
 , 陈勇
, 陈勇

湖北大学 中药生物技术省重点实验室 生物资源绿色转化湖北省协同创新中心,湖北 武汉 430062
收稿日期:2017-01-04;接收日期:2017-04-06; 网络出版时间:2017-04-18 基金项目:湖北省教育厅科学技术研究计划青年人才项目(No. Q20141002) 资助
摘要:构建了miR-29b2c重组腺病毒Ad-miR-29b2c,考察了其对HGC-27、MGC-803胃癌细胞增殖及迁移的抑制作用。采用PCR从基因组扩增miR-29b2c片段,并克隆至腺病毒穿梭载体pAdTrack-CMV中,构建穿梭质粒pAdT-29b2c,经酶切及测序鉴定。穿梭质粒经PmeⅠ线性化后与腺病毒骨架载体共转化BJ5183感受态,产生重组腺病毒质粒Ad-miR-29b2c,再经PacⅠ线性化后转染293A细胞进行包装。重组腺病毒扩增后感染HGC-27细胞,通过MTT及细胞迁移实验观察Ad-miR-29b2c对HGC-27、MGC-803细胞增殖及迁移的影响。采用Western blotting检测Ad-miR-29b2c对HGC-27、MGC-803细胞δ-catenin蛋白表达的影响。酶切、测序及荧光定量PCR结果表明重组腺病毒构建成功,miR-29b及miR-29c在HGC-27细胞过表达。MTT实验表明Ad-miR-29b2c能显著抑制HGC-27、MGC-803细胞增殖。细胞迁移实验表明Ad-miR-29b2c能显著抑制HGC-27、MGC-803细胞迁移。此外,Ad-miR-29b2c能显著降低HGC-27、MGC-803细胞δ-catenin蛋白表达水平。综上所述,构建了miR-29b2c的重组腺病毒,并发现其可以抑制胃癌细胞HGC-27和MGC-803的增殖及迁移,该作用可能与miR-29抑制HGC-27、MGC-803细胞δ-catenin蛋白表达有关。
关键词:miR-29 胃癌 重组腺病毒 基因治疗
Effects of recombinant adenovirus Ad-miR-29b2c on HGC-27 cell proliferation and migration
Ailing Luo, Chunyuan Du, Xuemei Zhao, Jichao Liang

 , Yong Chen
, Yong Chen

Hubei Province Key Laboratory of Biotechnology of Chinese Traditional Medicine, Hubei Collaborative Innovation Center for Green Transformation of Bio-resources, Hubei University, Wuhan 430062, Hubei, China
Received: January 4, 2017; Accepted: April 6, 2017; Published: April 18, 2017
Supported by: Young Talent Project of the Education Department of Hubei Province (No. Q20141002)
Corresponding author:Jichao Liang. Tel/Fax: +86-27-88663590; E-mail: liang529114@163.com
Yong Chen. Tel/Fax: +86-27-88663882; E-mail: cy101610@qq.com
Abstract: We constructed recombinant adenoviruses expressing miR-29b2c (Ad-miR29b2c), and analyzed their effects on the proliferation and migration of HGC-27 and MGC-803 cells. miR-29b2c gene was amplified by PCR from genomic DNA and cloned into the pAdTrack-CMV vector to create the shuttle plasmid pAdT-29b2c. The recombinant plasmid was verified by restriction enzyme digestion and sequencing. The linearized shuttle vector was mixed with an adenoviral backbone plasmid (pAdEasy-1), followed by cotransformation into competent BJ5183 cells to generate the recombinant plasmid pAd-miR-29b2c. Finally, recombinant adenoviral vectors were generated by transfecting the recombinant plasmid into 293A packaging cell line. HGC-27 and MGC-803 cells were infected with the recombinant adenoviruses expressing pAd-miR-29b2c, then MTT and wound-healing assay were used to analyze the effects of pAd-miR-29b2c on the proliferation and migration of HGC-27 and MGC-803 cells. The miR-29b and miR-29c levels were significantly increased in HGC-27 cells after infected with pAd-miR-29b2c. MTT and wound-healing analysis also revealed a significant decrease in proliferation and migration of HGC-27 and MGC-803 cells compared to the control Ad-GFP-infected cells. Furthermore, western blotting results demonstrated that the protein expression level of δ-catenin was reduced in pAd-miR-29b2c transfected HGC-27 and MGC-803 cells. Taken together, the recombinant adenoviral vector was generated, and it can significantly inhibit the proliferation and migration of HGC-27 and MGC-803 cells.
