陈敦武, 陈雄, 李欣


发酵工程教育部重点实验室, 工业发酵湖北省协同创新中心, 湖北工业大学生物工程与食品学院, 湖北 武汉 430068
收稿日期:2019-03-04;修回日期:2019-04-02;网络出版日期:2019-04-19
基金项目:国家自然科学基金(31871789);湖北省教育厅青年基金(6101-12131)
*通信作者:李欣, E-mail:51545530@qq.com.
摘要:作为一种天然稳定剂的双糖,海藻糖(Trehalose)在逆境下对生物体活性的保护功能既吸引了广泛的研究兴趣,也使其具有良好的应用价值和潜力。本文聚焦重要模式微生物和工业应用微生物酵母,结合组学研究最新进展,从海藻糖代谢途径、应激条件下的海藻糖代谢和转录特征以及提高胞内海藻糖含量策略等方面,对内源性海藻糖研究新进展进行了综述。
关键词:酵母内源性海藻糖代谢组转录组
Advances in regulation of endogenous trehalose metabolism in yeast
Dunwu Chen, Xiong Chen, Xin Li


Key Laboratory of Fermentation Engineering(Ministry of Education), Hubei Collaborative Innovation Center for Industrial Fermentation, School of Food and Biological Engineering, Hubei University of Technology, Wuhan 430068, Hubei Province, China
Received: 4 March 2019; Revised: 2 April 2019; Published online: 19 April 2019
*Corresponding author: Xin Li, E-mail:51545530@qq.com.
Foundation item: Supported by the National Natural Science Foundation of China (31871789) and by the Research Foundation of Education Bureau of Hubei Provice (6101-12131)
Abstract: As a naturally stable disaccharide, the protective function of trehalose on the activity of organisms under adverse conditions has attracted a wide range of research interests, and also has good application value and potential. This paper focuses on the important model microorganisms and industrial applied yeast, combined with the latest progress in the research of omics, and summarizes the new progress in the research of endogenous trehalose from the aspects of trehalose metabolic pathway, trehalose metabolism and transcription characteristics under stress conditions, and the strategy of improving intracellular trehalose.
Keywords: yeastendogenous trehalosemetabolomicstranscriptomics
海藻糖是一种由2个葡萄糖分子以1, 1-糖苷键构成的非还原性双糖,化学名称为α, D-吡喃葡糖基-α, D-吡喃葡糖苷,分子式是C12H22O11·2H2O,分子量为378.33[1-2]。海藻糖最初是从生活在沙漠中的一种甲虫蛹中分离得到的[3],后来发现它存在于植物、无脊椎动物、真菌和原核生物中,但不存在于脊椎动物中[4]。长期研究表明,海藻糖不仅能作为一种微生物储备碳源,更是一种天然保护剂,在保护细胞免受胁迫侵害方面发挥着重要功能。此外,稳定和友好亲和的特性使得海藻糖能作为一种外源添加物被用于食品工业、化妆品、医药等领域,具有十分广阔的应用前景。本文从酵母细胞内海藻糖代谢途径出发,重点介绍了酵母这种重要模式微生物和工业应用微生物在应激条件下的海藻糖代谢组学和转录组学特征以及提高胞内海藻糖含量的策略。
1 酵母细胞内源性海藻糖代谢 1.1 海藻糖代谢途径 海藻糖生物合成途径大致分为5类,TPP/TPS途径(古菌、细菌、真菌、植物和昆虫)、TreY/TreZ途径(古菌)、TreS途径(细菌)、TreP途径(真菌)和TreT途径(古菌)[5]。其中TPP/TPS途径是广泛存在的,该途径由少数几个代谢物组成却受到复杂而严密的调控机制控制,它在酵母细胞内的代谢途径如图 1所示。先由己糖激酶(或葡萄糖激酶)催化将葡萄糖转变为葡萄糖-6-磷酸,再由海藻糖-6-磷酸合成酶(Tps)催化UDP-葡萄糖(UDPG)和葡萄糖-6-磷酸(G-6-P)形成海藻糖-6-磷酸(T-6-P),海藻糖6-磷酸磷酸酯酶(Tpp)使T-6-P去磷酸化为海藻糖[6],而海藻糖的分解则取决于三种不同的海藻糖酶(Nth1、Nth2和Ath1),最终将其分解成2分子的葡萄糖。其中,由胁迫刺激所产生的内部海藻糖的降解依赖于中性海藻糖酶(Nth1),被转运出去的外部海藻糖的降解依赖于酸性海藻糖酶(Ath1)。
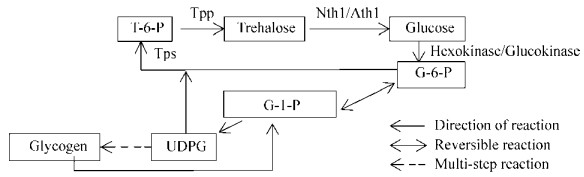 |
| 图 1 酵母细胞内海藻糖代谢途径(TPP/TPS途径) Figure 1 Trehalose metabolic pathway in yeast cells (TPP/TPS pathway). |
| 图选项 |
