
 , 张宝会1, 许建韧1, 王丹丹1, 白凤武2
, 张宝会1, 许建韧1, 王丹丹1, 白凤武21 大连理工大学 生命科学与技术学院, 辽宁 大连 116024;
2 上海交通大学 生命科学技术学院 微生物代谢国家重点实验室, 上海 200240
收稿日期:2017-12-04;接收日期:2018-04-02 基金项目:国家自然科学基金(No.21406029)资助
摘要:木质纤维素预处理过程中产生的有毒副产物严重影响了纤维素乙醇发酵, 提高酿酒酵母抑制物耐受性是提高纤维素乙醇发酵效率的有效方法。文中通过过表达LCB4基因, 研究了重组菌株S288C-LCB4在乙酸、糠醛和香草醛胁迫下的细胞生长和乙醇发酵性能。结果表明, LCB4过表达菌株在分别含有10 g/L乙酸、1.5 g/L糠醛和1 g/L香草醛的平板中生长均优于对照菌株;在分别含有10 g/L乙酸、3 g/L糠醛和2 g/L香草醛的液体乙醇发酵过程中, 重组菌株S288C-LCB4乙醇发酵产率分别为0.85 g/(L·h)、0.76 g/(L·h)和1.12 g/(L·h), 比对照菌株提高了34.9%、85.4%和330.8%;且糠醛和香草醛胁迫下发酵时间分别缩短了30 h和44 h。根据发酵终点发酵液代谢物分析发现重组菌株比对照菌株产生了更多甘油、海藻糖和琥珀酸, 这些物质有利于增强菌株的抑制物耐受性。综上所述, LCB4基因过表达可显著提高酿酒酵母S288C在乙酸、糠醛和香草醛胁迫下的乙醇发酵性能。
关键词:乙醇发酵 LCB4 乙酸胁迫 糠醛胁迫 香草醛胁迫
Improvement of inhibitors tolerance of Saccharomyces cerevisiae by overexpressing of long chain sphingoid kinases encoding gene LCB4
Yanyan He1, Lihan Zi1

 , Baohui Zhang1, Jianren Xu1, Dandan Wang1, Fengwu Bai2
, Baohui Zhang1, Jianren Xu1, Dandan Wang1, Fengwu Bai2 1 School of Life Science and Biotechnology, Dalian University of Technology, Dalian 116024, Liaoning, China;
2 State Key Laboratory of Microbial Metabolism, School of Life Science and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China
Received: December 4, 2017; Accepted: April 2, 2018
Supported by: National Natural Science Foundation of China (No.21406029)
Corresponding author:Lihan Zi.Tel:+86-411-84706785; E-mail:lihanzi@dlut.edu.cn
Abstract: By-products released from pretreatment process of lignocellulose seriously hinder the development of cellulosic fuel ethanol.Therefore, the great way to increase the efficiency of cellulosic ethanol production is improvement of Saccharomyces cerevisiae tolerance to these inhibitors.In this work, the effects of LCB4 gene overexpression on cell growth and ethanol fermentation in S.cerevisiae S288C under acetic acid, furfural and vanillin stresses were studied.Compared to the control strain S288C-HO, the recombinant strain S288C-LCB4 grew better on YPD solid medium containing 10 g/L acetic acid, 1.5 g/L furfural and 1 g/L vanillin.Ethanol yields of recombinant strain S288C-LCB4 were 0.85 g/(L·h), 0.76 g/(L·h) and 1.12 g/(L·h) when 10 g/L acetic acid, 3 g/L furfural and 2 g/L vanillin were supplemented into the fermentation medium respectively, which increased by 34.9%, 85.4% and 330.8% than the control strain S288C-HO.Meanwhile, ethanol fermentation time was reduced by 30 h and 44 h under furfural and vanillin stresses respectively.Further metabolites analysis in fermentation broth showed that the recombinant strain produced more protective compounds, such as glycerol, trehalose and succinic acid, than the control strain, which could be the reason for enhancing strain tolerance to these inhibitors from pretreatment process of lignocellulose.The results indicated that overexpression of LCB4 gene could significantly improve ethanol fermentation in S.cerevisiae S288C under acetic acid, furfural and vanillin stresses.
