The expression and function of netrin-4 in murine ocular tissues.Exp Eye Res, 2012, 96(1): 24–35.DOI: 10.1016/j.exer.2012.01.007 |
| [22] | Culotti JG, Merz DC. Dcc and netrins.Curr Opin Cell Biol, 1998, 10(5): 609–613.DOI: 10.1016/S0955-0674(98)80036-7 |
| [23] | Guijarro P, Simó S, Pascual M, et al. Netrin1 exerts a chemorepulsive effect on migrating cerebellar interneurons in a dcc-independent way.Mol Cell Neurosci, 2006, 33(4): 389–400.DOI: 10.1016/j.mcn.2006.08.010 |
| [24] | Kefeli U, Ucuncu Kefeli A, Cabuk D, et al. Netrin-1 in cancer:Potential biomarker and therapeutic target?.Tumour Biol, 2017, 39(4): 1010428317698388. |
| [25] | Delloye-Bourgeois C, Brambilla E, Coissieux MM, et al. Interference with netrin-1 and tumor cell death in non-small cell lung cancer.J Natl Cancer Inst, 2009, 101(4): 237–247.DOI: 10.1093/jnci/djn491 |
| [26] | Mazelin L, Bernet A, Bonod-Bidaud C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis.Nature, 2004, 431(7004): 80–84.DOI: 10.1038/nature02788 |
| [27] | Mille F, Llambi F, Guix C, et al. Interfering with multimerization of netrin-1 receptors triggers tumor cell death.Cell Death Differ, 2009, 16(10): 1344–1351.DOI: 10.1038/cdd.2009.75 |
| [28] | Arakawa H. P53, apoptosis and axon-guidance molecules.Cell Death Differ, 2005, 12(8): 1057–1065.DOI: 10.1038/sj.cdd.4401601 |
| [29] | Eveno C, Contreres JO, Hainaud P, et al. Netrin-4 overexpression suppresses primary and metastatic colorectal tumor progression.Oncol Rep, 2013, 29(1): 73–78.DOI: 10.3892/or.2012.2104 |
| [30] | Feng M. miR-196a overexpress in liver cancer and promote hepatoma cell proliferation[D]. Changchun: Jilin University, 2015 (in Chinese). 冯默. miR-196a在肝细胞肝癌中的表达和促增殖作用研究[D]. 长春: 吉林大学, 2015. |
| [31] | Jemal A, Bray F, Center MM, et al. Global cancer statistics.CA Cancer J Clin, 2011, 61(2): 69–90.DOI: 10.3322/caac.v61:2 |
| [32] | Fitamant J, Guenebeaud C, Coissieux MM, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer.Proc Natl Acad Sci USA, 2008, 105(12): 4850–4855.DOI: 10.1073/pnas.0709810105 |
| [33] | Qi Q, Li DY, Luo HR, et al. Netrin-1 exerts oncogenic activities through enhancing yes-associated protein stability.Proc Natl Acad Sci USA, 2015, 112(23): 7255–7260.DOI: 10.1073/pnas.1505917112 |
| [34] | Shin SK, Nagasaka T, Jung BH, et al. Epigenetic and genetic alterations in netrin-1 receptors unc5c and dcc in human colon cancer.Gastroenterology, 2007, 133(6): 1849–1857.DOI: 10.1053/j.gastro.2007.08.074 |
| [35] | Coissieux MM, Tomsic J, Castets M, et al. Variants in the netrin-1 receptor unc5c prevent apoptosis and increase risk of familial colorectal cancer.Gastroenterology, 2011, 141(6): 2039–2046.DOI: 10.1053/j.gastro.2011.08.041 |
| [36] | Xu B, Shen F, Cao J, et al. Angiogenesis in liver metastasis of colo-rectal carcinoma.Front Bioscience (Landmark Ed), 2013, 18: 1435–1443.DOI: 10.2741/4190 |
| [37] | Eveno C, Broqueres-You D, Feron JG, et al. Netrin-4 delays colorectal cancer carcinomatosis by inhibiting tumor angiogenesis.Ame J Pathol, 2011, 178(4): 1861–1869.DOI: 10.1016/j.ajpath.2010.12.019 |
| [38] | Chen WQ, Zheng RS, Baade PD, et al. Cancer statistics in China, 2015.CA:A Cancer J Clin, 2016, 66(2): 115–132.DOI: 10.3322/caac.21338 |
