
 , 何培民1
, 何培民11 上海海洋大学 水产与生命学院,上海 201306
2 Department of Molecular Membrane Biology, Max Planck Institute of Biophysics, Max-von-Laue-Str.3, D-60438 Frankfurt am Main, Germany
3 中国科学院植物研究所,北京 100093
网络出版时间:2016-02-03
基金项目:国家高技术研究发展计划(863计划) (No. 2014AA093506)资助
摘要: 工程聚球藻的psbA基因及其所用载体的启动子PpsbA均受光质调控,利用该机制可通过光质调控提高聚球藻的光合效率及其外源基因表达率。以转vp28基因聚球藻7002为实验材料,通过优化光强、温度及pH,解除光限制因素并提高光能利用率。通过改变白光、红光及蓝光的比例,调控光质组成及单色光的光强,检测细胞生长、外源基因的表达及psbA基因的转录。研究结果表明:高比例蓝光下,vp28基因的表达率达到2.4%,是纯白光下的3倍,重组蛋白VP28的积累量提高至2倍。高比例红光抑制了psbAⅡ、psbAⅢ基因及外源基因的表达,但促使生物量在3 d内突破1.5 g/L。本研究为蓝藻的生物制药和工程蓝藻代谢产物的产业化提供了理论基础。
关键词: 光生物反应器 聚球藻7002 psbA启动子 VP28 光质调控 psbA
Effects of light quality on cell growth and psbA promoter of engineered Synechococcus sp. PCC7002
Yihua Sun1, Chunli Zhang2, Dingji Shi3, Xiaohui Jia1, Rui Jia1

 , Peimin He1
, Peimin He1 1 College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China;
2 Department of Molecular Membrane Biology, Max Planck Institute of Biophysics, Max-von-Laue-Str.3, D-60438 Frankfurt am Main, Germany;
3 Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China
Received: January 3, 2016; Accepted: January 29, 2016
Supported by:National High Technology Research and Development Program of China (863 Program) (No. 2014AA093506)
Corresponding authors:Rui Jia. Tel: +86-21-61900449; E-mail: rjia@shou.edu.cn
Abstract: Light quality can regulate both psbA genes and vector promoter psbA of the engineered Synechococcus. Through light regulation, we tried to improve yield of the recombinant protein for vp28 gene-expressed Synechococcus sp. PCC7002. To drive photon-capturing efficiently, three limiting factors (irradiance, temperature and pH) were optimized by measuring net photosynthesis. High cell density cultures were performed with variant ratios of white, red and blue light in a 5-L photo-bioreactor. Yields of biomass, expressions of vp28 and transcription levels of psbA were compared. High ratio blue light-induced vp28 transcription had tripled and the relative accumulation of VP28 protein was doubled. The relative expressions of psbAⅡ and psbAⅢ had positive correlations with higher ratio of blue light, not the red light. With high ratio red light inducing, dry biomass reached 1.5 g/L in three days. Therefore, we speculated that red light accelerated biomass accumulation of the transgenic strain and blue light promoted transcription for PpsbA and psbA. These results provided useful information for mass production of cyanobacteria and its secondary metabolites.
Key words: biophotoreactor Synechococcus sp. PCC7002 psbA promoter VP28 light quality regulation psbA