Key words: ethanol fermentation LCB4 acetic acid stress furfural stress vanillin stress
纤维素燃料乙醇是替代化石资源的可持续能源之一,与淀粉质或糖质原料燃料乙醇生产相比,木质纤维素作为农业废弃物原料充足、成本低,因此木质纤维素乙醇成为国内外生物能源研究的热点[1]。然而木质纤维素原料在预处理过程中会产生大量的有机酸类、呋喃类和酚类等副产物[2-3]。这些化合物通过影响酵母细胞碳代谢过程、增加细胞膜透性和破坏酶活性等途径抑制酵母生长和乙醇发酵[4]。基因工程、随机诱变及代谢工程等手段选育高耐受性、高效生产乙醇的工业酿酒酵母是解决这一问题的主要方法[5-8]。其中DNA重组技术可以使特定的基因在酵母菌株中过表达或敲除,是生物育种简单有效的方法之一。
酿酒酵母中LCB4编码的长链鞘氨醇激酶[9],位于高尔基体和次级内体[10],与膜结构相连,调节长链鞘氨醇-1-磷酸(Sphingoid long chain base phosphates,LCBPs)的生物合成[11]。在哺乳动物细胞中,LCBPs是质膜脂类代谢具有生物活性的中间代谢物,可以与G-蛋白受体偶联,参与免疫细胞运输[12]。同时,LCBPs作为细胞中的第二信使,参与细胞增殖、存活、运动和细胞骨架形成[13-14]。在酵母中,细胞受到热激后,长链鞘氨醇(Sphingolipid long chain bases,LCBs)作为引起细胞周期阻滞的信号分子大量激增,长链鞘氨醇激酶通过将LCBs磷酸化为LCBPs,使处于细胞周期阻滞状态的热激细胞恢复正常[15-16]。然而,目前关于LCB4基因过表达与酿酒酵母抑制物耐受性的关系有待进一步研究。因此本文研究了LCB4基因过表达对酿酒酵母模式菌株S288C抑制物耐受性的影响,为进一步选育高抑制物耐受性乙醇发酵酵母菌株提供理论和实验技术基础。
1 材料与方法1.1 菌种大肠杆菌DH5α,酿酒酵母模式菌株S288C (本实验室保存)。
1.2 培养基抑制物耐受性检测固体培养基:YPD固体培养基中分别添加终浓度为10 g/L乙酸、1.5 g/L糠醛和1 g/L香草醛,pH 4.5;抑制物耐受性检测液体发酵培养基:在液体发酵培养基中分别添加终浓度为10 g/L乙酸、3 g/L糠醛和2 g/L香草醛,及其混合添加,pH 4.5。其中LB、YPD、酵母种子培养基和液体发酵培养基详见文献[17]。
1.3 构建重组菌株S288C-LCB4利用引物LCB4-F和引物LCB4-R (引物序列见表 1)从酿酒酵母S288C基因组中扩增获得目的基因LCB4,该基因GenBank登录号为NM_001183590.1。将所得LCB4基因片段克隆到pHO整合表达载体[18]。将验证正确的pHO-LCB4质粒经限制性内切酶NotⅠ线性化后回收目的条带,通过电转化法将LCB4基因同源整合到宿主酵母S288C染色体HO位点[19]。通过含G418抗生素(固体培养基添加300 μg/mL,液体培养基添加100 μg/mL)的YPD培养基筛选,并经富集培养后提取基因组作为模板,利用验证引物LCB4-F和PG418-R进行PCR初步验证。
表 1 本文所用引物列表Table 1 Primers used in this study
| Primer name | Primer sequence (5'-3') |
| LCB4-F | AAACCCGGGATGGTGGTGCAGAAAAAACTT |
| LCB4-R | CCCTTAATTAACTACATGGATTCAAACTCT |
| PG418-R | GGCCTCGAAACGTGAGTC |
| rtLCB4-F | CAAACTCTTCGCCGGATTTA |
| rtLCB4-R | AGGTACTGGTTCCGTCATCG |
| rtALG9-F | ATCGTGAAATTGCAGGCAGCTTGG |
| rtALG9-R | CATGGCAACGGCAGAAGGCAATAA |
表选项
1.4 菌种活化、耐受性检测及乙醇发酵1.4.1 菌种活化将含有空载体的对照菌株S288C-HO及重组菌株S288C-LCB4接种至种子培养基中进行活化,30 ℃、150 r/min条件下培养至对数期进行下一步实验。
1.4.2 重组菌株的耐受性检测将活化后的菌液OD620调至一致(1.0),以5倍梯度稀释为6个浓度,依次取2 μL菌液点样于抑制物耐受性检测平板,30 ℃倒置培养24-36 h,观察培养过程中菌株生长状况并拍照,具体方法参见文献[20]。
1.4.3 抑制物胁迫下的乙醇发酵比较对照菌株S288C-HO和重组菌株S288C- LCB4分别在抑制物单一和共同添加下的乙醇发酵性能。发酵方法如下:菌株经活化后用种子培养基调节OD620至1.0,按接种量10% (V/V)接种到含90 mL抑制物耐受性检测发酵培养基的250 mL摇瓶中,30 ℃、150 r/min条件下进行发酵实验。每隔6-12 h定时取样,测定发酵液菌体、残糖和乙醇浓度,每组进行3个平行实验。