1.2 参与海藻糖代谢的酶 在Eleutherio等的综述中,对酵母细胞在应激条件下参与合成和分解海藻糖的酶进行了详细的介绍[7]。参与海藻糖合成的酶是一个酶复合体,该复合体由4个亚基(Tps1,Tps2,Tps3,Tsl1)组成,分别由基因TPS1 (56 kDa)、TPS2 (100 kDa)、TPS3 (123 kDa)和TSL1 (123 kDa)编码,且分别位于酿酒酵母的Ⅱ、Ⅳ、Ⅹ、Ⅲ染色体上。TSL1是TPS复合物活性的决定性亚基,在其不存在时没有发生海藻糖合成[8]。Tps1为复合体中最小的亚基,具有海藻糖-6-磷酸合成酶活性,它对酿酒酵母细胞完整性至关重要[9],此外还能作为一种主要的促生存因子,在酿酒酵母生长期间对细胞活力具有关键作用[10]。多项研究证明,在胁迫条件下,T-6-P的含量对海藻糖的积累起着重要作用。T-6-P是Tps1的非竞争性抑制剂,这种抑制剂能够在浓度增加时将反应速率降低至零,从而使海藻糖合成停止[11],同时它还能抑制己糖激酶,使得酵母细胞在遭遇环境胁迫而启动海藻糖途径后,对葡萄糖进入糖酵解的代谢流有一定的限制作用。此外,葡萄糖抑制糖异生途径相关基因的作用也强烈依赖于T-6-P[12]。
参与海藻糖降解的酶为中性海藻糖酶(Nth1和Nth2)和酸性海藻糖酶(Ath1),分别由基因NTH1、NTH2和ATH1编码。NTH1、NTH2分别位于啤酒酵母的Ⅳ和Ⅱ染色体上[13],而ATH1在染色体上的具体定位还尚不清楚。在酿酒酵母中NTH2是NTH1的旁系同源物[14],NTH1基因缺失突变株不能代谢体内的海藻糖,也检测不到海藻糖酶活性,而NTH2基因缺失突变株的海藻糖酶活性和海藻糖含量都没有变化,ATH1基因缺失突变株在含海藻糖为唯一碳源的培养基上不能生长[13]。另有研究表明Ath1可能通过MVB途径(The multivesicular body pathway,多液泡通路途径)降低其他液泡蛋白的下调,使得它对酵母液泡蛋白活性的损害具有潜在的影响[15]。
2 应激条件对酵母细胞内海藻糖含量的影响 海藻糖作为生物体内的应激保护物质,在对抗多种环境胁迫方面发挥着重要生理功能。Auesukaree等对乙醇发酵过程中酵母细胞遇到各种应激(高糖浓度引起的高渗透压、增加的乙醇浓度、氧代谢衍生的活性氧和升高的温度)之后产生的细胞效应进行了介绍[16],随后Priyanka等也总结了在此过程中酵母细胞面对环境胁迫(热休克、乙醇胁迫和渗透胁迫)的生理变化[17]。在乙醇作为碳源的渗透胁迫下,两种野生型酿酒酵母菌株(W303-1A、BY4741)胞内海藻糖水平在90 min内分别增加至基础水平(未添加胁迫条件)的2倍和6倍[18]。在使用活化后的酿酒酵母(耐高温型)进行酒精发酵过程中,发酵起始pH值过高(6.5)或过低(3.5)都会导致发酵液中游离海藻糖含量/海藻糖总量增高,发酵起始pH值过低(3.5)还会导致酵母胞内海藻糖含量升高[19]。另外Gao等已经对酵母耐热性研究进行了详细的综述[20],当培养温度从低温到高温的过程中(从28 ℃转移到40 ℃),酿酒酵母(BY4741)胞内海藻糖水平增加了6倍以上[11]。除酿酒酵母外,胡梦蝶等的研究表明,对于鲁氏酵母(CCTCCM 2013310),渗透压胁迫(NaCl)对海藻糖积累没有明显的作用,添加乙醇和升高温度有利于海藻糖的积累,并且氧化胁迫(H2O2)可明显提高鲁氏酵母胞内海藻糖的含量[21]。此外,面对重金属胁迫,所改造的工程菌株在有Pb2+胁迫诱导的情况下比野生型A. subglaciale F134表现出海藻糖含量增加3倍以上[22]。
3 应激条件下海藻糖的代谢组分析 作为第一个分支点,葡萄糖-6-磷酸(G-6-P)在糖酵解途径的上部发挥重要作用。正常情况下,磷酸戊糖途径(Pentose phosphate pathway,PPP)和储存碳水化合物的合成观察到约三分之二的合成G-6-P用于糖酵解,约10%用于PPP,20%用于可逆转化为葡萄糖-1-磷酸(G-1-P),只有小部分用于生产T-6-P和其他途径[23-24]。研究表明,海藻糖生物合成中的T-6-P已经成为糖酵解的重要调节剂,它可以控制糖酵解途径的上半部分[25],同时还能调节细胞内的pH[26],对海藻糖的积累起着关键作用。
通过代谢组学分析可知整个代谢网络中,各物质的代谢通量是不断在变化的。多项研究表明,酵母细胞在受到胁迫后,其海藻糖代谢途径会被不同程度地激活,并且受其他多种代谢途径影响。李明达等观察到酿酒酵母在盐胁迫培养条件下糖酵解途径各支路的代谢产物通量均有所增加(包括海藻糖代谢途径),而三羧酸循环(Tricarboxylic acid cycle,TCA)中各代谢产物的通量均有所减少[27]。这与Roja等的实验现象是一致的,当适应压力环境后,更多的碳可以被导向糖异生并进入糖酵解途径的上部,流向海藻糖合成的分支途径[18]。Suarez-Mendez等在对酿酒酵母(CEN PK 113-7D)应用快速盛宴/饥荒扰动期间发现糖酵解中较低的代谢物通量表现出与糖酵解途径上部代谢中间体相反的动力学特征,向PPP的相对流量从3.8%增加到20%,相应地,糖酵解的流量从74%降低到61%,海藻糖代谢的相对通量从4%增加到5.2%[24],而中性海藻糖酶活性由糖酵解途径下部的中间体维持在高水平[28],用于将海藻糖降解成葡萄糖,使其稳定在合适的范围内。此外,研究表明升温能有效刺激鲁氏酵母(CCTCCM 2013310)胞内海藻糖的合成,同时也促进了糖酵解途径、丙酮酸溢流途径和三羧酸循环途径的活性,三者之间形成了耦合协调关系[29]。值得注意的是,多项研究均观察到酵母细胞在应激条件下海藻糖富集的显著延迟[18, 24, 30]。
此外,酵母细胞中海藻糖代谢与其生长周期是密切相关的。Chen等在利用酿酒酵母S288c进行乙醇发酵时,在停滞-指数相变过程中,酵母细胞适应压力,TCA循环下调,在指数-稳定相变期间,重新激活的TCA循环可以提供充足的能量,同时甘油、海藻糖、麦角甾醇和一些氨基酸的含量增加,它们可能共同赋予酵母细胞更高的乙醇耐受性[31]。酿酒酵母(W303-1A)在热应激反应过程中,能量(ATP)、还原当量(NADPH)和保护分子(海藻糖、甘油)的生成在前10 min内急剧增加,并且在10 min时分配给各个糖酵解通量分支的资源达到新的平衡,直到热应激后20 min趋于稳定[32]。裂殖酵母S. pombe氮饥饿后1 h内,最重要的变化发生在前15 min,海藻糖、2-氧戊二酸和琥珀酸增加,一些嘌呤生物合成途径中的中间体代谢物(如5-磷酸核糖-1-二磷酸、N-甲酰甘氨酰胺核糖核苷酸等)急剧下降[33]。另外,芽殖酵母(CEN.PK)在静止期还可以通过糖异生来消耗乙醇、甘油,特别是乙酸盐以产生乙酰CoA,乙酰CoA可以进入TCA循环,该模式取决于乙醛酸分流的活性,其能够从乙酸盐合成海藻糖[34]。