Key words: miR-29 gastric cancer recombinant adenovirus gene therapy
胃癌是一种发病率及死亡率极高的恶性肿瘤,每年约90万人被诊断为胃癌,约70万人死于胃癌[1]。目前对于胃癌的治疗以手术为主,但无论是早期还是中晚期胃癌均存在术后复发的情况,寻找有效的早期诊断指标及治疗标靶是胃癌处理中的一个难题。
miRNA是一类长18-25个核苷酸组成的内源性非蛋白编码RNA,在体内分布广泛,物种间高度保守。在病理及生理条件下,miRNA调节大量蛋白编码基因的表达,并形成复杂的调控网络,在发育、分化、增殖及凋亡等过程中起着非常重要的调节作用。miRNA是最大的一类基因表达转录后调控因子,自2002年发现miRNA表达异常与肿瘤相关以来,越来越多的研究表明多种miRNA均参与肿瘤的发生与发展[2-3]。
研究表明,miR-29家族与肿瘤密切相关。研究人员陆续报道了恶性胆管癌、鼻咽癌、肺癌、成神经细胞瘤、横纹肌肉瘤等多种肿瘤细胞中存在miR-29基因簇或单个基因表达异常[4-10]。miRNA-29家族是一类抑癌基因,对胃癌细胞的增殖和转移发挥负调控作用[11]。最近有研究显示,miR-29表达水平与胃癌患者生存率有密切关系,miR-29高表达胃癌患者与miR-29低表达胃癌患者相比,术后5年生存率更高,提示miR-29家族与胃癌的发生发展可能密切相关[12]。
miR-29家族包括miR-29a、miR-29b和miR-29c等3个成员,且分为miR-29b1a、miR-29b2c两个基因簇位于不同的染色体上。本研究克隆了miR-29b2c基因片段,并构建了重组腺病毒,发现Ad-miR-29b2c能显著抑制胃癌细胞HGC-27和MGC-803的增殖和迁移。
1 材料与方法1.1 材料及试剂pAdTrack-CMV穿梭质粒、pAdEasy-1腺病毒骨架质粒、HGC-27细胞、293A细胞、BJ5183感受态(实验室保存)。限制性内切酶Bgl Ⅱ、Xba Ⅰ (Promega公司);Pme Ⅰ、Pac Ⅰ (NEB公司);DL2000、λ DNA/Hind Ⅲ核酸分子量标准(TaKaRa公司);胶回收试剂盒(AXYGEN公司);质粒提取试剂盒(QIAGEN公司);Lipofectamine 2000 (Invitrogen公司);Taqman荧光定量PCR试剂盒(Applied Biosystems公司);DMEM细胞培养基、胎牛血清(GIBCO公司)。miRNA提取试剂盒(Ambion公司);miRNA反转录及荧光定量检测试剂盒(Applied Biosystems公司)。PVDF膜(Millipore公司),Anti-GAPDH、Anti-Catenin (BOSTER公司),鼠二抗、兔二抗(KPL公司)。BCA试剂盒(Thermo公司),超敏ECL化学发光试剂盒、RIPA裂解液、蛋白酶抑制剂PMSF、蛋白上样缓冲溶液(碧云天公司),蛋白酶抑制剂Cooktail (Roche公司)。
引物设计与合成:用于PCR扩增miR-29b2c片段的引物如下,下划线为引入的酶切位点。
29F:TTGCTCAGATCTGCCTACAGGGTCATGGGAT;29R:CCTTCTTCTAGACCAGGGACCACTTCTCATT。
插入载体的是前体序列,分别为miR-29b (GenBank Accession No. NR_029518.1) 和miR-29c (GenBank Accession No. NR_029832.1)。
1.2 穿梭表达载体pAdT-29b2c的构建PCR扩增出的miR-29b2c片段经BglⅡ/XbaⅠ双酶切后亚克隆到pAdTrack-CMV载体,构建出重组穿梭质粒pAdT-29b2c。重组质粒经测序鉴定,序列完全正确。
1.3 重组腺病毒Ad-miR-29b2c的构建pAdT-29b2c质粒经PmeⅠ线性化后,与pAdEasy-1腺病毒骨架质粒共转化BJ5183感受态细胞,在胞内进行同源重组,涂卡那霉素平板后培养18-24 h,挑取较小单克隆,提取质粒后经PacⅠ酶切鉴定。得到重组腺病毒质粒pAd-miR-29b2c。
1.4 重组腺病毒pAd-miR-29b2c的包装和扩增取4 μg重组腺病毒质粒pAd-miR-29b2c,经PacⅠ酶切线性化,用乙醇进行沉淀回收,按照Lipofectamine 2000转染试剂要求,转染至70%汇合度的293A细胞中,1-2周后可以观察到扩增斑。50%的细胞变圆并漂起后收集细胞,4次液氮/37 ℃反复冻融后离心得到第1代重组腺病毒。
1.5 细胞增殖实验取对数生长期的HGC-27和MGC-803细胞,胰酶消化后接种于96孔板。实验分为5组,对照组给予含pAd-GFP的完全培养基,实验组用完全培养基配置不同滴度的上述收集的重组腺病毒pAd-miR-29b2c,用pAd-GFP调整实验组病毒总量,保证每组细胞感染了相同总量的重组腺病毒以降低实验误差;每组设置5个平行复孔,在37 ℃、5% CO2条件下置于培养箱中培养24 h。然后,每孔加入20 μL 5 g/L MTT溶液,于培养箱中继续培养4 h后,弃掉培养液,每孔加入150 μL DMSO,室温振荡混匀,用酶标仪测定在570 nm处的吸光度,根据吸光值比较各组细胞增殖情况。
