王小敏1

 , 徐祥兰2, 徐鹏1, 王兴开1, 惠禄露1, 范红结3, 何孔旺2
, 徐祥兰2, 徐鹏1, 王兴开1, 惠禄露1, 范红结3, 何孔旺2

1. 遵义医科大学微生物学教研室, 贵州 遵义 563000;
2. 江苏省农业科学院兽医研究所, 江苏 南京 210014;
3. 南京农业大学动物医学院, 江苏 南京 210095
收稿日期:2019-11-12;修回日期:2020-04-01;网络出版日期:2020-06-17
基金项目:国家自然科学基金(31860706);遵义医学院博士科研启动资金(F-872);贵州省教育厅青年科技人才成长项目(黔教合KY字[2018]222);遵义医学院大学生创新创业训练计划(遵医201751051)
*通信作者:王小敏, Tel:+86-851-28642718;E-mail:WXM_ZMU@163.com;
何孔旺, E-mail:kwh2003@yeah.net.
摘要:[目的] 通过高通量测序的方法获得PCV2感染3D4/21细胞的miRNAs表达谱,并探讨miRNA-98在PCV2复制中的作用。[方法] 本研究以猪肺泡巨噬细胞系3D4/21细胞为细胞模型,对PCV2感染过程中的3D4/21细胞进行miRNAs差异表达分析,筛选与病毒复制相关的特异性miRNAs,并探讨其在PCV2复制中的作用。[结果] 经高通量测序,获得PCV2感染3D4/21细胞的miRNAs表达谱,结合实验室前期研究筛选获得miRNA-98。实验表明,miRNA-98的表达量随PCV2感染时间的延长而持续升高,其变化趋势与Cap蛋白表达变化基本一致,由此推测miRNA-98与PCV2复制正相关。过表达miRNA-98可显著上调Cap蛋白的表达量和PCV2的复制。进一步的研究表明,miRNA-98参与调节宿主免疫相关细胞因子的表达和PCV2的复制。[结论] miRNA-98可通过调节免疫相关细胞因子的表达调控宿主免疫功能,帮助PCV2逃逸宿主免疫,促进PCV2在3D4/21细胞中的复制。这些发现不仅为深入了解PCV2与宿主之间的关系提供了新视角,还有望为猪圆环病毒相关疾病的防控提供新的抗病毒策略。
关键词:猪圆环病毒2型微小RNA-98复制
miRNA-98 enhances the replication of porcine circovirus type 2 in 3D4/21 cells
Xiaomin Wang1

 , Xianglan Xu2, Peng Xu1, Xingkai Wang1, Lulu Hui1, Hongjie Fan3, Kongwang He2
, Xianglan Xu2, Peng Xu1, Xingkai Wang1, Lulu Hui1, Hongjie Fan3, Kongwang He2

1. Department of Microbiology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China;
2. Institute of Veterinary Medicine, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, Jiangsu Province, China;
3. College of Animal Medicine, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China
Received: 12 November 2019; Revised: 1 April 2020; Published online: 17 June 2020
*Corresponding author: Xiaomin Wang, Tel:+86-851-28642718;E-mail:WXM_ZMU@163.com;
Kongwang He, E-mail:kwh2003@yeah.net.
Foundation item: Supported by the National Natural Science Foundation of China (31860706), by the Doctoral Research Initiation Fund Project of Zunyi Medical University (F-872), by the Guizhou Provincial Education Department for Young Scientists and Engineers (KY[2018]222) and by the Students' Innovation Projects of Zunyi Medical University (201751051)
Abstract: [Objective] This study aims to obtain the miRNAs gene expression profiling of 3D4/21 cells infected with porcine circovirus type 2 (PCV2) and to explore the role of miRNA-98 in the replication of PCV2. [Methods] We used the porcine alveolar macrophage cell lines, 3D4/21 cells, as a cell model to analyze the different miRNAs of 3D4/21 cells infected or uninfected with PCV2. Combined with the results of bioinformatics and experiments, we screened the specific miRNAs related to virus replication and studied the role of miRNA-98 in PCV2 replication. [Results] Differential expression of miRNAs in the process of PCV2 infection was got by high-throughput sequencing technology and evaluated by qRT-RCR and preliminary study. The expression of miRNA-98 increased with the prolongation of PCV2 infection time, and the change trend of miRNA-98 was basically same as that of cap protein of PCV2. These results indicated that a significantly positive correlation between miRNA-98 expression and PCV2 replication. Overexpression of miRNA-98 significantly enhanced PCV2 replication and the expression of Cap. Further evidence supports a link between infection and the host immune regulatory cytokines, which is regulated by miRNA-98. [Conclusion] Overall, miRNA-98 can help PCV2 escape from immune surveillance by regulating the host immune system and enhance PCV2 replication. These results not only supply new insights about relationship between PCV2 infection and host but also provide a potential target for antiviral therapy against PCVAD (porcine circovirus-associated diseases).