4 应激条件下海藻糖的转录组分析 在面对胁迫条件时,酵母细胞内涉及到海藻糖代谢的基因表达量会发生显著的变化,控制合成途径的酶基因TPS1、TPS2和TLS1表现出上调,而控制其降解的酶基因ATH1、NTH1和NTH2却有不同的变化趋势,但最终都表现出胞内海藻糖水平的增加。南极酵母Pseudozyma sp. JCC207的TPS1在高盐度(96‰,12 h)和低温度(0 ℃,36 h)胁迫条件下有最高的表达量,导致海藻糖增加来保护酵母细胞抵抗极端环境的变化[35]。酵母菌株Pichia kudriavzevii A16在盐胁迫(10–80 g/L,NaCl)诱导下,与海藻糖合成相关的基因(TPS1和TSL1)丰富表达,以及盐胁迫显著抑制镉摄取基因ZRT1的表达,多重因素保护细胞免受复杂胁迫条件的影响,提高了镉耐受性[36]。当酿酒酵母W303在暴露于热应激和其他蛋白质损伤应激(氧化和乙醇胁迫)时,海藻糖合成和分解代谢相关基因(包括TPS1、TPS2和NTH1)的表达迅速上调[37],这与另一些研究中的酿酒酵母在渗透胁迫下或者当温度降至10 ℃ (或更低)时TPS1和TPS2的mRNA水平显著增加的实验现象是一致的[38-39]。另外,基因倍型也会对应激条件下酵母细胞内海藻糖代谢产生影响。在单倍体和三倍体酿酒酵母菌株中,基因TPS1、TPS2和TLS1的上调程度大于ATH1、NTH1和NTH2的上调程度,导致两者海藻糖含量升高[40]。同样地,海藻糖代谢途径的相关基因表达量也与其生长阶段密切相关。在酿酒酵母生物乙醇发酵的过程中,总体而言,指数期和稳定期的细胞具有更高的海藻糖含量。与停滞期相比,指数期的NTH1、NTH2基因表达有所下调,而稳定期的TPS1、ATH1、NTH1、NTH2的基因表达上调,TPS2有所下降。在停滞-指数相变期,NTH1、NTH2的基因表达下调导致胞内海藻糖含量有所升高,而在指数-稳定的相变期,TPS1的基因表达上调,使得海藻糖含量进一步提高,而在整个分批发酵过程中,TPS3的基因表达无明显变化[41]。
同代谢通量一致,除了与海藻糖代谢途径相关的酶基因表达量发生变化外,某些其他代谢途径中的酶基因也会相应发生变化。酿酒酵母在高渗透压下(乙醇作为碳源),典型的应激分子(包括海藻糖)和编码PPP中的酶基因强烈上调[18]。同样地,在酵母Issatchenkia orientalis中发现NAD(P)+依赖的酶基因被上调,使得NAD+的需求增加,以保持氧化磷酸化运行[42]。另有研究表明诱导糖原合成途径可能赋予酵母更强的耐热性,Atsushi等将耐热的酿酒酵母菌株YK60-1分别在30 ℃和40 ℃温育时,编码参与葡萄糖转化为UDPG的酶基因(HXK1、PGM2和UGP1)被上调,编码参与海藻糖合成和分解代谢途径的酶基因(NTH1,TPS1,TPS2和TSL1)也被上调[43]。由于糖原合成途径提供海藻糖合成所需的糖基单元,因此YK60-1中糖原合成途径的激活也可有助于增强海藻糖的积累。Mevlüt等也观察到突变菌株SRM11 (基于高通量方法以及进化工程筛选出的长寿命菌株)在应激条件下(重金属、H2O2)糖原-海藻糖合成途径相关基因的上调[44]。此外,酿酒酵母菌株Sc131在乙醇(10%V/V,4h)胁迫下,除了编码海藻糖合成的基因(TPS1和TPS2)上调外,麦角甾醇生物合成基因(ERG2,ERG3,ERG24)也显著上调,参与脯氨酸生物合成过程的基因PRO1显著下调,编码色氨酸的基因显著上调[45]。但是在早前的研究中,酿酒酵母S288C在乙醇胁迫下(9%,V/V)海藻糖含量在6 h内持续增加的同时(超过3.0%的水平),胞内脯氨酸含量超过了5倍[46],表明海藻糖代谢与某些氨基酸代谢也存在一定的关联,但是氨基酸的种类和变化趋势可能会因菌种的不同而呈现出差异性。
5 提高酵母内源性海藻糖的方法 提高酵母细胞内海藻糖含量的方法有多种,如适当的应激胁迫,添加营养元素(生物素[47]、游离氨基酸[48]等),以及通过基因工程手段来对野生型菌株进行改良。近年来,提高酵母细胞海藻糖含量的基因工程改良方法主要集中在两方面,一是增强其合成代谢,TPS1基因的过表达可以显著提高优选前后面包酵母BY6-9a中细胞内海藻糖的含量[49]。二是抑制其降解速率,Nagahisa等构建了海藻糖酶基因ATH1、NTH1和NTH2的所有组合的缺失菌株,在盐胁迫条件下,与亲本酿酒酵母菌株FY834相比,缺陷菌株显示海藻糖积累增加。特别是三重缺失菌株,在盐胁迫条件下显示出比亲本菌株更高的生长速率[50]。同时,通过过量表达TPS1和缺失NTH1两者结合起来对提高海藻糖含量也有十分显著的效果,黄酒酵母菌株RY-1在10%乙醇胁迫下,工程菌株比原始菌株的海藻糖积累提高了1倍[51],也使面包酵母(TPS1的过表达和ATH1的缺失)在冷冻条件下海藻糖积累效果更加显著(提升了1.5倍)[52]。Liu等通过实验证明,与野生型菌株相比,突变菌株(TPS1的过量表达和ATH1的缺失)胞内的海藻糖含量增加了3倍,显著提高了其对辐射和重金属的耐受性[22]。
多项研究表明,通过一些其他基因的过量表达或缺失也会对酵母细胞内海藻糖含量产生影响。通过过量表达ARI1(编码醛还原酶)基因使得工程菌株对环境胁迫和醛抑制剂更耐受,与野生酿酒酵母菌株BCRC 21685相比,工程菌株表现出更高的海藻糖积累[53]。过表达MAL62基因(编码麦芽糖通透酶)的酵母菌株BY14a在优选冷冻和长期冷冻后显示出增加的海藻糖含量和细胞活力[54]。过表达GRX5(编码谷氧还蛋白)的重组酿酒酵母菌株(GRX5-Sc4126)分泌的海藻糖在不同时期均高于对照菌株(Sc4126)[55]。过表达长链鞘氨醇激酶基因LCB4使得酿酒酵母菌株S288C在乙酸(10 g/L)、糠醛(3 g/L)和香草醛(2 g/L)胁迫下,胞内海藻糖含量分别提高了6.9%、33.2%、60.8%[56]。在经过乙酸和糠醛筛选的酿酒酵母突变菌株AFb.01 (TRX1的过表达,编码硫氧还蛋白)中观察到获得性抑制剂耐受性能增强应激保护分子的生物合成(海藻糖等),以减轻酵母细胞在甘蔗渣培养基(预处理)分批发酵过程中的氧化应激[57]。张黎杰等发现在乙酸处理1 h和2 h后,基因JJJ1 (也被称为Hsp40s,是热休克蛋白Hsp70的分子伴侣)缺失的工程菌株其海藻糖含量分别比野生型酿酒酵母菌株BY4741高36.7%和54.3%[58]。另外,PUT1 (编码脯氨酸)的缺失提高了面包酵母BY-14a的海藻糖含量,使其耐冻性显著提高[59]。然而这些基因与海藻糖代谢过程的内在联系尚不清楚。
6 结语和展望 自公布了酿酒酵母的完整基因组顺序后,因其基因组易于操作以及能够在基础培养基上被完全控制生长等诸多内在优势,而使得酿酒酵母成为最典型的模式微生物之一,已对其内源性海藻糖代谢特征有了较深入的了解,组学研究显示内源性海藻糖在不同应激条件下的积累机制呈现出多样性。迄今为止所作的研究更多是支持海藻糖对应激条件下的酵母细胞起保护作用,然而近期有研究人员提出了新的观点,认为酿酒酵母对各种应激(氧化,渗透压,乙醇)的耐受性依赖于海藻糖代谢途径而非海藻糖物质本身[9],但对此结论还需要更多的研究支撑。此外,已证实酿酒酵母在热激条件下Msn2/Msn4(转录激活蛋白)有助于海藻糖合成和降解酶(Nth1,Tps1,Tps2和Tsl1)的表达[43, 60],但是在其他条件下尚未发现信号调控蛋白参与海藻糖代谢调控。