1.6 细胞迁移实验取对数生长期的HGC-27和MGC-803细胞,胰酶消化后接种于6孔板(过夜铺满)。翌日用干净的蓝枪头均匀划痕,将不同浓度的重组腺病毒pAd-miR-29b2c及空白对照置于含pAd-GFP的无血清培养基中继续培养,观察24 h和48 h后迁移到划痕区细胞的情况,每组设3个重复孔。
1.7 实时定量PCR采用miRNA分离提取试剂盒提取总miRNA,miRNA经Applied Biosystems miRNA反转录试剂盒反转录后采用Taqman探针法定量分析miR-29b及miR-29c水平。
1.8 蛋白印迹实验取对数生长期的胃癌细胞,胰酶消化后接种于6孔板。翌日,分别转染Ad-GFP和Ad-miR-29b2c。细胞融合至90%左右,取出6孔板,预冷PBS清洗2-3次,再加入预先配好的细胞裂解液,充分裂解后,经BCA蛋白测定试剂盒测得总蛋白浓度。裂解液中加入蛋白上样缓冲溶液,沸水浴10 min变性,进行10% SDS-PAGE。电泳结束后,用湿转法将凝胶上的蛋白转移到PVDF膜上,电转结束后用含5%脱脂奶粉的TBST溶液封闭2 h,将PVDF膜移至配好的GAPDH和Catenin一抗(1:1 000) 中,4 ℃孵育过夜。TBST溶液洗膜6次,8 min/次,分别加入鼠二抗、兔二抗(1:5 000) 于28 ℃孵育1 h,TBST溶液洗膜6次,8 min/次,加入超敏ECL化学发光液后置于暗室曝光。
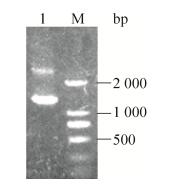
2 结果与分析2.1 pAdT-29b2c穿梭质粒的构建与鉴定PCR扩增出miR-29b2c片段,凝胶电泳检测,如图 1所示,片段大小与设计一致。目标片段及pAd-Track-CMV空载体经BglⅡ/XbaⅠ双酶切后用T4 DNA连接酶进行连接,转化大肠杆菌后挑取单克隆,小量提取质粒,并用BglⅡ/XbaⅠ双酶切进行初步鉴定。如图 2所示,质粒经BglⅡ/XbaⅠ双酶切后,出现了一条约1.3 kb的小片段。取阳性克隆送公司测序,结果证明插入序列与设计完全一致,没有突变,插入方向正确。结果表明重组穿梭质粒pAdT-29b2c构建成功。
 |
| 图 1 miR-29b2c基因序列的扩增 Figure 1 PCR amplification of miR-29b2c gene sequence. M: DL2000 marker; 1, 2: miR-29b2c fragments. |
| 图选项 |
 |
| 图 2 重组穿梭质粒pAdT-29b2c的酶切鉴定 Figure 2 Restriction endonuclease analysis of recombinant shuttle plasmid pAdT-29b2c. |
| 图选项 |
2.2 重组腺病毒载体的构建及鉴定重组穿梭质粒pAdT-29b2c经PmeⅠ酶切线性化后凝胶电泳回收。回收片段与100 ng腺病毒骨架载体pAdEasy-1混合后,电转化BJ5183感受态细胞,挑菌、提取重组质粒,重组质粒经PacⅠ酶切,电泳。如图 3所示,根据重组腺病毒包装系统说明书,酶切产生4.5 kb的片段,说明腺病毒重组成功[13],命名为pAd-miR-29b2c。
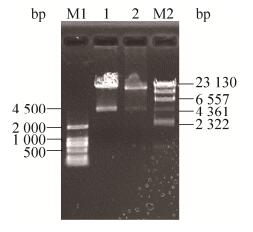 |
| 图 3 Pac Ⅰ酶切重组质粒pAd-miR-29b2c Figure 3 Pac Ⅰ digestion of recombinant plasmid pAd-miR-29b2c. M1: DL2000 marker; M2: λ-Hind Ⅲ digest DNA marker. |
| 图选项 |
2.3 重组腺病毒的包装及扩增腺病毒重组质粒经PacⅠ酶切线性化后,转染293A细胞进行包装,48 h后荧光显微镜下可见少量GFP (绿色荧光蛋白)的表达,1周后细胞基本都变绿,如图 4所示。50%的细胞漂起后收集细胞,反复冻融释放病毒(Ad-miR-29b2c),重新感染新鲜的293A细胞进行病毒扩增。
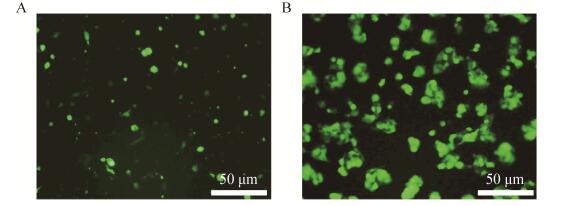 |
| 图 4 线性化重组质粒pAd-miR-29b2c转染293A细胞3 d (A)和5 d (B)后的绿色荧光蛋白表达 Figure 4 Green fluorescent protein expression in 293A cells 3 d (A) and 5 d (B) after transfected by linearized recombinant plasmid pAd-miR-29b2c. |