Keywords: porcine circovirus type 2microRNA-98replication
猪圆环病毒2型(porcine circovirus type 2,PCV2)被认为是流行于全球的猪圆环病毒相关疾病(porcine circovirus-associated diseases,PCVAD)的主要致病病原,给全世界的养猪业带来了严重的经济损失[1]。PCV2是单链环状DNA病毒,属于圆环病毒科(Circoviridae)的圆环病毒属(Circovirus),主要包含5个开放阅读框(open reading frames,ORFs)。ORF1相对保守,编码病毒DNA复制必需蛋白Rep和Rep'[2];ORF2具有较高的突变率,编码唯一的结构蛋白,即Cap蛋白[1, 3];ORF3编码病毒复制非必需蛋白,参与调控PCV2毒力和PCV2介导的细胞凋亡[4];ORF4能够抑制天冬氨酸特异性半胱氨酸蛋白酶活性和调节感染过程中CD4+和CD8+ T淋巴细胞的数量,与凋亡抑制有关[5];ORF5能够影响细胞周期,诱导细胞内质网应激、细胞自噬和促进病毒复制[6]。
MicroRNAs (miRNAs)是一种长约23个核苷酸、保守的内源性非编码单链RNA,参与调节基因表达[7]。研究表明,miRNAs能够与mRNA的3'UTR (untranslated region,UTR)结合,通过降解靶基因mRNA或抑制翻译,在转录后水平调节病毒或宿主基因的表达,调控一系列基本的细胞事件,从而调节病毒复制[7-9]。Hong等[10]在PCV2感染的PK15细胞中鉴定出51个ORF1参与调控的miRNAs、74个ORF2参与调控的miRNAs和32个ORF3参与调控的miRNAs,其中一些miRNAs参与转录后翻译的调控并可激活MAPK信号通路。在PCV2感染的过程中,纵隔淋巴结中miRNA-126-3p、miRNA-126-5p、let-7d-3p、miRNA-129a和let-7b-3p表达上调,而miRNA-193a-5p、miRNA-574-5p和miRNA-34a表达下调,这些miRNAs可能通过免疫系统相关途径参与调节PCV2的发病机制[11]。然而,深度测序数据表明,PCV2基因组在体内亚临床感染中并不编码miRNAs[12]。
猪肺泡巨噬细胞(porcine alveolar macrophage,PAM)不仅是PCV2感染猪的靶细胞之一,而且是猪肺脏重要的防御和免疫细胞。PCV2感染PAM后,可以不引起细胞凋亡,逃避细胞免疫识别引起免疫抑制。研究表明,病毒可通过miRNAs干扰宿主细胞的病毒抗原递呈、免疫细胞激活等正常免疫过程,逃避免疫监视。因此,我们设想PCV2感染后可能会通过miRNAs调控PAM免疫功能,帮助其实现免疫逃逸,引起免疫抑制。本研究以猪肺泡巨噬细胞系3D4/21细胞作为细胞模型,借助高通量测序筛选得到PCV2感染3D4/21细胞过程中高表达的miRNAs,通过定量PCR验证其在PCV2感染3D4/21细胞中的表达量,并进一步探讨其在PCV2体外复制中的作用。
1 材料和方法 1.1 细胞和病毒 3D4/21细胞和PCV2 2010AHCY株[13] (GenBank accession No:KX828581)均由遵义医科大学微生物学教研室保存。
1.2 试剂 DMEM培养基、胎牛血清(FBS)、转染试剂(LipofectamineTM 2000)、anti-β-actin抗体和FITC标记的羊抗鼠二抗均购自赛默飞世尔科技(中国)有限公司;血液/细胞/组织基因组DNA提取试剂盒、总RNA提取试剂盒、快速SDS-PAGE凝胶制备试剂盒、脱脂奶粉和ECL化学发光检测试剂盒均购自北京庄盟国际生物基因科技有限公司;PrimeScriptTM RT Master Mix和TB Green? Premix Ex TaqTM均购自TaKaRa公司;miRcute miRNA提取分离试剂盒、miRcute miRNA cDNA第一链合成试剂盒和miRcute miRNA荧光定量检测试剂盒均购自天根生化科技(北京)有限公司;PVDF膜购自Milipore公司;RIPA裂解液和DAPI染色液均购自上海碧云天生物技术有限公司;miRNA mimic和inhibitor由广州锐博生物技术有限公司合成;PCV2 Cap蛋白单克隆抗体由江苏省农业科学院兽医研究所制备和馈赠[14];HRP标记的羊抗鼠二抗和HRP标记的羊抗兔二抗均购自上海雅酶生物科技有限公司。
1.3 转染 取处在对数生长期的3D4/21细胞1×105个,接种于六孔板培养,置于5% CO2的37 ℃细胞培养箱中培养至长满单层细胞。按照LipofectamineTM 2000转染试剂盒说明书,分别将60 nmol/L的miRNA-98 mimic、miRNA-98 inhibitor、mimic control和inhibitor control转染3D4/21细胞。