随着蓝藻的产业化发展,光质调控已成为提高生物量及目的产物表达的重要途径[1-3],转录因子介导的光调控机制逐步被揭示[4]。光质是光源所包含的所有单色光的光强及波长的总称,直接影响藻细胞的光合作用及代谢途径。聚球藻光系统Ⅱ的捕光天线主要受psbA基因的调控[5],psbA基因对光质具有较敏感的响应机制[6]。同时,工程蓝藻常用的载体启动子PpsbA[7]与蓝藻的psbA基因同源[8],蓝光与红光分别诱导的信号通路可同时调节蓝藻本身的psbA基因及其载体启动子PpsbA[9]。然而,相对于野生蓝藻,转基因蓝藻的最适光合条件已有显著改变[10],若要研究光质对于psbA基因及PpsbA的调控,必须先排除光合作用的干扰因素(总光强、温度与pH),以达到最大光合效率。
新型光生物反应器(SDJ,上海联环生物工程设备有限公司)可对总光强、温度、pH、光质进行精确调控,能够使藻细胞达到最大光合效率,是工程蓝藻研究及产业化探索的重要工具。光生物反应器配备的LED光源可提供稳定的光质(红光615-630 nm,蓝光440-475 nm),已普遍应用于微藻培养[11]。研究发现,相同LED光质对于不同藻种的影响显示出差异,例如蓝光促进拟绿球藻Nannochloropsis sp.细胞增殖[12],却会抑制小球藻Chlorella sp.的生长并促进其油脂积累[13]。因此,研究LED光质对工程蓝藻生长的影响是产业化的必要途径。
白斑综合症病毒(WSSV)于1992年首先在中国台湾地区发现,20年来WSSV在亚洲、南北美洲、欧洲和非洲造成的经济损失已超过70亿美元,尚未见在规模生产中应用有效药物来防治[14]。Witteveldt等[15]首次用大肠杆菌表达的VP28 (WSSV被膜中的结构蛋白),经口服和注射接种,可提高对虾抗WSSV的能力。Jia等[10]使用转vp28基因蓝藻,经口服后攻毒,成功提高了对虾的成活率,证明口服转基因蓝藻能有效抵抗WSSV。工程蓝藻口服剂的研制已进入中试阶段,其中工程蓝藻的规模培养是产业化的基础。聚球藻属Synechococcus sp.由于适合光反应器培养,已被广泛用于规模培养的研究[16],因此转vp28基因聚球藻7002[17]在产业化上非常具有前景。
1 材料与方法1.1 转vp28基因聚球藻的摇床培养转vp28基因聚球藻S. PCC7002由中国科学院植物研究所提供,装在含150 mL BG-11培养基[18]的250 mL摇瓶中进行培养,温度为30 ℃,摇床转速为130 r/min,光照为50 μmol/(m2·s),连续光照。
1.2 净光合作用的单因素试验聚球藻7002的叶绿素浓度通过公式(1)[19]测定:
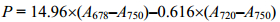 | (1) |
净光合速率使用氧电极(Hansatech,UK)测定,温度由恒温水浴箱(Julubo,Germany)通过循环水控制。测定样品在不同条件下的净光合速率(光强,温度,pH)[20]。净光合速率计算采用公式(2):
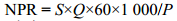 | (2) |
1.3 转vp28基因聚球藻的光生物反应器培养封闭式光生物反应器为SDJ-5L型光合发酵系统(上海联环生物工程设备有限公司),由全自动圆柱形玻璃反应器及外置LED光源构成。该系统可对pH、温度、光照进行恒定控制,培养条件(光照、温度、pH)按照优化后的指标((300±10) μmol/(m2·s),37 ℃,pH 7.5)进行设置。工作体积为3.5 L,初始接种量使藻液A750达到0.1±0.02,平均空气流量为1 L/min,平均CO2的体积分数为0.5%。培养时长为72 h。LED光源采用白、红、蓝进行调控,平均光强测定采用公式(3)[21]:
 | (3) |
 | (4) |
调控白光、红光、蓝光的配比并设置White、Red、Blue、Balance共4个实验组(表 1),通过公式(3)测定并调控平均光强,使得平均光强符合优化的标准((300±10) μmol/(m2·s))。
表 1 光质配比及平均光强Table 1 The proportions of light quality and average irradiance
| Item | White light: Red light: Blue light | Iav (μmol/(m2·s)) |
| White | 6:0:0 | 294-146 |
| Red | 3:2:1 | 308-138 |
| Blue | 3:1:2 | 292-171 |
| Balance | 2:2:2 | 303-154 |
表选项
1.4 vp28表达率测定及VP28蛋白检测Trizol法提取转vp28基因聚球藻7002的总RNA。以总RNA为模板,用试剂盒反转录为cDNA(Tiangen,China)。vp28序列参照文献[17]并设计vp28的qPCR引物(表 2)。管家基因16s rRNA的引物采用已有模板[23]。20 μL PCR反应体系包括2 μL cDNA,0.6 μL正向引物与反向引物,10 μL SYBRFast qPCR Master Mix (2X) (KAPA,USA)。参照Jia等[10]的方法,计算vp28表达率并用Western blotting检测蛋白条带。
表 2 本实验所用引物Table 2 Primers used in this study
| Primer name | Primer sequence (5'–3') |
| vp28-FW | AACCTCCGCATTCCTGTGACTG |
| vp28-RV | TCCGCATCTTCTTCCTTCATCTGTG |
| psbAI-FW | CGTATTCCAGGCAGAGCACAACAT |
| psbAI-RV | GCAGTGAACCAGATACCGACTACAG |
| psbAⅡ-FW | ATGTTGTGCTCTGCCTGGAATACG |
| psbAⅡ-RV | TCACTGCCACCACCTGCTTCA |
| psbAⅢ-FW | GCGACTGCTGTGCTCTTGGTT |
| psbAⅢ-RV | TCCGTTTCTGTGGTCTCCCGAAT |