| 图选项 |
2.4 重组腺病毒在HGC-27细胞中的表达培养HGC-27细胞,在对数生长期分别感染Ad-GFP及重组腺病毒Ad-miR-29b2c。24及48 h后收集细胞,提取总miRNA,采用Taqman探针法对miR-29b及miR-29c进行定量分析。如图 5所示,与感染Ad-GFP组相比,感染Ad-miR-29b2c组miR-29b及miR-29c表达水平明显升高。
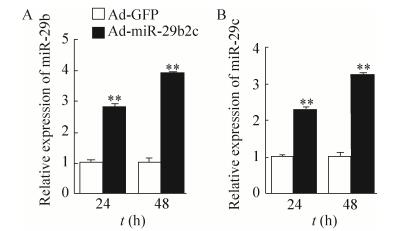 |
| 图 5 Real-time PCR分析经重组腺病毒感染后的HGC-27细胞中miR-29b和miR-29c表达水平 Figure 5 Real-time PCR analysis of miR-29b and miR-29c expression levels in HGC-27 cells after infected by indicated adenovirus (**P < 0.01). |
| 图选项 |
2.5 重组腺病毒对HGC-27和MGC-803细胞增殖的影响HGC-27细胞分别感染Ad-GFP及Ad-miR-29b2c,24、48及72 h后MTT法检测抑制效果。如图 6所示,与感染Ad-GFP组相比,感染Ad-miR-29b2c组对HGC-27和MGC-803细胞有明显的抑制作用,48 h及72 h比24 h抑制作用更强。
 |
| 图 6 HGC-27和MGC-803细胞感染重组腺病毒后MTT分析细胞活力 Figure 6 Cell viability analysis of HGC-27 and MGC-803 cells treated with indicated adenovirus was measured using an MTT assay. The Ad-GFP in experimental group was used to normalize the total amount of adenoviruses. |
| 图选项 |
2.6 重组腺病毒对HGC-27和MGC-803细胞迁移能力的影响划痕实验结果显示(图 7),与感染Ad-GFP组相比,感染Ad-miR-29b2c组HGC-27和MGC-803细胞迁移明显受到了抑制。
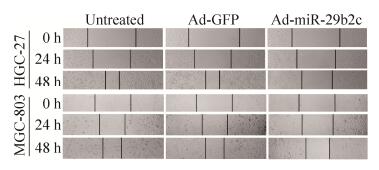 |
| 图 7 HGC-27和MGC-803细胞中细胞迁移分析 Figure 7 Cell migration assay was conducted in HGC-27 and MGC-803 cells. |
| 图选项 |
2.7 重组腺病毒对HGC-27和MGC-803细胞Catenin蛋白表达的影响Western blotting结果显示,与感染Ad-GFP组相比,感染Ad-miR-29b2c组Catenin蛋白表达水平明显降低,如图 8所示。
 |
| 图 8 蛋白印迹分析δ-catenin在HGC-27和MGC-803细胞中的表达 Figure 8 Western blotting analysis of δ-catenin protein expression in HGC-27 and MGC-803 cells. |
| 图选项 |
3 讨论胃癌是最常见的消化道恶性肿瘤之一[14],全球范围内发病率高,仅次于肺癌、乳腺癌及结直肠癌[15]。胃癌死亡率更是位居恶性肿瘤第二[16]。我国每年新发病例大约40万,约占世界总发病例数的42%[17]。胃癌已经严重威胁我国居民的生活健康。
miRNA的发现及其调控机制的阐明为深入理解很多生理病理过程提供了新的重要线索及视野[3, 18-20]。近年来,研究发现,多种miRNA的异常表达与胃癌的发生发展密切相关(miR-448[21]、miR-let-7a[22]、miR-133[23]、miR-206[24])。
miR-29家族包含miR-29a、miR-29b及miR-29c等3个成员。研究表明,miR-29家族通常扮演抑癌基因的角色,抑制多种肿瘤细胞的生长[25]。
目前,虽然miR-29c已被发现与胃癌细胞增殖相关,但miR-29家族在体内分布在不同的染色体上,miR-29b与miR-29a及miR-29c分别形成两个miRNA簇,即miR-29b1a及miR-29b2c。miRNA通常在物种之间非常保守,主要通过其种子序列靶向结合于靶mRNA 3′非翻译区,抑制靶基因的翻译。miR-29a、miR-29b及miR-29c种子序列完全一致,即5′-UAGCACCAU-3′。研究表明,miR-29家族成员在多种细胞内可以同时靶向同1个靶mRNA,该调控模式的意义还有待研究。miR-29b1a和miR-29b2c存在于不同的基因簇,加工后分别表达miR-29b、miR-29a及miR-29b、miR-29c。为了研究miR-29b2c与胃癌之间的关系,本研究克隆了其片段,并包装成重组腺病毒,模拟体内miR-29b与miR-29c共表达的现象,通过细胞增殖及迁移实验,发现miR-29b与miR-29c能显著抑制胃癌细胞增殖及迁移,为实现腺病毒基因治疗胃癌提供线索。miR-29b1a是否具有相同或类似的影响胃癌细胞增殖及迁移的活性,需要单独包装其腺病毒,作进一步的研究。