值得注意的是,对内源性海藻糖合成研究的对象主要聚焦于酿酒酵母,而对其他酵母关注较少,特别是作为食品工业上酱油酿造中的主发酵酵母鲁氏酵母和球拟酵母(耐盐性酵母,其代谢产物如酯类、多元醇、琥珀酸等是酱油香味的主要来源),其内源性海藻糖在不同逆境下的代谢调控机制还缺乏充足的研究,应该给予关注。
References
| [1] | Peng YF, Zhou YB, Li Q, Xue F, Feng J. Application prospect of trehalose. China Food Additives, 2009(1): 65-69. (in Chinese) 彭亚锋, 周耀斌, 李勤, 薛峰, 冯俊. 海藻糖的特性及其应用. 中国食品添加剂, 2009(1): 65-69. DOI:10.3969/j.issn.1006-2513.2009.01.008 |
| [2] | Feofilova EP, Usov AI, Mysyakina IS, Kochkina GA. Trehalose: chemical structure, biological functions, and practical application. Microbiology, 2014, 83(3): 184-194. |
| [3] | Zhou QF. Application of trehalose in food processing. China Food Industry, 1996(2): 22-23. (in Chinese) 周青峰. 海藻糖在食品加工上的应用. 中国食品工业, 1996(2): 22-23. |
| [4] | Tang B, Wang S, Wang SG, Wang HJ, Zhang JY, Cui SY. Invertebrate trehalose-6-phosphate synthase gene: genetic architecture, biochemistry, physiological function, and potential applications. Frontiers in Physiology, 2018, 9: 30. DOI:10.3389/fphys.2018.00030 |
| [5] | Gong T, Liu DH, Wang JW, Yang WL, Xie FH. Advances in trehalose biosynthesis pathways and application of molecular biology technique. Chinese Agricultural Science Bulletin, 2016, 32(14): 62-67. (in Chinese) 巩涛, 刘德海, 王继雯, 杨文玲, 解复红. 海藻糖合成途径及分子生物学研究进展. 中国农学通报, 2016, 32(14): 62-67. DOI:10.11924/j.issn.1000-6850.casb15120111 |
| [6] | Paul MJ, Primavesi LF, Jhurreea D, Zhang YH. Trehalose metabolism and signaling. Annual Review of Plant Biology, 2008, 59: 417-441. DOI:10.1146/annurev.arplant.59.032607.092945 |
| [7] | Eleutherio E, Panek A, de Mesquita JF, Trevisol E, Magalh?es R. Revisiting yeast trehalose metabolism. Current Genetics, 2015, 61(3): 263-274. |
| [8] | Trevisol ETV, Panek AD, de Mesquita JF, Eleutherio ECA. Regulation of the yeast trehalose-synthase complex by cyclic AMP-dependent phosphorylation. Biochimica et Biophysica Acta (BBA)-General Subjects, 2014, 1840(6): 1646-1650. DOI:10.1016/j.bbagen.2013.12.010 |
| [9] | Petitjean M, Teste MA, Fran?ois JM, Parrou JL. Yeast tolerance to various stresses relies on the trehalose-6P synthase (Tps1) protein, not on trehalose. Journal of Biological Chemistry, 2015, 290(26): 16177-16190. DOI:10.1074/jbc.M115.653899 |
| [10] | Petitjean M, Teste MA, Léger-Silvestre I, Fran?ois JM, Parrou JL. A new function for the yeast trehalose-6P synthase (Tps1) protein, as key pro-survival factor during growth, chronological ageing, and apoptotic stress. Mechanisms of Ageing and Development, 2017, 161: 234-246. DOI:10.1016/j.mad.2016.07.011 |
| [11] | Magalh?es RSS, de Lima KC, de Almeida DSG, de Mesquita JF, Eleutherio ECA. Trehalose-6-phosphate as a potential lead candidate for the development of Tps1 inhibitors: insights from the trehalose biosynthesis pathway in diverse yeast species. Applied Biochemistry and Biotechnology, 2017, 181(3): 914-924. DOI:10.1007/s12010-016-2258-6 |