表选项
1.5 psbA的表达分析在NCBI (http://www.ncbi.nlm.nih.gov/)上找到对应的蛋白序列psbAI (ID: 501262777)、psbAⅡ (ID: 501264014)、psbAⅢ (ID: 501264746),并利用tblastn反向定位到聚球藻7002基因组全序列(CP000951)的对应片段psbAI (region: 1489906-1490985)、psbAⅡ (region: 161804-162883)、psbAⅢ (region: 2245055-2246134)。分别设计qPCR引物(表 2)。取接种前的样品(藻种)作为参照,每隔24 h对White、Red、Blue三个实验组的样品进行检测,其中qPCR方法及反应体系同1.5。
2 结果与讨论2.1 pH、光强、温度对藻细胞光合作用的影响光合作用中,除了光强直接供给电子激活的能量,温度与pH对酶活力的影响决定了光合作用的效率[24]。因此,必须首先排除温度与pH的干扰,使得光强的能量最大限度地输入光系统Ⅱ,才能进一步分析出光的成分(光质)对于捕光效果的影响。在温度为35 ℃、pH为7.3的条件下,研究光强(50-800 μmol/(m2·s))对净光合速率的影响(图 1A)。随着光强的增加,净光合速率不断增加。当光强达到400 μmol/(m2·s)时,净光合速率出现下降,从320 μmol O2/mg Chlah左右下降至281 μmol O2/mg Chlah。在光强为300 μmol/(m2·s),pH为7.3的条件下,研究温度(15-50 ℃)对净光合速率的影响(图 1B)。随着温度增加,净光合速率不断增加。当温度达到40 ℃后,净光合速率突然下降,50 ℃时净光合速率从320 μmol O2/mg Chlah变为0。在光强为300 μmol/(m2·s),温度为35 ℃的条件下,研究pH 5.5-9.0对净光合速率的影响(图 1C)。随着pH增加,净光合速率不断增加。当pH达到7.5后,净光合速率开始下降,净光合作用从326 μmol O2/mg Chlah下降至210 μmol O2/mg Chlah。
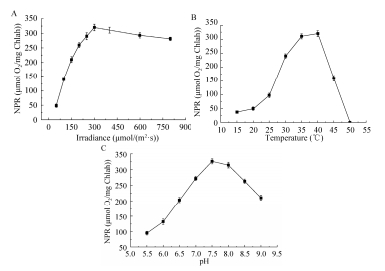 |
| 图 1 光强、温度、pH对净光合速率的影响 Figure 1 Effect of irradiance, temperature and pH on net photosynthetic rates. (A) Irradiance. (B) Temperature. (C) pH. |
| 图选项 |
2.2 藻细胞干生物量的积累光生物反应器培养的实验中,我们尝试用单色光(蓝光)来培养聚球藻,但细胞生长速度极慢,细胞样品的采集与处理遇到较大困难,并且细胞浓度过低严重影响到基因与蛋白的检测。因此,为了方便实验以及产业化生产的需要,本研究以3 d内干生物量达到1 g/L为最低标准,进行光质配比的设定。随着细胞密度的升高,入射光强并不能实时反映藻细胞的光能利用率[22]。从开始培养到结束,藻细胞的光能利用率处于一个由高至低的动态的变化过程。本研究采用平均光强代替入射光作为计量参数[26],计算出了平均光强的变化范围(表 1),以求反应实际的光能利用率[11]。White、Red、Blue、Balance四个实验组72 h的干生物量分别为1.48 g/L、1.68 g/L、1.22 g/L、1.38 g/L。图 3中,培养前36 h内,4组藻细胞进入对数生长期,干生物量积累速度差异不显著。培养36 h后,Red组与Blue、Balance组的干生物量积累速度差异显著,Red组生长速度最快;Red与Blue组差异极显著,Blue组明显生长较慢;White组与Balance组差异不显著,生长速度较接近。光生物反应器的验证培养结果表明,在生物量积累上,已经超过了同类实验[22, 25],3 d内平均干重超过1.2 g/L。
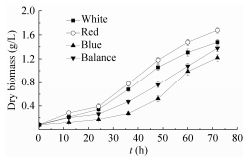 |
| 图 2 不同光质组成下干生物量积累曲线 Figure 2 Variations of dry biomass with various proportions of light quality. |
| 图选项 |
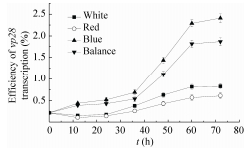 |
| 图 3 不同光质组成下vp28表达率变化曲线 Figure 3 Variations of vp28 transcription with various proportions of light quality. |
| 图选项 |
2.3 vp28基因的表达量与VP28蛋白的积累宁文艳等[27]提出光调控启动子PpsbA是鱼腥藻的高效表达载体,未经光调控的PpsbA表达效率是IPTG调控下Ptac的1.17倍。然而尚未有报道指出转基因鱼腥藻的载体启动子PpsbA可接受光质调控,未经光调控的转vp28基因鱼腥藻7120的表达效率只有0.1%-1%[10]。转基因聚球藻7002所用表达载体为prl-489,启动子为PpsbA[17]。本文中高比例蓝光促进了vp28基因的表达,并促进了psbAⅡ、psbAⅢ的转录,这与Tsinoremas等[9]研究转基因聚球藻7942结果一致。图 3与图 5的结果证明,载体启动子PpsbA与光合基因psbA的转录在时空效应上具有一致性,均受到蓝光的诱导作用。然而外源蛋白(VP28)的积累量(图 4)差异不如外源基因表达差异明显(图 3),这可能是DNA转录与mRNA翻译的时空差异造成的。图 3中,White、Red、Blue、Balance四组vp28基因表达率总体均呈现上升趋势,前36 h内4组vp28表达率都较低。其中,Blue与Balance组表达率较高,White与Red组表达率较低,36 h后前两组与后两组相比差异明显。Blue与Balance组在前36 h及60 h后差异不显著,而在36 h至60 h之间差异明显。White与Red组在72 h内差异都不明显。Blue组中vp28基因表达率最高达到2.41%,出现于第72 h。Red组中vp28基因表达率最低达到0.11%,出现于12 h。图 4中,总蛋白条带在PAGE上无明显差别,而VP28条带在PVDF上差异较为明显。灰度值从少到多分别为Red、White、Balance、Blue,说明Blue组蛋白积累量最高,Red组蛋白积累量最少,并且Blue组与Red组差异明显。