研究表明,胃癌细胞的增殖、迁移受到多个信号通路的影响,如PI3K/Akt信号途径。在胃癌、乳腺癌、肺癌等多种恶性肿瘤中均能检测到PI3K/Akt途径的活化[26-28],并通过其下游效应分子调节肿瘤细胞的分化、侵袭转移等,与临床肿瘤预后密切相关。此外,HIF-1α/VEGF途径在胃癌的发生、发展及侵袭转移方面也发挥了重要作用。胃癌组织的低氧状态诱导HIF-1α的活化,从而调节包括葡萄糖转运蛋白、血管内皮生长因子等多种基因的表达水平,导致胃癌恶化、转移[29]。
δ-catenin最初作为1个神经特异性表达蛋白被发现。研究表明,δ-catenin可以与经典的钙粘素、钙调节的细胞-细胞链接蛋白等相互作用,参与多种人类疾病的发生发展过程,如δ-catenin的表达异常与近视、阿尔兹海默病及精神分裂症相关[30]。最新的研究表明,δ-catenin影响Wnt信号通路及参与Rho GTP酶的调节,其突变及表达紊乱与包括胃癌在内的多种肿瘤的增殖及转移密切相关[31]。鉴于δ-catenin在胃癌发生发展中的重要性,以及其被预测为miR-29家族的潜在靶基因,我们在胃癌细胞中检测了其表达水平是否受到miR-29家族的调控。
综上所述,腺病毒介导的过表达miR-29b2c抑制HGC-27及MGC-803胃癌细胞增殖和迁移,该活性可能与δ-catenin蛋白表达水平有关。
参考文献
| [1] | Herrero R, Parsonnet J, Greenberg ER. Prevention of gastric cancer.JAMA, 2014, 312(12): 1197–1198.DOI: 10.1001/jama.2014.10498 |
| [2] | Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay.Nat Rev Genet, 2010, 11(9): 597–610. |
| [3] | David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged.Genes Dev, 2010, 24(21): 2343–2364.DOI: 10.1101/gad.1973010 |
| [4] | Mott JL, Kobayashi S, Bronk SF, et al. miR-29 regulates Mcl-1 protein expression and apoptosis.Oncogene, 2007, 26(42): 6133–6140.DOI: 10.1038/sj.onc.1210436 |
| [5] | Sengupta S, den Boon JA, Chen IH, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins.Proc Natl Acad Sci USA, 2008, 105(15): 5874–5878.DOI: 10.1073/pnas.0801130105 |
| [6] | Garzon R, Liu SJ, Fabbri M, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1.Blood, 2009, 113(25): 6411–6418.DOI: 10.1182/blood-2008-07-170589 |
| [7] | Pekarsky Y, Santanam U, Cimmino A, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181.Cancer Res, 2006, 66(24): 11590–11593.DOI: 10.1158/0008-5472.CAN-06-3613 |
| [8] | Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B.Proc Natl Acad Sci USA, 2007, 104(40): 15805–15810.DOI: 10.1073/pnas.0707628104 |
| [9] | Xu H, Cheung IY, Guo HF, et al. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors.Cancer Res, 2009, 69(15): 6275–6281.DOI: 10.1158/0008-5472.CAN-08-4517 |