| [39] | Ramesh G, Berg A, Jayakumar C. Plasma netrin-1 is a diagnostic biomarker of human cancers.Biomarkers, 2011, 16(2): 172–180.DOI: 10.3109/1354750X.2010.541564 |
| [40] | Yildirim ME, Kefeli U, Aydin D, et al. The value of plasma netrin-1 in non-small cell lung cancer patients as diagnostic and prognostic biomarker.Tumor Biology, 2016, 37(9): 11903–11907.DOI: 10.1007/s13277-016-5025-y |
| [41] | Link BC, Reichelt U, Schreiber M, et al. Prognostic implications of netrin-1 expression and its receptors in patients with adenocarcinoma of the pancreas.Ann Surg Oncol, 2007, 14(9): 2591–2599.DOI: 10.1245/s10434-007-9469-6 |
| [42] | Dumartin L, Quemener C, Laklai H, et al. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells.Gastroenterology, 2010, 138(4): 1595–1606, 1606.e8.DOI: 10.1053/j.gastro.2009.12.061 |
| [43] | Huang Q, Hua HW, Jiang F, et al. Netrin-1 promoted pancreatic cancer cell proliferation by upregulation of mdm2.Tumour Biol, 2014, 35(10): 9927–9934.DOI: 10.1007/s13277-014-2195-3 |
| [44] | An XZ, Zhao ZG, Luo YX, et al. Netrin-1 suppresses the mek/erk pathway and itgb4 in pancreatic cancer.Oncotarget, 2016, 7(17): 24719–24733. |
| [45] | Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012.CA Cancer J Clin, 2015, 65(2): 87–108.DOI: 10.3322/caac.21262 |
| [46] | Chen JM, Wang SH, Xu SX, et al. Netrin-1 protein expression in gastric cancer and its correlation with clinicopathologic features and prognosis.Sichuan Univ:Med Sci Ed, 2011, 42(6): 802–806.(in Chinese). 陈金明, 王沈华, 徐善祥, 等. Netrin-1蛋白在胃癌组织中的表达与临床病理及预后的关系.四川大学学报:医学版, 2011, 42(6): 802-806. |
| [47] | Yin K, Wang LJ, Zhang X, et al. Netrin-1 promotes gastric cancer cell proliferation and invasion via the receptor neogenin through pi3k/akt signaling pathway.Oncotarget, 2017, 8(31): 51177–51189. |
| [48] | Lv B, Song CH, Wu LJ, et al. Netrin-4 as a biomarker promotes cell proliferation and invasion in gastric cancer.Oncotarget, 2015, 6(12): 9794–9806. |
| [49] | Hibi K, Sakata M, Sakuraba K, et al. Methylation of the dcc gene is lost in advanced gastric cancer.Anticancer Res, 2010, 30(1): 107–109. |
| [50] | Hibi K, Sakata M, Sakuraba K, et al. Changes in UNC5C gene methylation during human gastric carcinogenesis.Anticancer Res, 2009, 29(11): 4397–4399. |
| [51] | Kefeli U, Yildirim ME, Aydin D, et al. Netrin-1 concentrations in patients with advanced gastric cancer and its relation with treatment.Biomarkers, 2012, 17(7): 663–667.DOI: 10.3109/1354750X.2012.709882 |
| [52] | Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China.Lancet Oncol, 2014, 15(7): e279–e289.DOI: 10.1016/S1470-2045(13)70567-9 |
| [53] | Fidler IJ. The pathogenesis of cancer metastasis:The 'seed and soil' hypothesis revisited.Nat Rev Cancer, 2003, 3(6): 453–458.DOI: 10.1038/nrc1098 |
| [54] | Hu J, Li G, Zhang PY, et al. A cd44v+ subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity.Cell Death Dis, 2017, 8(3): e2679.DOI: 10.1038/cddis.2017.72 |
| [55] | Gordon AC, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for breast cancer liver metastases.J Vasc Interv Radiol, 2016, 27(9): 1316–1319.DOI: 10.1016/j.jvir.2016.06.016 |