 |
| 图 5 不同光质组成下psbA的相对表达量 Figure 5 Relative transcription of psbA genes with various proportions of light quality. |
| 图选项 |
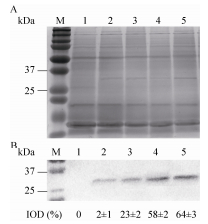 |
| 图 4 同光质组成下VP28的相对含量 Figure 4 Relative accumulation of VP28 in engineered Synechococcus sp. PCC7002 with various proportions of light quality. (A) SDS-PAGE analysis of total protein. (B) Western blotting of VP28. M: marker; 1: wild type; 2: red; 3: white; 4: balance; 5: blue. |
| 图选项 |
2.4 psbA的表达差异图 5显示,接种后光能利用率较高,psbA转录效率比接种前提高了50%-75%。White与Red组psbA的总mRNA表达量高于Blue组。随时间变化,总psbA mRNA含量呈下降趋势。psbAI、psbAⅡ、psbAⅢ在3组中都呈下降趋势,其中psbAⅡ、psbAⅢ在White与Red组中下降速度较快,psbAⅡ分别从第1天的0.72、0.48下降至第3天的0.32、0.28,psbAⅢ分别从0.72、0.42下降至0.21、0.11。而在Blue组中,psbAⅡ与psbAⅢ的下降速度明显较慢,从第1天的0.34、0.78分别下降至第3天的0.20、0.40。Blue组的psbAⅡ与psbAⅢ始终高于White与Red组,而Red组的psbAⅡ与psbAⅢ始终处于较低水平。与纯白光相比,高比例红光并不影响psbA基因总mRNA含量,但最终提高了转基因聚球藻7002的干生物量积累;高比例蓝光抑制了psbA总mRNA的含量,从而抑制了干生物量的积累,但促进了psbAⅡ与psbAⅢ的转录。这可能是由于不同比例的红光、蓝光影响了Rubisco与碳酸酐酶的活性[28],从而影响了碳循环途径。本文中高比例红光促进了生物量的积累,这可能是因为红光诱导了一种以钙调蛋白为二级信使的信号通路[29],并促进了psbAI的表达。
3 结论本文研究了光质调控对工程聚球藻7002生长及vp28表达的影响,确定最优光能利用条件,使得生物量在3 d内突破1.2 g/L。当白光,红光与蓝光的光强比为3:1:2时,重组蛋白VP28表达翻倍。发现蓝光可促进启动子PpsbA表达vp28,红光可提高生物量的积累。通过检测基因转录及目的蛋白表达,证明psbA基因响应了光质调控。研究结果对工程蓝藻的规模培养、重组蛋白的高效表达、转基因生物制药具有重要的参考价值。
参考文献
| [1] | Aguilera J, Francisco J, Gordillo L. Light quality effect on photosynthesis and efficiency of carbon assimilation in the red alga Porphyra leucosticta.J Plant Physiol,2000, 157(1): 86–92.DOI: 10.1016/S0176-1617(00)80140-6 |
| [2] | Niizawa I, Heinrich JM, Irazoqui HA. Modeling of the influence of light quality on the growth of microalgae in a laboratory scale photo-bio-reactor irradiated by arrangements of blue and red LEDs.Biochem Eng J,2014, 90(5): 214–223. |
| [3] | Rashid N, Rehman MSU, Han JI. Enhanced growth rate and lipid production of freshwater microalgae by adopting two-stage cultivation system under diverse light and nutrients conditions.Water Environ J,2015, 29(4): 533–540.DOI: 10.1111/wej.12110 |
| [4] | Nesbit AD, Whippo C, Hangarter RP. Translation initiation factor 3 families: what are their roles in regulating cyanobacterial and chloroplast gene expression?.Photosynth Res,2015, 126(1): 147–159.DOI: 10.1007/s11120-015-0074-4 |
| [5] | Zouni A, Witt HT, Kern J, et al. Crystal structure of photosystem Ⅱ from Synechococcus elongatus at 3.8 ? resolution.Nature,2001, 409(6821): 739–743.DOI: 10.1038/35055589 |