1.4 实时荧光定量PCR 按照miRcute miRNA提取分离试剂盒说明书提取细胞总miRNA,随后,按照miRcute miRNA cDNA第一链合成试剂盒和miRcute miRNA荧光定量检测试剂盒的要求进行cDNA的合成和miRNA-98实时荧光定量PCR的扩增。该实验设定3个重复孔,以U6为内参,实验重复3次,以2-△△Ct法计算miRNA-98的表达量。
按照血液/细胞/组织基因组DNA提取试剂盒和总RNA提取试剂盒说明书提取细胞总DNA和总RNA,随后参照Wang等[13]建立的方法测定PCV2的病毒载量和细胞因子表达量。
1.5 Western blotting实验 以RIPA裂解液裂解细胞,进行细胞总蛋白的提取,随后,使用BCA法测定蛋白含量。每个样品取30 μg蛋白进行SDS-PAGE、转膜、5%脱脂奶粉封闭1 h、一抗4 ℃孵育过夜、二抗室温孵育1 h以及ECL化学发光后的显影分析。该实验以β-actin作为内参。
1.6 间接免疫荧光检测 将60 nmol/L的miRNA-98 mimic和mimic control分别转染3D4/21细胞,24 h后接种PCV2 2010AHCY (MOI=1),感染后36 h,PBS洗涤2次,加入4%多聚甲醛,4 ℃固定30 min;PBS洗涤2次,加入含0.5% BSA (牛血清白蛋白)的PBS,置于室温孵育封闭30 min;PBS洗涤3次,加入针对PCV2 Cap蛋白的单克隆抗体,37 ℃孵育2 h;PBS洗涤5次,加入FITC标记的羊抗鼠二抗,37 ℃室温避光孵育1 h;PBS洗涤5次后,DAPI避光室温下染色10 min;PBS洗涤5次后,倒置荧光显微镜下观察并拍照。
2 结果和分析 2.1 miRNA的筛选 收集0 h未经处理的空白细胞(Mock)、PCV2感染后4 h、36 h和48 h的样本及相应时间点的mock细胞样本(每个时间点3个生物学重复),提取细胞miRNA进行高通量测序。筛选差异表达的特异性miRNAs的原则为:A:表达水平变化倍数(fold change) > 2;B:比较相应时间点mock和PCV2感染细胞间的miRNAs表达谱,找出同一时间点两组间表达差异显著的miRNAs;C:优先选择已报道与病毒复制相关的miRNAs;D:选择与PCV2复制变化趋势相一致的miRNAs。基于图 1的初步筛选结果以及前期研究结果,最终确定了在PCV2感染后表达上调的miRNA-98作为本文研究对象。
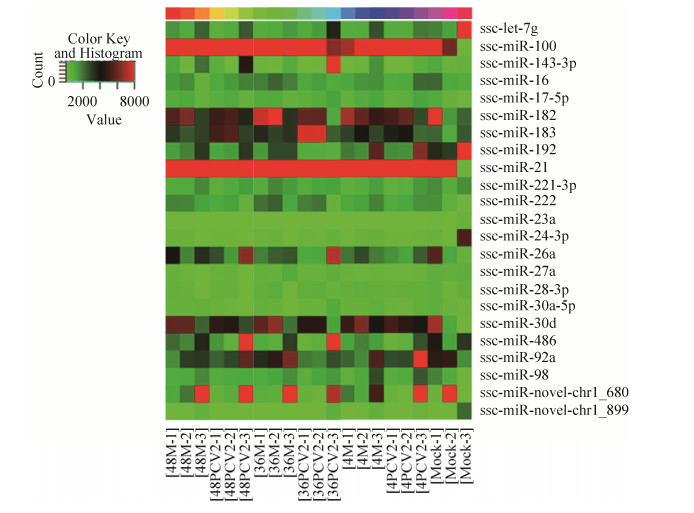 |
| 图 1 PCV2感染3D4/21细胞miRNAs的表达 Figure 1 miRNAs profiles differentiate the 3D4/21 cells with PCV2 infection from the 3D4/21 cells with no viral infection (Mock). Both down-regulated (green) and up-regulated (red) miRNAs were identified in 3D4/21 cells. |
| 图选项 |
2.2 miRNA-98的验证 为了鉴定PCV2感染3D4/21细胞中miRNA-98表达丰度的变化,初步探索其变化与PCV2感染之间的关系,本研究利用Western blotting和实时荧光定量PCR分别检测PCV2 Cap蛋白和miRNA-98在PCV2感染3D4/21细胞后不同时间点的表达量。由图 2-A[13]可知,PCV2感染3D4/21后Cap蛋白的表达量呈波动性变化,约在36 h达到最大值。如图 2-B,miRNA-98的表达量随PCV2感染时间的延长而持续升高,其变化趋势与Cap蛋白变化基本一致。由此推测,miRNA-98可能促进病毒的复制。
 |
| 图 2 PCV2感染3D4/21细胞后不同时间点PCV2 ORF2和miRNA-98的表达 Figure 2 The expression of ORF2 of PCV2 and miRNA-98 in PCV2 infected 3D4/21 cells. A: Western blotting analysis of the expression of ORF2 during the course of PCV2 infection; B: Quantitative PCR expression of miRNA-98 during the course of PCV2 infection. The fold change from the qRT-PCR was determined using the 2-△△Ct method and miRNA expression values were normalized against the U6 endogenous control. The value expressed by each bar represents the mean±SD (n=3). |