| [10] | Wang HT, Garzon R, Sun H, et al. NF-κB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma.Cancer Cell, 2008, 14(5): 369–381.DOI: 10.1016/j.ccr.2008.10.006 |
| [11] | Gong JN, Li JX, Wang Y, et al. Characterization of microRNA-29 family expression and investigation of their mechanistic roles in gastric cancer.Carcinogenesis, 2014, 35(2): 497–506.DOI: 10.1093/carcin/bgt337 |
| [12] | Chen L, Chen XH, Li XC, et al. Expression of miR-29 in serum of gastric cancer patients and its effect on prognosis.J Mol Diagn Ther, 2011, 3(2): 90–92.(in Chinese). 陈陵, 陈先华, 李学成, 等. miR-29在胃癌患者血清中的表达及其对预后的影响.分子诊断与治疗杂志, 2011, 3(2): 90-92. |
| [13] | Liang JC, Liu CZ, Qiao AJ, et al. MicroRNA-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis.J Hepatol, 2013, 58(3): 535–542.DOI: 10.1016/j.jhep.2012.10.024 |
| [14] | Jemal A, Bray F, Center MM, et al. Global cancer statistics.CA Cancer J Clin, 2011, 61(2): 69–90.DOI: 10.3322/caac.v61:2 |
| [15] | Dong ZW, Qiao YL, Li LD, et al. Report of Chinese cancer control strategy.Bull Chin Cancer, 2002, 11(5): 250–260.(in Chinese). 董志伟, 乔友林, 李连弟, 等. 中国癌症控制策略研究报告.中国肿瘤, 2002, 11(5): 250-260. |
| [16] | Wu WKK, Lee CW, Cho CH, et al. MicroRNA dysregulation in gastric cancer: a new player enters the game.Oncogene, 2010, 29(43): 5761–5771.DOI: 10.1038/onc.2010.352 |
| [17] | Zou WB, Li ZS. Progress in the incidence and mortality of gastric cancer in China.Chin J Prac Intern Med, 2014, 34(4): 408–415.(in Chinese). 邹文斌, 李兆申. 中国胃癌发病率及死亡率研究进展.中国实用内科杂志, 2014, 34(4): 408-415. |
| [18] | Link A, Kupcinskas J, Wex T, et al. Macro-role of microRNA in gastric cancer.Dig Dis, 2012, 30(3): 255.DOI: 10.1159/000336919 |
| [19] | Xie Y, Todd NW, Liu ZQ, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer.Lung Cancer, 2010, 67(2): 170–176.DOI: 10.1016/j.lungcan.2009.04.004 |
| [20] | Chen YC, Zhu XD, Zhang XJ, et al. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy.Mol Ther, 2010, 18(9): 1650–1656.DOI: 10.1038/mt.2010.136 |
| [21] | Wu XS, Tang HR, Liu GB, et al. miR-448 suppressed gastric cancer proliferation and invasion by regulating ADAM10.Tumor Biol, 2016, 37(8): 10545–10551.DOI: 10.1007/s13277-016-4942-0 |