| [56] | Custódio-Santos T, Videira M, Brito MA. Brain metastasization of breast cancer.Biochim Biophys Acta Rev Cancer, 2017, 1868(1): 132–147.DOI: 10.1016/j.bbcan.2017.03.004 |
| [57] | Jackson W, Sosnoski DM, Ohanessian SE, et al. Role of megakaryocytes in breast cancer metastasis to bone.Cancer Res, 2017, 77(8): 1942–1954.DOI: 10.1158/0008-5472.CAN-16-1084 |
| [58] | Gao YS, Wang CJ, Cai SP, et al. Expression of netrin-1 in invasive ductal breast cancer and its relationship with tumor metastasis.J Shandong Univ:Health Sci, 2015, 53(7): 39–42.(in Chinese). 高永生, 王春健, 蔡淑萍, 等. Netrin-1在乳腺浸润性导管癌中的表达及与转移的相关性.山东大学学报:医学版, 2015, 53(7): 39-42. |
| [59] | Grandin M, Mathot P, Devailly G, et al. Inhibition of DNA methylation promotes breast tumor sensitivity to netrin-1 interference.EMBO Mol Med, 2016, 8(8): 863–877.DOI: 10.15252/emmm.201505945 |
| [60] | Delloye-Bourgeois C, Fitamant J, Paradisi A, et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma.J Exp Med, 2009, 206(4): 833–847.DOI: 10.1084/jem.20082299 |
| [61] | Paradisi A, Creveaux M, Gibert B, et al. Combining chemotherapeutic agents and netrin-1 interference potentiates cancer cell death.EMBO Mol Med, 2013, 5(12): 1821–1834.DOI: 10.1002/emmm.201302654 |
| [62] | Grandin M, Meier M, Delcros JG, et al. Structural decoding of the netrin-1/unc5 interaction and its therapeutical implications in cancers.Cancer Cell, 2016, 29(2): 173–185.DOI: 10.1016/j.ccell.2016.01.001 |
| [63] | Esseghir S, Kennedy A, Seedhar P, et al. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins.Clin Cancer Res, 2007, 13(11): 3164–3173.DOI: 10.1158/1078-0432.CCR-07-0224 |
| [64] | Xu XP, Yan QY, Wang YA, et al. NTN4 is associated with breast cancer metastasis via regulation of emt-related biomarkers.Oncol Rep, 2017, 37(1): 449–457.DOI: 10.3892/or.2016.5239 |
| [65] | Yuan Y, Leszczynska M, Konstantinovsky S, et al. Netrin-4 is upregulated in breast carcinoma effusions compared to corresponding solid tumors.Diagnostic Cytopathol, 2011, 39(8): 562–566. |
| [66] | Larrieu-Lahargue F, Welm AL, Thomas KR, et al. Netrin-4 induces lymphangiogenesis in vivo.Blood, 2010, 115(26): 5418–5426.DOI: 10.1182/blood-2009-11-252338 |
| [67] | iegel R, Ward E, Brawley O, et al. Cancer statistics, 2011:The impact of eliminating socioeconomic and racial disparities on premature cancer deaths.CA:A Cancer J Clin, 2011, 61(4): 212–236.DOI: 10.3322/caac.v61:4 |
| [68] | Papanastasiou AD, Pampalakis G, Katsaros D, et al. Netrin-1 overexpression is predictive of ovarian malignancies.Oncotarget, 2011, 2(5): 363–367. |
| [69] | Li Y, Xiao M, Guo FC. The role of sox6 and netrin-1 in ovarian cancer cell growth, invasiveness, and angiogenesis.Tumour Biol, 2017, 39(5): 1010428317705508. |
| [70] | Qin YR, Tang H, Xie FJ, et al. Characterization of tumor-suppressive function of SOX6 in human esophageal squamous cell carcinoma.Clin Cancer Res, 2011, 17(1): 46–55.DOI: 10.1158/1078-0432.CCR-10-1155 |
| [71] | Carter T, Shaw H, Cohn-Brown D, et al. Ipilimumab and bevacizumab in glioblastoma.Clin Oncol, 2016, 28(10): 622–626.DOI: 10.1016/j.clon.2016.04.042 |
| [72] | Ylivinkka I, Sihto H, Tynninen O, et al. Motility of glioblastoma cells is driven by netrin-1 induced gain of stemness.J Experim Clin Cancer Res, 2017, 36(1): 9.DOI: 10.1186/s13046-016-0482-0 |