| 图选项 |
2.3 miRNA-98对PCV2复制的影响 为探讨miRNA-98在PCV2复制过程中的作用,委托广州锐博生物技术有限公司合成miRNA-98 mimic和miRNA-98 inhibitor,分别转染3D4/21细胞后感染PCV2 2010AHCY,设定转染mimic control或inhibitor control后感染PCV2 2010AHCY的细胞作为对照,同时设定未经处理的空白细胞(mock)作为阴性对照。由图 3-A和3-B可知,相对于mimic control,miRNA-98过表达能够显著促进PCV2的复制,PCV2的拷贝数在感染48时增加约8倍,miRNA-98 inhibitor抑制内源性miRNA-98的表达则显著抑制了PCV2的复制。为了进一步确定miRNA-98对PCV2复制过程的影响,本文设计了免疫荧光实验,观察miRNA-98过表达对PCV2 Cap蛋白表达的作用。如图 3-C所示,与mimic control相比,miRNA-98过表达的细胞中可观察到更多针对PCV2 Cap蛋白的特异性绿色荧光。这一结果与上述病毒载量检测结果一致,表明miRNA-98过表达能够显著促进PCV2的复制。
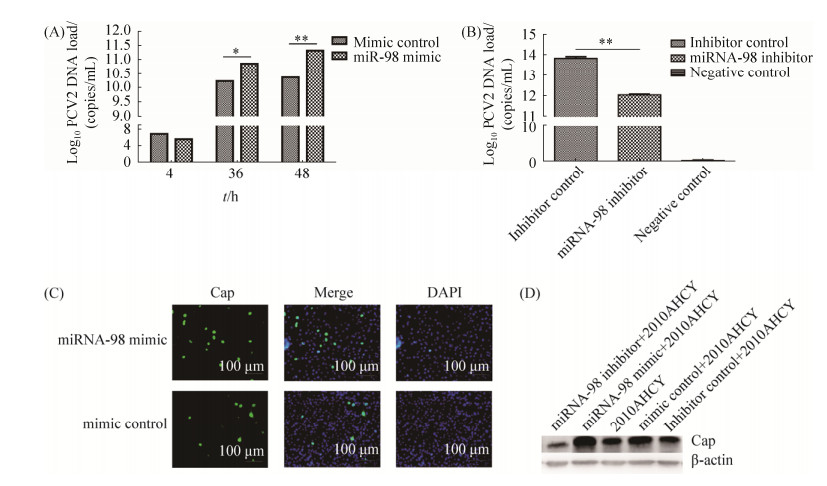 |
| 图 3 miRNA-98对PCV2复制的作用 Figure 3 The effect of miRNA-98 on the replication of PCV2. A, B: Quantitative PCR expression of PCV2. The copies of PCV2 were measured by qRT-PCR. Data are present the mean±SD (n=3). Statistical significance was analyzed by t-test. Significance compared to mimic control or inhibitor control. **: P < 0.01. C: 3D4/21 cells transfected with miRNA-98 mimic or mimic control were infected with PCV2, then detected by immunofluorescence assay using a monoclonal antibody against the PCV2 Cap protein. D: Western blotting analysis of the expression of Cap during the course of PCV2 infection. |
| 图选项 |
Western blotting检测结果显示(图 3-D),mimic control组、inhibitor control组和2010AHCY组几乎无差异,这也表明转染并未对PCV2的感染产生影响。miRNA-98 mimic转染后过表达miRNA-98增强了Cap蛋白的表达,相反,miRNA-98 inhibitor抑制了Cap蛋白的表达。这一结果与病毒载量和免疫荧光结果一致,进一步证实miRNA-98能够促进PCV2的复制,上调Cap蛋白表达水平。
2.4 miRNA-98对宿主免疫相关细胞因子的调节作用 为了探讨miRNA-98是否参与调节宿主免疫相关细胞因子的表达,本文将3D4/21细胞分别转染miRNA-98 mimic及mimic control,随后,感染PCV2 2010AHCY株(MOI=1)。感染后36 h,应用qRT-PCR检测与细胞凋亡、炎症、细胞周期和抗病毒功能相关细胞因子的mRNA[13, 15]表达水平,引物参考Wang等建立的实验方法[13]。如图 4所示,PCV2感染显著上调Apaf-2 (apoptotic protease activating factor 2,凋亡蛋白酶活化因子2)的表达,miRNA-98过表达则显著下调Apaf-2的表达。与mimic control相比,miRNA-98过表达能够上调IL-1β (human interleukin-1 beta,人白细胞介素1β)、LAMP2 (lysosomal-associated membrane protein 2,溶酶体关联膜蛋白2)、IFNβ(interferon β,干扰素β)、BCL2L2 (B-cell CLL/Lymphoma 2 like protein 2,B-细胞淋巴瘤因子2样蛋白2)、MAPK (mitogen-activated protein kinase,丝裂原活化蛋白激酶)、SKP2 (S期激酶相关蛋白2)和CyclinA (细胞周期素A)的表达;相反,miRNA-98过表达能够下调TRAM1 (translocation associated membrane protein 1,易位关联膜蛋白1)、IL-8 (human interleukin-8,人白细胞介素8)等的表达。这些结果表明,miR-98参与调节宿主免疫相关细胞因子的表达,在宿主免疫应答中发挥作用,调节病毒的复制。