| [6] | Tsinoremas NF, Schaefer M, Golden SS. Blue and red light reversibly control psbA expression in the cyanobacterium Synechococcus sp. strain PCC 7942.J Biol Chem,1994, 269(23): 16143–16147. |
| [7] | Ma WM, Shi DJ, Wang QX, et al. Exogenous expression of the wheat chloroplastic fructose-1, 6-bisphosphatase gene enhances photosynthesis in the transgenic cyanobacterium, Anabaena PCC7120.J Appl Phycol,2005, 17(3): 273–280.DOI: 10.1007/s10811-005-4850-y |
| [8] | Bergsland KJ, Haselkorn R. Evolutionary relationships among eubacteria, cyanobacteria, and chloroplasts: evidence from the rpoC1 gene of Anabaena sp. strain PCC7120.J Bacterial,1991, 173(11): 3446–3455. |
| [9] | Tsinoremas NF, Kawakami A, Christopher DA. High-fluence blue light stimulates transcription from a higher plant chloroplast psbA promoter expressed in a cyanobacterium, Synechococcus (sp. strain PCC7942).Plant Cell Physiol,1999, 40(4): 448–452.DOI: 10.1093/oxfordjournals.pcp.a029562 |
| [10] | Jia XH, Zhang CL, Shi DJ, et al. Oral administration of Anabaena-expressed VP28 for both drug and food against white spot syndrome virus in shrimp.J Appl Phycol,2015.DOI: 10.1007/s10811-015-0607-4 |
| [11] | Cornet JF, Dussap CG, Gros JB. Kinetics and energetics of photosynthetic micro-organisms in photobioreactors// Bioprocess and Algae Reactor Technology, Apoptosis.Berlin Heidelberg: Springer,2007: 153–224.DOI: 10.1007/BFb0102299 |
| [12] | Das P, Wang L, Sarah AS, et al. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light.Bioresour Technol,2011, 102(4): 3883–3887.DOI: 10.1016/j.biortech.2010.11.102 |
| [13] | Shu CH, Tsai CC, Liao WH, et al. Effects of light quality on the accumulation of oil in a mixed culture of Chlorella sp. and Saccharomyces cerevisiae.J Chem Technol Biotechnol,2012, 87(5): 601–607.DOI: 10.1002/jctb.v87.5 |
| [14] | Seibert CH, Pinto AR. Challenges in shrimp aquaculture due to viral diseases: distribution and biology of the five major penaeid viruses and interventions to avoid viral incidence and dispersion.Braz J Microbiol,2012, 43(3): 857–864.DOI: 10.1590/S1517-83822012000300002 |
| [15] | Witteveldt J, Cifuentes CC, Vlak JM, et al. Protection of Penaeus monodon against white spot syndrome virus by oral vaccination.J Virol,2004, 78(4): 2057–2061.DOI: 10.1128/JVI.78.4.2057-2061.2004 |
| [16] | Niki K, Aikawa S, Yokono M, et al. Differences in energy transfer of a cyanobacterium, Synechococcus sp. PCC 7002, grown in different cultivation media.Photosynth Res,2015, 125(1/2): 201–210. |
| [17] | Zhang CL. Cloning of envelope protein vp28 gene of WSSV and its expression in cyanobacteria[D]. Qingdao: Institute of Oceanology, Chinese Academy of Sciences, 2002 (in Chinese). 张春莉. WSSV囊膜蛋白VP28基因在蓝藻中克隆和表达[D].青岛:中国科学院海洋研究所, 2002.http://cdmd.cnki.com.cn/article/cdmd-80068-2002128073.htm |