| [73] | Ylivinkka I, Hu YZ, Chen P, et al. Netrin-1-induced activation of notch signaling mediates glioblastoma cell invasion.J Cell Sci, 2013, 126(11): 2459–2469.DOI: 10.1242/jcs.120022 |
| [74] | Wan XL. Role of Netrin-1 in Human Glioma[D]. Changchun: Northeast Normal University, 2013 (in Chinese). 万茜淋. Netrin-1在人类神经胶质瘤中表达的研究[D]. 长春: 东北师范大学, 2013. |
| [75] | Chen JY, He XX, Ma C, et al. Netrin-1 promotes glioma growth by activating nf-κB via UNC5A.Sci Rep, 2017, 7(1): 5454.DOI: 10.1038/s41598-017-05707-0 |
| [76] | Sanvoranart T, Supokawej A, Kheolamai P, et al. Targeting netrin-1 in glioblastoma stem-like cells inhibits growth, invasion, and angiogenesis.Tumour Biol, 2016, 37(11): 14949–14960.DOI: 10.1007/s13277-016-5314-5 |
| [77] | Li L, Hu YZ, Ylivinkka I, et al. Netrin-4 protects glioblastoma cells from temozolomide induced senescence.PLoS ONE, 2013, 8(11): e80363.DOI: 10.1371/journal.pone.0080363 |
| [78] | Li L, Teng C, Liu JY, et al. Influence from Netrin-4 to ERK/MAPK signal pathway in GBM cell lines.Mod Oncol, 2017, 25(5): 697–700.(in Chinese). 李里, 滕冲, 刘佳音, 等. Netrin-4对胶质母细胞瘤细胞erk/mapk信号通路的影响.现代肿瘤医学, 2017, 25(5): 697-700. |
| [79] | Cheung NKV, Dyer MA. Neuroblastoma:Developmental biology, cancer genomics and immunotherapy.Nat Rev Cancer, 2013, 13(6): 397–411.DOI: 10.1038/nrc3526 |
| [80] | Li L, Hu YZ, Ylivinkka I, et al. Netrin-4 protects glioblastoma cells from temozolomide induced senescence.PLoS ONE, 2013, 8(11): e80363.DOI: 10.1371/journal.pone.0080363 |
| [81] | Picard M, Petrie RJ, Antoine-Bertrand J, et al. Spatial and temporal activation of the small gtpases rhoa and rac1 by the netrin-1 receptor unc5a during neurite outgrowth.Cellul Signall, 2009, 21(12): 1961–1973.DOI: 10.1016/j.cellsig.2009.09.004 |
| [82] | Li XD, Saint-Cyr-Proulx E, Aktories K, et al. Rac1 and Cdc42 but not RhoA or rho kinase activities are required for neurite outgrowth induced by the netrin-1 receptor DCC (Deleted in Colorectal Cancer) in n1e-115 neuroblastoma cells.J Biol Chem, 2002, 277(17): 15207–15214.DOI: 10.1074/jbc.M109913200 |
| [83] | Zhu YY, Li YY, Haraguchi S, et al. Dependence receptor unc5d mediates nerve growth factor depletion-induced neuroblastoma regression.J Clin Investigat, 2013, 123(7): 2935–2947.DOI: 10.1172/JCI65988 |
| [84] | Wang H, Zhang B, Gu M, et al. Overexpression of the dependence receptor UNC5H4 inhibits cell migration and invasion, and triggers apoptosis in neuroblastoma cell.Tumour Biol, 2014, 35(6): 5417–5425.DOI: 10.1007/s13277-014-1706-6 |
| [85] | Wang H, Gu M, Li S, et al. Netrin-1 prevents apoptosis on SH-SH5Y cells induced by dependence receptor Unc5H4.Chin J Mod Appl Pharm, 2014, 31(12): 1431–1434.(in Chinese). 王弘, 顾敏, 李爽, 等. Netrin-1抑制受体unc5h4诱导人神经母细胞瘤细胞凋亡.中国现代应用药学, 2014, 31(12): 1431-1434. |
| [86] | Milla LA, Arros A, Espinoza N, et al. Neogenin1 is a sonic hedgehog target in medulloblastoma and is necessary for cell cycle progression.Int J Cancer, 2014, 134(1): 21–31.DOI: 10.1002/ijc.v134.1 |
| [87] | Lejmi E, Bouras I, Camelo S, et al. Netrin-4 promotes mural cell adhesion and recruitment to endothelial cells.Vasc Cell, 2014, 6(1): 1–13.DOI: 10.1186/2045-824X-6-1 |