1.5 发酵参数及代谢物的测定不同抑制物添加下乙醇发酵过程中发酵液残糖浓度、乙醇浓度、乙醇得率(YE/CS)、乙醇产率(PE/T)以及发酵液代谢物(甘油、海藻糖和琥珀酸)测定及分析方法参见文献[17]。
1.6 RNA提取及实时定量分析利用天根RNAsimple总RNA提取试剂盒分别提取不添加抑制物条件下发酵过程中对照菌S288C-HO和重组菌S288C-LCB4对数期细胞的总RNA。参照文献[21]进行RNA反转录、RT- PCR检测及基因相对表达量的分析。目的基因引物分别为rtLCB4-F和rtLCB4-R;管家基因ALG9作为内参,引物分别为rtALG9-F和rtALG9-R[22]。
2 结果与分析2.1 LCB4表达水平分析及重组菌株抑制物平板耐受性检测为研究LCB4基因过表达能否提高酿酒酵母S288C抑制物耐受性,根据1.3方法构建了LCB4基因过表达重组菌株S288C-LCB4。提取酵母转化子基因组DNA作为模板,用引物LCB4-F和PG418-R进行PCR验证。LCB4全长1 875 bp,PG418-R结合于其下游G418编码基因的ORF上,距LCB4的ORF终点500 bp,故验证时PCR产物理论大小约为2.3 kb,图 1中阳性对照及目的片段的条带与该理论值大小相吻合,表明重组菌株S288C-LCB4构建成功。
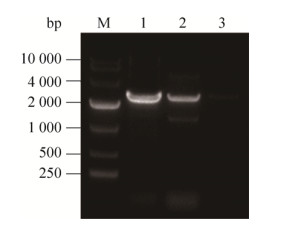 |
| 图 1 重组菌株S288C-LCB4的PCR分析验证 Figure 1 Verification of recombinant strain S288C-LCB4 by PCR analysis. M: DNA marker (DL10000); 1: positive control; 2: target fragment (LCB4+G418); 3: negative control. |
| 图选项 |
取未添加抑制物的发酵对数期重组菌株S288C-LCB4和对照菌株S288C-HO细胞进行RT-PCR检测,分析LCB4基因在2个菌株中的表达量。结果显示(图 2) LCB4基因在S288C-LCB4中的相对表达量是对照菌株的2.9倍,表明LCB4基因在S288C-LCB4中成功过表达。
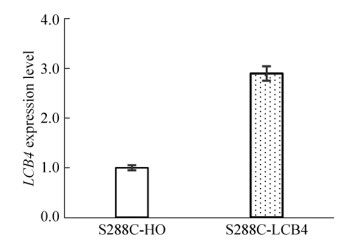 |
| 图 2 S288C-HO与S288C-LCB4菌株中LCB4基因的表达量 Figure 2 Comparison of LCB4 expression between recombinant strain S288C-LCB4 and the control strain S288C-HO. |
| 图选项 |
进一步对获得的转化子进行抑制物耐受性评价。检测了重组菌株与对照菌株分别在10 g/L乙酸、1.5 g/L糠醛和1 g/L香草醛抑制物平板上的生长状况,结果如图 3所示。在添加10 g/L乙酸平板上,对照菌株在第4个稀释梯度基本不生长,重组菌株在第6个稀释梯度仍有生长,重组菌株的生长状况明显优于对照菌株,乙酸耐受性显著提高。在1.5 g/L糠醛和1 g/L香草醛单独添加下,重组菌株和对照菌株的生长均受到抑制,但重组菌株生长状态较好。
 |
| 图 3 基因LCB4过表达对酵母菌株S288C胁迫耐受性的影响 Figure 3 Effect of LCB4 overexpression on stress tolerance of S. cerevisiae S288C. The yeast cell serial dilutions of 50-5-5 (from left to right) were spotted onto YPD solid medium with 10 g/L acetic acid, 1.5 g/L furfural or 1 g/L vanillin was supplemented and incubated at 30 ℃. |
| 图选项 |
平板耐受性检测结果表明,无论添加乙酸、糠醛或香草醛,重组菌株生长的耐受性均高于对照菌株。因此后续进一步研究了乙酸、糠醛和香草醛单一胁迫下S288C-LCB4的乙醇发酵性能。
2.2 抑制物胁迫对重组菌株S288C-LCB4乙醇发酵过程的影响2.2.1 重组菌株S288C-LCB4抑制物胁迫下乙醇发酵性能乙酸胁迫下重组菌乙醇发酵性能:从图 4A看出,在乙醇发酵过程中,重组菌株S288C-LCB4和对照菌株S288C-HO于12 h同时进入对数期,但重组菌株生长速度更快,菌体浓度高,相比对照菌株的菌液浓度提高了9.3%。重组菌株葡萄糖消耗速率及乙醇生成速率均高于对照菌株(图 4B),耗糖时间比对照菌缩短了12 h,重组菌株乙醇最大浓度为40.7 g/L (48 h),比对照菌株提高了10.5%。表明重组菌株S288C-LCB4在含有10 g/L乙酸的发酵培养基中生长状态及乙醇发酵性能均优于S288C-HO。