| [12] | Vicente RL, Spina L, Gómez JPL, Dejean S, Parrou JL, Fran?ois JM. Trehalose-6-phosphate promotes fermentation and glucose repression in Saccharomyces cerevisiae. Microbial Cell, 2018, 5(10): 444-459. DOI:10.15698/mic2018.10.651 |
| [13] | Chi ZM, Liang LK, Zhu KL, Zhang FL. Advanced in metabolism and regulation of trehalose in yeast. Periodical of Ocean University of China, 2006, 36(2): 209-214. (in Chinese) 池振明, 梁丽琨, 朱开玲, 张风丽. 酵母海藻糖的代谢与调控研究进展. 中国海洋大学学报, 2006, 36(2): 209-214. |
| [14] | Jules M, Beltran G, Fran?ois J, Parrou JL. New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p-dependent trehalose mobilization. Applied and Environmental Microbiology, 2008, 74(3): 605-614. DOI:10.1128/AEM.00557-07 |
| [15] | Tran LM, Bang SH, Yoon J, Kim YH, Min J. Effect of acid trehalase (ATH) on impaired yeast vacuolar activity. Enzyme and Microbial Technology, 2016, 93-94: 44-50. DOI:10.1016/j.enzmictec.2016.07.010 |
| [16] | Auesukaree C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. Journal of Bioscience and Bioengineering, 2017, 124(2): 133-142. |
| [17] | Saini P, Beniwal A, Kokkiligadda A, Vij S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochemistry, 2018, 72: 1-12. DOI:10.1016/j.procbio.2018.07.001 |
| [18] | Babazadeh R, Lahtvee PJ, Adiels CB, Goks?r M, Nielsen JB, Hohmann S. The yeast osmostress response is carbon source dependent. Scientific Reports, 2017, 7(1): 990. DOI:10.1038/s41598-017-01141-4 |
| [19] | Xu HX, Zhou P, Wang X, Zhang XP, Duan G. Effects of stress factors on trehalose accumulation in the process of ethanol fermentation. Liquor-Making Science & Technology, 2016(5): 65-69. (in Chinese) 许宏贤, 周鹏, 王欣, 张晓萍, 段钢. 酒精发酵过程中胁迫因子对酵母海藻糖积累的影响. 酿酒科技, 2016(5): 65-69. |
| [20] | Gao LM, Liu YQ, Sun H, Li C, Zhao ZP, Liu GY. Advances in mechanisms and modifications for rendering yeast thermotolerance. Journal of Bioscience and Bioengineering, 2016, 121(6): 599-606. DOI:10.1016/j.jbiosc.2015.11.002 |
| [21] | Hu MD, Chen X, Li X, Wang Z, Dai J, Huang Z, Liu CC. Intracellular trehalose metabolism characteristics of Zygosaccharomyces rouxii under different stresses. Science and Technology of Food Industry, 2016, 37(11): 130-133. (in Chinese) 胡梦蝶, 陈雄, 李欣, 王志, 代俊, 黄珍, 刘翠翠. 不同胁迫条件对鲁氏酵母胞内海藻糖积累的影响研究. 食品工业科技, 2016, 37(11): 130-133. |
| [22] | Liu TT, Zhu LY, Zhang ZP, Huang H, Zhang ZD, Jiang L. Protective role of trehalose during radiation and heavy metal stress in Aureobasidium subglaciale F134. Scientific Reports, 2017, 7(1): 17586. DOI:10.1038/s41598-017-15489-0 |
| [23] | Voit EO. Biochemical and genomic regulation of the trehalose cycle in yeast: review of observations and canonical model analysis. Journal of Theoretical Biology, 2003, 223(1): 55-78. DOI:10.1016/S0022-5193(03)00072-9 |