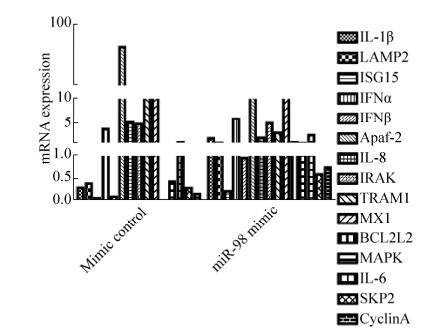 |
| 图 4 miRNA-98对PCV2感染3D4/21过程中细胞因子表达的影 Figure 4 Effect of miRNA-98 on cytokines expression in 3D4/21 cells infected with PCV2. The relative abundance of 15 mRNAs of cytokines which related to apoptosis, cell cycle or antiviral function were measured by qRT-PCR. The fold change from the qRT-PCR was determined using the 2-△△Ct method and miRNA expression values were normalized against the beta-actin control. The value expressed by each bar represents the mean±SD (n=3). |
| 图选项 |
3 讨论 miRNAs能够通过与宿主和病毒的靶mRNA结合,从而调节宿主细胞功能、影响病毒RNA功能、限制病毒复制,甚至灭活病毒[8, 15-16]。研究证实,在PCV2体内感染过程中,一系列miRNAs参与调控宿主免疫系统和PCV2发病机制,然而,深度测序揭示PCV2在体内亚临床感染时,其基因组本身并不编码任何miRNAs[10-12, 17]。研究表明,miRNA-139-5p和let-7e能够分别调控与PCV2病毒编码蛋白相互作用的锌指蛋白265(zinc finger protein 265,ZNF265)和G蛋白信号调节子16 (regulator of G-protein 16,RGS16)的表达[10-11]。李等[18]使用茎环定量PCR检测比较了PCV2感染组和对照组之间多个热点miRNAs的差异表达水平,并结合蛋白质组学构建了差异miRNA和mRNA的互作网络。综上所述,miRNAs在PCV2感染过程中发挥着重要作用,为进一步探讨miRNAs在细胞和病毒生命周期中的作用奠定了基础。为深入了解miRNAs在PCV2感染猪肺泡巨噬细胞中的作用,本文对PCV2感染3D4/21细胞不同时间点的细胞和空白对照组细胞进行miRNAs的高通量测序和生物信息学分析,筛选获得PCV2感染过程中高表达的miRNA-98。
miRNA-98是Let-7家族的成员之一,已被证实与多种癌症正相关。有研究表明,miRNA-98能够负调控高迁移率族蛋白2(high mobility group AT-hook 2,HMGA2)癌基因的表达[19],调控结直肠癌细胞增殖、侵袭和上皮间质转化。研究证实,miRNA-98过表达能够靶向调控N-RAS抑制鼻咽癌CNE-1细胞的增殖和迁移[20]。miRNA-98亦可通过调控ABCC5和ABCC10影响药物的敏感性。本研究中,miRNA-98的表达量随PCV2感染时间的延长而持续升高,其变化趋势与Cap蛋白变化基本一致。病毒载量结果表明,过表达miRNA-98能够显著增强PCV2的复制能力;抑制miRNA-98的表达,能够显著抑制PCV2的复制。PCV2 Cap蛋白能够通过接触宿主细胞表面受体进入细胞,还能影响病毒的装配或结构稳定[21-22]。本研究中的免疫荧光和Western blotting结果证实,miRNA-98能够促进PCV2 Cap蛋白的表达,这也暗示miRNA-98可能通过影响PCV2的组装,促进病毒增殖。