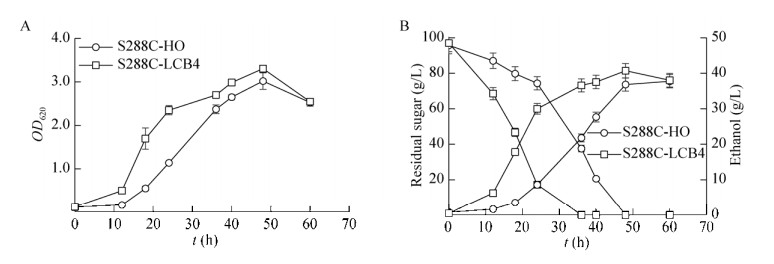 |
| 图 4 酿酒酵母S288C-HO与S288C-LCB4在10 g/L乙酸添加下的生长(A)、残糖及乙醇浓度(B)的变化 Figure 4 The profiles of cells growth, residual sugar and ethanol during ethanol fermentation by S. cerevisiae S288C-HO and S288C-LCB4 when 10 g/L acetic acid was supplemented. (A) Growth curves. (B) Residual sugar and ethanol production. |
| 图选项 |
糠醛胁迫下重组菌乙醇发酵性能:3 g/L糠醛添加下(图 5A),重组菌株S288C-LCB4的延迟期比对照菌株S288C-HO缩短了30 h,且最大菌液浓度比对照菌株提高了47.8%。葡萄糖消耗速率及乙醇产率均高于对照菌株,相比对照菌株,重组菌株提前42 h消耗完葡萄糖,乙醇最大浓度提高了21.7%,发酵时间缩短了30 h (图 5B)。表明在糠醛胁迫下,重组菌株生长及乙醇发酵性能均明显优于对照菌株,LCB4基因过表达提高了酵母细胞对糠醛的耐受性。
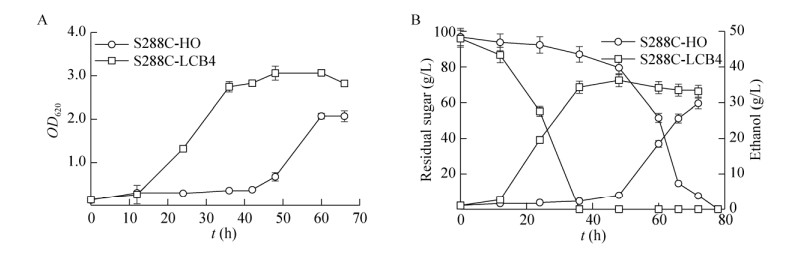 |
| 图 5 酿酒酵母S288C-HO与S288C-LCB4在3 g/L糠醛添加下的生长(A)、残糖及乙醇浓度(B)的变化 Figure 5 The profiles of cells growth, residual sugar and ethanol during ethanol fermentation by S. cerevisiae S288C-HO and S288C-LCB4 when 3 g/L furfural was supplemented. (A) Growth curves. (B) Residual sugar and ethanol production. |
| 图选项 |
香草醛胁迫下重组菌乙醇发酵性能:S288C- LCB4与S288C-HO在2 g/L香草醛胁迫下乙醇发酵情况如图 6所示。重组菌株比对照菌株提前24 h进入对数期,且最大菌体浓度提高了59.3%。重组菌株耗糖速率显著提高,最大乙醇浓度比对照菌提高了56.2%,且发酵时间缩短了44 h。重组菌株S288C-LCB4香草醛耐受性显著增强。
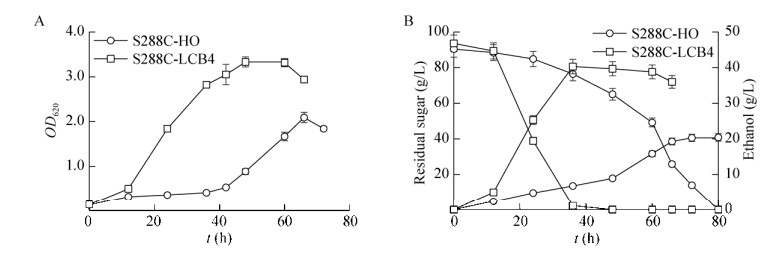 |
| 图 6 酿酒酵母S288C-HO与S288C-LCB4在2 g/L香草醛添加下的生长(A)、残糖及乙醇浓度(B)的变化 Figure 6 The profiles of cells growth, residual sugar and ethanol during ethanol fermentation by S. cerevisiae S288C-HO and S288C-LCB4 when 2 g/L vanillin was supplemented. (A) Growth curves. (B) Residual sugar and ethanol production. |
| 图选项 |
重组菌株在抑制物胁迫下乙醇发酵性能比较:重组菌株S288C-LCB4与对照菌株S288C-HO在乙酸、糠醛和香草醛胁迫下乙醇得率和乙醇产率如表 2所示。乙酸、糠醛和香草醛胁迫下重组菌株乙醇得率比对照菌株分别提高7.7%、22.6%和87.0%,乙醇产率分别提高34.9%、85.4%和330.8%,其中2 g/L香草醛胁迫下重组菌株乙醇得率和乙醇产率提高最为明显。重组菌株提高了菌株在乙酸、糠醛和香草醛胁迫下的乙醇发酵效率,表明LCB4基因过表达提高了重组菌的乙酸、糠醛和香草醛的胁迫耐受性。
表 2 抑制物胁迫下LCB4基因过表达对酿酒酵母S288C乙醇得率和产率的影响Table 2 Impact of LCB4 overexpression on ethanol yield and productivity of S. cerevisiae S288C with inhibitors supplemented
| Item | 10 g/L acetic acid | 3 g/L furfural | 2 g/L vanillin | |||
| YE/CS (g ethanol/g glucose) | PE/T (g ethanol/(L·h)) | YE/CS (g ethanol/ g glucose) | PE/T (g ethanol/ (L·h)) | YE/CS (g ethanol/ g glucose) | PE/T (g ethanol/ (L·h)) | |