| [18] | Stanier RY, Kunisawa R, Mandel M, et al. Purification and properties of unicellular blue-green algae (order Chroococcales).Bacteriol Rev,1971, 35(2): 171–205. |
| [19] | Packer L, Glazer AN. Methods in Enzymology: Cyanobacteria.San Diego: Academic Press,1988: 766–778. |
| [20] | Shi DJ, Zhou GF, Fang SX, et al. Studies on photosynthesis, respiration and morphology of Nostoc flagelliforme.Acta Bot Sin,1992, 34(7): 507–514. |
| [21] | Grima EM, Camacho FG, Pérez JAS, et al. Evaluation of photosynthetic efficiency in microalgal cultures using averaged irradiance.Enzyme Microb Tech,1997, 21(5): 375–381.DOI: 10.1016/S0141-0229(97)00012-4 |
| [22] | Kang RJ, Zhou WQ, Cai ZL, et al. Photoautotrophic cultivation of Synechococcus sp. PCC7002 in photobioreactor.Chin J Biotech,2000, 16(5): 618–622.(in Chinese). 康瑞娟, 周文齐, 蔡昭铃, 等. 聚球藻7002在光生物反应器中的光自养培养.生物工程学报, 2000, 16(5): 618-622. |
| [23] | Pinto F, Pacheco CC, Ferreira D, et al. Selection of suitable reference genes for RT-qPCR analyses in cyanobacteria.PLoS ONE,2012, 7(4): e34983.DOI: 10.1371/journal.pone.0034983 |
| [24] | Moore LR, Goericke R, Chisholm SW. Comparative physiology of synechococcus and prochlorococcus-influence of light and temperature on growth, pigments, fluorescence and absorptive properties.Mar Ecol Prog Ser,1995, 116: 259–275.DOI: 10.3354/meps116259 |
| [25] | Zeng WL, Zhao FF, Cao ZG, et al. Medium optimization by response surface method for transgenic Synechococcus sp. PCC 7002 with mouse metallothionein-I gene.Chin J Biotech,2008, 24(1): 130–136.(in Chinese). 曾文炉, 赵飞飞, 曹照根, 等. 利用响应面方法优化转小鼠金属硫蛋白-I基因聚球藻7002的培养基成分.生物工程学报, 2008, 24(1): 130-136. |
| [26] | Camacho-Rubio F, Padial-Vico A, Martinez-Sancho ME. The effect of the mean intensity of light on the cultivation of Chlorella pyrenoidosa.Int Chem Eng,1985, 25(2): 283–288. |
| [27] | Ning WY, Wu XM, Wang CM, et al. Efficiency comparison of promoters Ptac & PsbA driving hG-CSF expression in Anabaena sp. PCC7120.J Mirobiol,2014, 34(3): 36–41.(in Chinese). 宁文艳, 吴先敏, 王春梅, 等. 启动子Ptac与PsbA在鱼腥藻7120中表达hG-CSF的效率比较.微生物学杂志, 2014, 34(3): 36-41. |
| [28] | Roscher E, Zetsche K. The effects of light quality and intensity on the synthesis of ribulose-1, 5-bisphosphate carboxylase and its mRNAs in the green alga Chlorogonium elongatum.Planta,1986, 167(4): 582–586.DOI: 10.1007/BF00391236 |
| [29] | Alizadeh D, Cohen A. Red light and calmodulin regulate the expression of the psbA binding protein genes in Chlamydomonas reinhardtii.Plant Cell Physiol,2010, 51(3): 312–322. |