| [88] | Matsunaga E, Tauszig-Delamasure S, Monnier PP, et al. Rgm and its receptor neogenin regulate neuronal survival.Nat Cell Biol, 2004, 6(8): 749–755.DOI: 10.1038/ncb1157 |
| [89] | O'Leary CJ, Bradford D, Chen M, et al. The netrin/rgm receptor, neogenin, controls adult neurogenesis by promoting neuroblast migration and cell cycle exit.Stem Cells, 2015, 33(2): 503–514.DOI: 10.1002/stem.1861 |
| [90] | Villanueva AA, Falcón P, Espinoza N, et al. The netrin-4/Neogenin-1 axis promotes neuroblastoma cell survival and migration.Oncotarget, 2016, 8(6): 9767–9782. |
| [91] | Akino T, Han XZ, Nakayama H, et al. Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma.Cancer Res, 2014, 74(14): 3716–3726.DOI: 10.1158/0008-5472.CAN-13-3116 |
| [92] | Huang ZQ, Yao HX, Lin LE, et al. Expression of Netrin-1 in multiple myeloma and its clinical significance.J Clin Exp Med, 2017, 15(21): 2121–2124.(in Chinese). 黄昭前, 姚红霞, 林丽娥, 等. Netrin-1在多发性骨髓瘤患者骨髓细胞中的表达水平和临床意义.临床和实验医学杂志, 2017, 15(21): 2121-2124.DOI:10.3969/j.issn.1671-4695.2017.21.012 |
| [93] | Chen HW, Chen Q, Luo QD. Expression of netrin-1 by hypoxia contributes to the invasion and migration of prostate carcinoma cells by regulating yap activity.Exp Cell Res, 2016, 349(2): 302–309.DOI: 10.1016/j.yexcr.2016.10.023 |
| [94] | Kaufmann S, Kuphal S, Schubert T, et al. Functional implication of netrin expression in malignant melanoma.Cell Oncol, 2009, 31(6): 415–422. |
| [95] | Zuo YL, Yang JF, He J, et al. An uncoordinated-5 homolog b receptor monoclonal antibody regulates a375 melanoma cell migration.Monocl Antibod Immunod Immunoth, 2014, 33(4): 280–286.DOI: 10.1089/mab.2013.0077 |
| [96] | Zuo YL, Yang JF, He Y, et al. Preparation of a uncoordinated-5 homolog b monoclonal antibody and its effect on melanoma cell migration in vitro.Chin J Cellul Mol Immunol, 2015, 31(9): 1259–1262.(in Chinese). 左元玲, 杨剑峰, 何杨, 等. UNC5B单克隆抗体的制备及对黑素瘤细胞体外迁移能力的影响.细胞与分子免疫学杂志, 2015, 31(9): 1259-1262. |
| [97] | Broutier L, Creveaux M, Vial J, et al. Targeting netrin-1/dcc interaction in diffuse large B-cell and mantle cell lymphomas.Embo Mol Med, 2016, 8(2): 96–104.DOI: 10.15252/emmm.201505480 |
| [98] | Zhang J, Zheng FX, Yu G, et al. Mir-196a targets netrin 4 and regulates cell proliferation and migration of cervical cancer cells.Biochem Biophys Res Commun, 2013, 440(4): 582–588.DOI: 10.1016/j.bbrc.2013.09.142 |
| [99] | Zhao M, Tang QQ, Wu W, et al. Mir-20a contributes to endometriosis by regulating ntn4 expression.Mol Biol Rep, 2014, 41(9): 5793–5797.DOI: 10.1007/s11033-014-3452-7 |
| [100] | Jia Y. Roles and mechanism of Netrin4 and neogenin infibrosarcoma cell HT1080[D]. Xiamen: Xiamen University, 2014 (in Chinese). 贾茵. Netrin4和neogenin在成纤维肉瘤细胞ht1080中的作用及其机制探讨[D]. 厦门: 厦门大学, 2014. |
| [101] | Leibinger M, Andreadaki A, Fischer D. Role of mtor in neuroprotection and axon regeneration after inflammatory stimulation.Neurobiol Dis, 2012, 46(2): 314–324.DOI: 10.1016/j.nbd.2012.01.004 |
| [102] | Weichhart T, Hengstschl ger M, Linke M. Regulation of innate immune cell function by mTOR.Nat Rev Immunol, 2015, 15(10): 599–614.DOI: 10.1038/nri3901 |