| S288C-HO | 0.39 | 0.63 | 0.31 | 0.41 | 0.23 | 0.26 |
| S288C-LCB4 | 0.42 | 0.85 | 0.38 | 0.76 | 0.43 | 1.12 |
表选项
2.2.2 重组菌株在抑制物胁迫下发酵液中代谢物的变化上述研究表明,相比对照菌株重组菌株S288C-LCB4乙酸、糠醛和香草醛的耐受性显著提高。为研究重组菌株耐受性提高的机制对发酵液中与抑制物耐受性相关的代谢物进行了检测(甘油、海藻糖和琥珀酸),结果如图 7所示。
 |
| 图 7 抑制物对重组菌株S288C-LCB4乙醇发酵胞外代谢物的影响 Figure 7 Impact of inhibitors on extracellular metabolites of S. cerevisiae S288C-LCB4 during ethanol fermentation. |
| 图选项 |
在10 g/L乙酸胁迫下,重组菌株发酵液中甘油、琥珀酸和海藻糖的含量分别比对照菌株提高了32.7%、39.7%和6.9%。乙酸的存在会导致胞内环境酸化,细胞需要更多的ATP将H+泵出细胞来维持胞内正常的pH[23-24]。琥珀酸含量的提高,促进细胞TCA循环产生了更多的ATP。甘油是乙醇发酵过程中代谢副产物,大部分存在于细胞膜,具有保护细胞的作用[25]。海藻糖不仅对细胞具有保护作用,并且有助于提高酿酒酵母乙酸耐受性[26-27]。表明细胞需要更多的ATP和保护性物质来应对乙酸对细胞的毒性,因此LCB4过表达可以促进细胞产生更多的甘油、琥珀酸来提高菌株对乙酸的耐受性。
重组菌株在3 g/L糠醛胁迫下,其甘油、琥珀酸和海藻糖的含量分别比对照菌株提高了21.5%、52.6%和33.2%。糠醛抑制糖酵解途径和TCA循环,降低细胞内NAD(P)H浓度,造成细胞ROS的积累导致细胞膜损伤[6-7]。细胞通过降低细胞生长速率将NADPH和ATP用于细胞修复而使细胞进入漫长的延迟期[4]。琥珀酸的增加可以促进细胞产生大量的ATP和NADPH,用于细胞损伤后修复和生长消耗,NADPH可以提供还原力将糠醛还原为糠醇,达到解毒效果,琥珀酸还可以参与到γ-氨基丁酸(γ-aminobutyric acid,GABA)途径,减弱糠醛造成的ROS积累[28]。甘油不仅具有保护细胞的作用,还能在糠醛胁迫下维持细胞内氧化还原平衡[25]。海藻糖的提高能在糠醛胁迫下维持细胞膜、蛋白质和核酸等大分子物质的稳定性[29]。表明细胞需要更多的还原力来应对糠醛对细胞的毒性,因此LCB4过表达可以促进细胞产生更多的琥珀酸来提高菌株对糠醛的耐受性。
与对照菌株相比,在2 g/L香草醛胁迫下重组菌株的甘油、琥珀酸和海藻糖的含量分别提高了19.1%、64.5%和60.8%。香草醛会导致细胞内ROS的积累,破坏线粒体,通过抑制核糖体组装而影响翻译,细胞内以NAD(P)H为辅因子的还原酶可以将香草醛转化成香草醇,降低香草醛的毒性[30]。香草醛胁迫下,琥珀酸的生成促进了TCA循环产生更多的NAD(P)H作为香草醛转化成香草醇的还原力,且琥珀酸能够降低ROS的积累。海藻糖作为酵母细胞内的能量储藏物质,在环境胁迫下通过水解,为蛋白质复性和香草醛还原提供能量[29]。因此LCB4过表达可以促进细胞产生更多的琥珀酸和海藻糖来提高菌株对香草醛的耐受性。
综上所述,基因LCB4过表达后,使菌株分泌更多的保护性物质甘油和海藻糖,增加代谢中间物琥珀酸的含量,通过维持细胞pH平衡、维持氧化还原平衡或将抑制物转化为低毒物质,从而提高细胞对抑制物(乙酸、糠醛和香草醛)的耐受性。
2.2.3 抑制物混合添加对重组菌株乙醇发酵性能的影响以上研究结果表明,重组菌株S288C-LCB4在乙醇发酵过程中对乙酸、糠醛和香草醛耐受性均优于对照菌株S288C-HO。而实际纤维素水解液中存在抑制物种类较多且成分复杂,因此在单一乙酸、糠醛和香草醛添加实验基础上,初步研究了乙酸、糠醛和香草醛混合添加对重组菌株生长及乙醇生成的影响(图 8)。重组菌株在单一抑制物和混合抑制物胁迫下的细胞生长和乙醇生成均低于对照空白组。与单一抑制物添加相比,抑制物混合添加后重组菌生长和乙醇生成几乎完全被抑制,而乙酸、糠醛和香草醛单独添加时达到最大菌体浓度与对照组基本接近,乙醇生成分别比对照组低6.4%、21.2%和13.5%。因此相比于对照组,乙酸、糠醛和香草醛对重组菌生长的影响基本一致,而3种抑制物混合添加对重组菌生长的抑制率接近90%,表明乙酸、糠醛和香草醛3种抑制物混合添加对重组菌株S288C-LCB4的生长存在一定的协同抑制作用。
 |
| 图 8 10 g/L乙酸、3 g/L糠醛和2 g/L香草醛及其混合添加对重组菌S288C-LCB4生长及乙醇生成的影响 Figure 8 The profiles of cells growth and ethanol of S. cerevisiae S288C-LCB4 when 10 g/L acetic acid, 3 g/L furfural, 2 g/L vanillin and their mixture were supplemented respectively. |