炎症反应是机体的一种免疫防御反应,是病原微生物侵犯机体后,机体的一种自我保护反应[23]。PCV2感染影响机体免疫系统功能的发挥,抑制促炎症细胞因子和抗病毒细胞因子等的分泌,造成免疫抑制。IL-1β、IL-8和IL-6是宿主重要的促炎症细胞因子,IFNα和IFNβ是宿主产生的具有抗病毒作用的重要细胞因子。miRNA-98过表达后,IL-8表达略有下调、IL-6表达变化不明显、细胞因子IL-1β、IFNα和IFNβ的表达略有上调,这也表明宿主细胞保留着较低水平的促炎和抗病毒作用。PCV2感染后凋亡蛋白酶活化因子2(Apaf-2)高表达,而miRNA-98的过表达则可以显著降低Apaf-2的表达;B-细胞淋巴瘤因子2样蛋白2(BCL2L2)是细胞凋亡信号转导的主要调节物,能够抑制细胞凋亡,miRNA-98过表达能够显著上调BCL2L2的表达;因此,推测miRNA-98过表达能够抑制3D4/21细胞的凋亡。CyclinA作为细胞周期蛋白家族的一员,控制着细胞DNA合成的起始,是调控细胞周期S期和G2/M期的关键蛋白。miRNA-98过表达上调CyclinA的表达,可能改变3D4/21细胞原有的细胞周期,使其更有利于PCV2的生存和复制。溶酶体关联膜蛋白2 (LAMP2)可用于监测自噬体与溶酶体的融合,miRNA-98过表达能够显著上调LAMP2的表达,这也暗示miRNA-98可能参与调控细胞自噬。与对照组相比,miRNA-98过表达后,参与调控TLR (Toll-like receptors,Toll样受体)信号通路的易位关联膜蛋白(TRAM1)表达显著下调,这也显示miRNA-98可能参与调控TLR免疫,帮助PCV2逃逸宿主免疫监视。综上,miRNA-98可能参与调节TLR免疫、细胞凋亡、细胞周期和细胞自噬等,从而调控PCV2在3D4/21细胞上的生命周期,促进PCV2的复制,在病毒的感染过程中具有重要的调节作用。这些发现不仅为研究PCV2和宿主之间的关系提供了新的思路,也为PCVAD的防控提供了新策略。
References
| [1] | Finsterbusch T, Mankertz A. Porcine circoviruses--small but powerful. Virus Research, 2009, 143(2): 177-183. DOI:10.1016/j.virusres.2009.02.009 |
| [2] | Cheung AK. Transcriptional analysis of porcine circovirus type 2. Virology, 2003, 305(1): 168-180. |
| [3] | Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. Journal of General Virology, 2000, 81(9): 2281-2287. DOI:10.1099/0022-1317-81-9-2281 |
| [4] | Liu J, Zhu Y, Chen I, Lau J, He F, Lau A, Wang ZL, Karuppannan AK, Kwang J. The ORF3 protein of porcine circovirus type 2 interacts with porcine ubiquitin E3 ligase Pirh2 and facilitates p53 expression in viral infection. Journal of Virology, 2007, 81(17): 9560-9567. DOI:10.1128/JVI.00681-07 |
| [5] | He JL, Cao JJ, Zhou N, Jin YL, Wu JS, Zhou JY. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. Journal of Virology, 2013, 87(3): 1420-1429. |
| [6] | Choi CY, Choi YC, Park IB, Lee CH, Kang SJ, Chun T. The ORF5 protein of porcine circovirus type 2 enhances viral replication by dampening type I interferon expression in porcine epithelial cells. Veterinary Microbiology, 2018, 226: 50-58. DOI:10.1016/j.vetmic.2018.10.005 |
| [7] | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell, 2009, 136(2): 215-233. |
| [8] | Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annual Review of Microbiology, 2010, 64(1): 123-141. DOI:10.1146/annurev.micro.112408.134243 |