| [22] | Tang R, Yang C, Ma X, et al. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in gastric cancer.Oncotarget, 2016, 7(5): 5972–5984. |
| [23] | Liu YF, Zhang X, Zhang YJ, et al. Identification of miRNomes in human stomach and gastric carcinoma reveals miR-133b/a-3p as therapeutic target for gastric cancer.Cancer Lett, 2015, 369(1): 58–66.DOI: 10.1016/j.canlet.2015.06.028 |
| [24] | Zheng ZQ, Yan DS, Chen XY, et al. MicroRNA-206: effective inhibition of gastric cancer progression through the c-met pathway.PLoS ONE, 2015, 10(7): e0128751.DOI: 10.1371/journal.pone.0128751 |
| [25] | Zhao JJ, Lin JH, Lwin T, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma.Blood, 2010, 115(13): 2630–2639.DOI: 10.1182/blood-2009-09-243147 |
| [26] | Han ZW, Rong RZ, Kong PZ, et al. Expression of MUC15 and PI3K/Akt in gastric carcinoma and its association with clini-copathological characteristics and prognosis.Chin J Clin Oncol, 2016, 43(2): 56–61.(in Chinese). 韩志伟, 戎瑞洲, 孔鹏洲, 等. MUC15和PI3K/Akt表达与胃癌临床病理特征及预后的相关性研究.中国肿瘤临床, 2016, 43(2): 56-61. |
| [27] | Lin XD, Hu D, Chen G, et al. Association of SNP in the PI3K-PTEN-AKT-mTOR pathway with genetic susceptibility of gastric cancer.J Clin Exp Pathol, 2016, 32(4): 361–365,369.(in Chinese). 林贤东, 胡丹, 陈刚, 等. PI3K-AKT-mTOR信号通路基因多态性与胃癌遗传易感相关性.临床与实验病理学杂志, 2016, 32(4): 361-365,369. |
| [28] | Murakami D, Tsujitani S, Osaki T, et al. Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy.Gastric Cancer, 2007, 10(1): 45–51.DOI: 10.1007/s10120-006-0410-7 |
| [29] | Urano N, Fujiwara Y, Doki Y, et al. Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma.Gastric Cancer, 2006, 9(1): 44–49.DOI: 10.1007/s10120-005-0356-1 |
| [30] | Lu Q, Aguilar BJ, Li MC, et al. Genetic alterations of δ-catenin/NPRAP/Neurojungin (CTNND2): functional implications in complex human diseases.Hum Genet, 2016, 135(10): 1107–1116.DOI: 10.1007/s00439-016-1705-3 |
| [31] | Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer.Oncogene, 2004, 23(48): 7947–7956.DOI: 10.1038/sj.onc.1208161 |