| 图选项 |
3 结论酿酒酵母中LCB4编码长链鞘氨醇激酶,参与细胞周期阻滞状态热激细胞的恢复。通过DNA重组技术将LCB4在酿酒酵母S288C中进行过表达。对重组菌株S288C-LCB4进行了抑制物耐受性评价,发现S288C-LCB4能够提高酿酒酵母在含10 g/L乙酸、1.5 g/L糠醛和1 g/L香草醛平板的耐受性。在含10 g/L乙酸、3 g/L糠醛和2 g/L香草醛的液体发酵中,重组菌株S288C-LCB4的乙醇得率比对照菌株分别提高了7.7%、22.6%和87.0%,乙醇发酵产率提高了34.9%、85.4%和330.8%。研究结果表明基因LCB4过表达使重组菌株在3种抑制物胁迫下,通过生成更多的保护性物质,通过维持细胞pH平衡、氧化还原平衡、生物大分子结构稳定性或将抑制物转化为低毒的物质,提高重组菌S288C-LCB4对乙酸、糠醛和香草醛的耐受性。该研究为选育高耐受性、高效生产乙醇的工业酿酒酵母提供了新的研究方向和参考依据。
参考文献
| [1] | Zhao XQ, Zi LH, Bai FW, et al. Bioethanol from lignocellulosic biomass.Adv Biochem Eng Biotechnol, 2012, 128(1): 25–51. |
| [2] | Larsson S, Palmqvist E, Hahn-Hgerdal B, et al. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood.Enzyme Microb Technol, 1999, 24(3/4): 151–159. |
| [3] | Jnsson LJ, Alriksson B, Nilvebrant NO. Bioconversion of lignocellulose:inhibitors and detoxification.Biotechnol Biofuels, 2013, 6: 16.DOI: 10.1186/1754-6834-6-16 |
| [4] | Almeida JR, Modig T, Petersson A, et al. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae.J Chem Technol Biotechnol, 2007, 82(4): 340–349.DOI: 10.1002/(ISSN)1097-4660 |
| [5] | Parawira W, Tekere M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production:review.Crit Rev Biotechnol, 2011, 31(1): 20–31.DOI: 10.3109/07388551003757816 |
| [6] | Do?an A, Demirci S, Aytekin A?, et al. Improvements of tolerance to stress conditions by genetic engineering in Saccharomyces cerevisiae during ethanol production.Appl Biochem Biotechnol, 2014, 174(1): 28–42. |
| [7] | Kim D, Hahn JS. Roles of the Yap1 Transcription factor and antioxidants in Saccharomyces cerevisiae's tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress.Appl Environ Microbiol, 2013, 79(16): 5069–5077.DOI: 10.1128/AEM.00643-13 |
| [8] | Liu ZL, Ma MG, Song MZ. Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways.Mol Genet Genom, 2009, 282(3): 233–244.DOI: 10.1007/s00438-009-0461-7 |
| [9] | Nagiec MM, Skrzypek M, Nagiec EE, et al. The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases.J Biol Chem, 1998, 273(31): 19437–19442.DOI: 10.1074/jbc.273.31.19437 |