| [9] | Schlegel A, Giddings TH, Ladinsky MS, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. Journal of Virology, 1996, 70(10): 6576-6588. DOI:10.1128/JVI.70.10.6576-6588.1996 |
| [10] | Hong JS, Kim NH, Choi CY, Lee JS, Na D, Chun T, Lee YS. Changes in cellular microRNA expression induced by porcine circovirus type 2-encoded proteins. Veterinary Research, 2015, 46(1): 39. DOI:10.1186/s13567-015-0172-5 |
| [11] | Nú?ez-Hernández F, Pérez LJ, Mu?oz M, Vera G, Tomás A, Egea R, Córdoba S, Segalés J, Sánchez A, Nú?ez JI. Identification of microRNAs in PCV2 subclinically infected pigs by high throughput sequencing. Veterinary Research, 2015, 46(1): 18. DOI:10.1186/s13567-014-0141-4 |
| [12] | Nú?ez-Hernández F, Pérez LJ, Vera G, Córdoba S, Segalés J, Sánchez A, Nú?ez JI. Evaluation of the capability of the PCV2 genome to encode miRNAs: lack of viral miRNA expression in an experimental infection. Veterinary Research, 2015, 46(1): 48. DOI:10.1186/s13567-015-0181-4 |
| [13] | Wang XM, Xu XL, Wang W, Yu ZY, Wen LB, He KW, Fan HJ. MicroRNA-30a-5p promotes replication of porcine circovirus type 2 through enhancing autophagy by targeting 14-3-3. Archives of Virology, 2017, 162(9): 2643-2654. DOI:10.1007/s00705-017-3400-7 |
| [14] | Wang W, Wang XM, Wen LB, He KW, Zhou JM, Guo RL, Wang F, Ni YX, Zhang XH, Lv LX, Yu ZY, Mao AH, Li B. Preparation and identification of specific monoclonal antibody against porcine circovirus type 2. Agricultural Science & Technology, 2014, 15(2): 173-176. (in Chinese) 汪伟, 王小敏, 温立斌, 何孔旺, 周俊明, 郭容利, 王芳, 倪艳秀, 张雪寒, 吕立新, 俞正玉, 茅爱华, 李彬. 猪圆环病毒2型特异性单克隆抗体的制备与鉴定. 农业科学与技术(英文版), 2014, 15(2): 173-176. |
| [15] | Fu YX, Xu WT, Chen DY, Feng CH, Zhang L, Wang XH, Lv XW, Zheng N, Jin Y, Wu ZW. Enterovirus 71 induces autophagy by regulating has-miR-30a expression to promote viral replication. Antiviral Research, 2015, 124: 43-53. DOI:10.1016/j.antiviral.2015.09.016 |
| [16] | Zhang Q, Guo XK, Gao L, Huang C, Li N, Jia XJ, Liu WJ, Feng WH. MicroRNA-23 inhibits PRRSV replication by directly targeting PRRSV RNA and possibly by upregulating type I interferons. Virology, 2014, 450-451: 182-195. DOI:10.1016/j.virol.2013.12.020 |