| [24] | Suarez-Mendez CA, Ras C, Wahl SA. Metabolic adjustment upon repetitive substrate perturbations using dynamic 13C-tracing in yeast. Microbial Cell Factories, 2017, 16(1): 161. DOI:10.1186/s12934-017-0778-6 |
| [25] | Fraenkel D, Nielsen J. Trehalose-6-phosphate synthase and stabilization of yeast glycolysis. FEMS Yeast Research, 2016, 16(1): fov100. DOI:10.1093/femsyr/fov100 |
| [26] | Walther T, Mtimet N, Alkim C, Vax A, Loret MO, Ullah A, Gancedo C, Smits GJ, Fran?ois MJ. Metabolic phenotypes of Saccharomyces cerevisiae mutants with altered trehalose 6-phosphate dynamics. Biochemical Journal, 2013, 454(2): 227-237. |
| [27] | Li MD, Chen X, Han D, Mo XY, Zhao CX. Metabolic flux analysis of Saccharomyces cerevisiae under salt stress conditions. Journal of Jilin University (Science Edition), 2010, 48(4): 699-703. (in Chinese) 李明达, 陈霞, 韩丹, 莫新迎, 赵长新. 盐胁迫条件下酿酒酵母的代谢通量分析. 吉林大学学报(理学版), 2010, 48(4): 699-703. |
| [28] | Leite FCB, da R Leite DV, Pereira LF, de Barros Pita W, de Morais MA Jr. High intracellular trehalase activity prevents the storage of trehalose in the yeast Dekkera bruxellensis. Letters in Applied Microbiology, 2016, 63(3): 210-214. DOI:10.1111/lam.12609 |
| [29] | Liu CC, Hu MD, Wang Z, Yao J, Li P, Li ZJ, Chen X, Li X. Metabolic characteristics of intracellular trehalose accumulation in Zygosaccharomyces rouxii. China Biotechnology, 2017, 37(9): 41-47. (in Chinese) 刘翠翠, 胡梦蝶, 王志, 姚娟, 李沛, 李志军, 陈雄, 李欣. 鲁氏酵母胞内海藻糖积累过程的代谢特征分析. 中国生物工程杂志, 2017, 37(9): 41-47. |
| [30] | Jordà J, Rojas HC, Carnicer M, Wahl A, Ferrer P, Albiol J. Quantitative metabolomics and instationary 13C-metabolic flux analysis reveals impact of recombinant protein production on trehalose and energy metabolism in Pichia pastoris. Metabolites, 2014, 4(2): 281-299. DOI:10.3390/metabo4020281 |
| [31] | Chen Z, Zheng Z, Yi CF, Wang FL, Niu YP, Li H. Intracellular metabolic changes in Saccharomyces cerevisiae and promotion of ethanol tolerance during the bioethanol fermentation process. RSC Advances, 2016, 6(107): 105046-105055. DOI:10.1039/C6RA19254H |
| [32] | Pereira T, Vilaprinyo E, Belli G, Herrero E, Salvado B, Sorribas A, Altés G, Alves R. Quantitative operating principles of yeast metabolism during adaptation to heat stress. Cell Reports, 2018, 22(9): 2421-2430. DOI:10.1016/j.celrep.2018.02.020 |
| [33] | Sajiki K, Pluskal T, Shimanuki M, Yanagida M. Metabolomic analysis of fission yeast at the onset of nitrogen starvation. Metabolites, 2013, 3(4): 1118-1129. DOI:10.3390/metabo3041118 |
| [34] | Erkut C, Gade VR, Laxman S, Kurzchalia TV. The glyoxylate shunt is essential for desiccation tolerance in C. elegans and budding yeast. eLife, 2016, 5: e13614. DOI:10.7554/eLife.13614 |
| [35] | 王帅.南极酵母Pseudozyma sp. JCC207海藻糖基因及其适应南极极端环境分子机制研究.山东大学硕士学位论文, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10422-1016162249.htm |