| [10] | Hait NC, Fujita K, Lester RL, et al. Lcb4p sphingoid base kinase localizes to the Golgi and late endosomes.FEBS Lett, 2002, 532(1/2): 97–102. |
| [11] | Funato K, Lombardi R, Vallée B, et al. Lcb4p is a key regulator of ceramide synthesis from exogenous long chain sphingoid base in Saccharomyces cerevisiae.J Biol Chem, 2003, 278(9): 7325–7334.DOI: 10.1074/jbc.M209925200 |
| [12] | Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity.Nat Rev Immunol, 2008, 8(10): 753–763.DOI: 10.1038/nri2400 |
| [13] | Olivera A, Kohama T, Edsall T, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival.J Cell Biol, 1999, 147(3): 545–558.DOI: 10.1083/jcb.147.3.545 |
| [14] | Pyne S, Pyne NJ. Sphingosine-1-phosphate signaling in mammalian cells.Biochem J, 2000, 349(2): 385–402.DOI: 10.1042/bj3490385 |
| [15] | Jenkins GM, Hannun YA. Role for de Novo sphingoid base biosynthesis in the heat-induced transient cell cycle arrest of Saccharomyces cerevisiae.J Biol Chem, 2001, 276(11): 8574–8581.DOI: 10.1074/jbc.M007425200 |
| [16] | Ferguson-Yankey SR, Skrzypek MS, Lester RL, et al. Mutant analysis reveals complex regulation of sphingolipid long chain base phosphates and long chain bases during heat stress in yeast.Yeast, 2002, 19(7): 573–586.DOI: 10.1002/(ISSN)1097-0061 |
| [17] | Fang Q, Zhang MM, Chen HQ, et al. Improvement of acetic acid tolerance of Saccharomyces cerevisiae by overexpressing glutaredoxin encoding gene GRX5.CIESC J, 2015, 66(4): 1434–1439.(in Chinese). 方青, 张明明, 陈洪奇, 等. 过表达谷氧还蛋白基因GRX5提高酿酒酵母乙酸耐性.化工学报, 2015, 66(4): 1434-1439.DOI:10.11949/j.issn.0438-1157.20141174 |
| [18] | He LY, Zhao XQ, Bai FW. Engineering industrial Saccharomyces cerevisiae strain with the FLO1-derivative gene isolated from the flocculating yeast SPSC01 for constitutive flocculation and fuel ethanol production.Appl Energy, 2012, 100: 33–40.DOI: 10.1016/j.apenergy.2012.03.052 |
| [19] | Voth WP, Richards JD, Shaw JM, et al. Yeast vectors for integration at the HO locus.Nucleic Acids Res, 2001, 29(12): E59.DOI: 10.1093/nar/29.12.e59 |