| [17] | Zhang P, Wang LY, Li YP, Jiang P, Wang YC, Wang PF, Kang L, Wang YD, Sun Y, Jiang YL. Identification and characterization of microRNA in the lung tissue of pigs with different susceptibilities to PCV2 infection. Veterinary Research, 2018, 49(1): 18. DOI:10.1186/s13567-018-0512-3 |
| [18] | Li C, Li J, He QG. Integrated analysis of miRNA and mRNA network of porcine circovirus 2 infecting porcine kidney cell line. Acta Veterinaria et Zootechnica Sinica, 2019, 50(1): 115-125. (in Chinese) 李畅, 李静, 何启盖. 猪圆环病毒2型感染PK-15细胞差异表达miRNA和mRNA互作网络. 畜牧兽医学报, 2019, 50(1): 115-125. |
| [19] | Xiang Q, Tang HF, Yu J, Yin J, Yang XY, Lei XY. MicroRNA-98 sensitizes cisplatin-resistant human lung adenocarcinoma cells by up-regulation of HMGA2. Die Pharmazie - An International Journal of Pharmaceutical Sciences, 2013, 68(4): 274-281. |
| [20] | Song H, Zhao H, Bian ZG, Gu ZW, Cao ZW. Effects of microRNA98 targeting N-RAS on the proliferation and migration of nasopharyngeal carcinoma CNE-1 cells. Progress of Anatomical Sciences, 2019, 25(3): 252-255. (in Chinese) 宋辉, 赵鹤, 边志刚, 顾兆伟, 曹志伟. microRNA98靶向N-RAS对鼻咽癌CNE-1细胞增殖与迁移的影响. 解剖科学进展, 2019, 25(3): 252-255. |
| [21] | Misinzo G, Delputte PL, Meerts P, Lefebvre DJ, Nauwynck HJ. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. Journal of Virology, 2006, 80(7): 3487-3494. DOI:10.1128/JVI.80.7.3487-3494.2006 |
| [22] | O'Dea MA, Hughes AP, Davies LJ, Muhling J, Buddle R, Wilcox GE. Thermal stability of porcine circovirus type 2 in cell culture. Journal of Virological Methods, 2008, 147(1): 61-66. DOI:10.1016/j.jviromet.2007.07.029 |
| [23] | Wang W, Wang XM, He KW, Wen LB, Ni YX. Inflammation-associated cytokines mRNA transcriptional profiles of porcine alveolar macrophages cell lines 3D4/21 stimulated by porcine circovirus type 2. Southwest China Journal of Agricultural Sciences, 2016, 29(5): 1225-1228. (in Chinese) 汪伟, 王小敏, 何孔旺, 温立斌, 倪艳秀. 猪圆环病毒2型体外刺激3D4/21猪肺泡巨噬细胞的炎症相关细胞因子mRNA转录分析. 西南农业学报, 2016, 29(5): 1225-1228. |