| [36] | Li CS, Yang XQ, Xu Y, Li LH, Wang YQ. Cadmium detoxification induced by salt stress improves cadmium tolerance of multi-stress-tolerant Pichia kudriavzevii. Environmental Pollution, 2018, 242: 845-854. DOI:10.1016/j.envpol.2018.07.058 |
| [37] | Kitichantaropas Y, Boonchird C, Sugiyama M, Kaneko Y, Harashima S, Auesukaree C. Cellular mechanisms contributing to multiple stress tolerance in Saccharomyces cerevisiae strains with potential use in high-temperature ethanol fermentation. AMB Express, 2016, 6(1): 107. DOI:10.1186/s13568-016-0285-x |
| [38] | Capece A, Votta S, Guaragnella N, Zambuto M, Romaniello R, Romano P. Comparative study of Saccharomyces cerevisiae wine strains to identify potential marker genes correlated to desiccation stress tolerance. FEMS Yeast Research, 2016, 16(3): fow015. DOI:10.1093/femsyr/fow015 |
| [39] | Kandror O, Bretschneider N, Kreydin E, Cavalieri D, Goldberg AL. Yeast adapt to near-freezing temperatures by STRE/Msn2, 4-dependent induction of trehalose synthesis and certain molecular chaperones. Molecular Cell, 2004, 13(6): 771-781. DOI:10.1016/S1097-2765(04)00148-0 |
| [40] | Zhang K, Fang YH, Gao KH, Sui Y, Zheng DQ, Wu XC. Effects of genome duplication on phenotypes and industrial applications of Saccharomyces cerevisiae strains. Applied Microbiology and Biotechnology, 2017, 101(13): 5405-5414. DOI:10.1007/s00253-017-8284-7 |
| [41] | Yi CF, Wang FL, Dong SJ, Li H. Changes of trehalose content and expression of relative genes during the bioethanol fermentation by Saccharomyces cerevisiae. Canadian Journal of Microbiology, 2016, 62(10): 827-835. DOI:10.1139/cjm-2015-0832 |
| [42] | Miao YJ, Xiong GT, Li RY, Wu ZF, Zhang X, Weng PF. Transcriptome profiling of Issatchenkia orientalis under ethanol stress. AMB Express, 2018, 8: 39. DOI:10.1186/s13568-018-0568-5 |
| [43] | Satomura A, Katsuyama Y, Miura N, Kuroda K, Tomio A, Bamba T, Fukusaki E, Ueda M. Acquisition of thermotolerant yeast Saccharomyces cerevisiae by breeding via stepwise adaptation. Biotechnology Progress, 2013, 29(5): 1116-1123. DOI:10.1002/btpr.1754 |
| [44] | Arslan M, Holyavkin C, K?sakesen H?, Topalo?lu A, Sürmeli Y, ?akar ZP. Physiological and transcriptomic analysis of a chronologically long-lived Saccharomyces cerevisiae strain obtained by evolutionary engineering. Molecular Biotechnology, 2018, 60(7): 468-484. DOI:10.1007/s12033-018-0087-2 |
| [45] | Li RY, Xiong GT, Yuan SK, Wu ZF, Miao YJ. Investigating the underlying mechanism of Saccharomyces cerevisiae in response to ethanol stress employing RNA-seq analysis. World Journal of Microbiology and Biotechnology, 2017, 33(11): 206. DOI:10.1007/s11274-017-2376-5 |
| [46] | Kaino T, Takagi H. Gene expression profiles and intracellular contents of stress protectants in Saccharomyces cerevisiae under ethanol and sorbitol stresses. Applied Microbiology and Biotechnology, 2008, 79(2): 273-283. |