| [20] | Wei XW, Ma C, Xiong L, et al. Effect of vacuolar proteinase B on high temperature ethanol fermentation of Saccharomyces cerevisiae.Microbiol China, 2015, 42(10): 1841–1846.(in Chinese). 魏小文, 马翠, 熊亮, 等. 液泡蛋白酶B对酿酒酵母高温乙醇发酵效率的影响.微生物学通报, 2015, 42(10): 1841-1846. |
| [21] | Fang Q. Effect of overexpression of key enzyme genes on stress tolerance of Saccharomyces cerevisiae[D]. Dalian: Dalian University of Technology, 2016 (in Chinese). 方青. 过表达关键酶基因对酿酒酵母胁迫耐性的影响[D]. 大连: 大连理工大学, 2016. |
| [22] | Teste MA, Duquenne M, Fran ois JM, et al. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae.BMC Mol Biol, 2009, 10: 99.DOI: 10.1186/1471-2199-10-99 |
| [23] | Geng P, Zhang L, Shi GY. Omics analysis of acetic acid tolerance in Saccharomyces cerevisiae.World J Microbiol Biotechnol, 2017, 33(5): 94.DOI: 10.1007/s11274-017-2259-9 |
| [24] | Mira NP, Palma M, Guerreiro JF, et al. Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid.Microb Cell Factor, 2010, 9: 79.DOI: 10.1186/1475-2859-9-79 |
| [25] | Wang X, Li BZ, Ding MZ, et al. Metabolomic analysis reveals key metabolites related to the rapid adaptation of Saccharomyce cerevisiae to multiple inhibitors of furfural, acetic acid, and phenol.OMICS, 2013, 17(3): 150–159.DOI: 10.1089/omi.2012.0093 |
| [26] | Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate.Antonie van Leeuwenhoek, 1990, 58(3): 209–217.DOI: 10.1007/BF00548935 |
| [27] | Yoshiyama Y, Tanaka K, Yoshiyama K, et al. Trehalose accumulation enhances tolerance of Saccharomyces cerevisiae to acetic acid.J Biosci Bioeng, 2015, 119(2): 172–175.DOI: 10.1016/j.jbiosc.2014.06.021 |
| [28] | Cao J, Barbosa JM, Singh NK, et al. GABA shunt mediates thermotolerance in Saccharomyces cerevisiae, by reducing reactive oxygen production.Yeast, 2013, 30(4): 129–144.DOI: 10.1002/yea.v30.4 |
| [29] | Voit EO. Biochemical and genomic regulation of the trehalose cycle in yeast:review of observations and canonical model analysis.J Theoret Biol, 2003, 223(1): 55–78.DOI: 10.1016/S0022-5193(03)00072-9 |
| [30] | Wang XN, Liang ZZ, Jin H, et al. Identification and functional evaluation of the reductases and dehydrogenases from Saccharomyces cerevisiae involved in vanillin resistance.BMC Biotechnol, 2016, 16: 31.DOI: 10.1186/s12896-016-0264-y |