| [47] | Chen X, Yan M, Xie FL, Dai J, Li DS, Wang Z, Li ZJ, Yao J, Li X, Zhao XJ. Biotin enhances salt tolerance of Torulopsis mogii. Annals of Microbiology, 2015, 65(1): 393-398. |
| [48] | Lei HJ, Li F, Peng F, Xu HD. Amino acid supplementations enhance the stress resistance and fermentation performance of lager yeast during high gravity fermentation. Applied Biochemistry and Biotechnology, 2019, 187(2): 540-555. DOI:10.1007/s12010-018-2840-1 |
| [49] | Tan HG, Dong J, Wang GL, Xu HY, Zhang CY, Xiao DG. Enhanced freeze tolerance of baker's yeast by overexpressed trehalose-6-phosphate synthase gene (TPS1) and deleted trehalase genes in frozen dough. Journal of Industrial Microbiology & Biotechnology, 2014, 41(8): 1275-1285. |
| [50] | Mahmud SA, Nagahisa K, Hirasawa T, Yoshikawa K, Ashitani K, Shimizu H. Effect of trehalose accumulation on response to saline stress in Saccharomyces cerevisiae. Yeast, 2010, 26(1): 17-30. |
| [51] | Divate NR, Chen GH, Wang PM, Ou BR, Chung YC. Engineering Saccharomyces cerevisiae for improvement in ethanol tolerance by accumulation of trehalose. Bioengineered, 2016, 7(6): 445-458. DOI:10.1080/21655979.2016.1207019 |
| [52] | Wu MY, Zhang CY, Sun X, Wang GL, Liu YW, Xiao DG. Effects of NTH1 gene deletion and overexpressing TPS1 gene on freeze tolerance in baker's yeast//Zhang TC, Ouyang PK, Kaplan S, Skarnes B. Proceedings of the 2012 International Conference on Applied Biotechnology. Berlin, Heidelberg: Springer, 2014: 447–454. |
| [53] | Divate NR, Chen GH, Divate RD, Ou BR, Chung YC. Metabolic engineering of Saccharomyces cerevisiae for improvement in stresses tolerance. Bioengineered, 2017, 8(5): 524-535. DOI:10.1080/21655979.2016.1257449 |
| [54] | Sun X, Zhang CY, Wu MY, Fan ZH, Liu SN, Zhu WB, Xiao DG. MAL62 overexpression and NTH1 deletion enhance the freezing tolerance and fermentation capacity of the baker's yeast in lean dough. Microbial Cell Factories, 2016, 15: 54. DOI:10.1186/s12934-016-0453-3 |
| [55] | Fang Q, Zhang MM, Chen HQ, Xiong L, Zhao XQ, Bai FW. Improvement of acetic acid tolerance of Saccharomyces cerevisiae by overexpressing glutaredoxin encoding gene GRX5. CIESC Journal, 2015, 66(4): 1434-1439. (in Chinese) 方青, 张明明, 陈洪奇, 熊亮, 赵心清, 白凤武. 过表达谷氧还蛋白基因GRX5提高酿酒酵母乙酸耐性. 化工学报, 2015, 66(4): 1434-1439. |
| [56] | He YY, Zi LH, Zhang BH, Xu JR, Wang DD, Bai FW. Improvement of inhibitors tolerance of Saccharomyces cerevisiae by overexpressing of long chain sphingoid kinases encoding gene LCB4. Chinese Journal of Biotechnology, 2018, 34(6): 906-915. (in Chinese) 何艳艳, 孜力汗, 张宝会, 许建韧, 王丹丹, 白凤武. 过表达长链鞘氨醇激酶基因LCB4提高酿酒酵母抑制物耐受性. 生物工程学报, 2018, 34(6): 906-915. |
| [57] | Unrean P, G?tgens J, Klein B, Noack S, Champreda V. Elucidating cellular mechanisms of Saccharomyces cerevisiae tolerant to combined lignocellulosic-derived inhibitors using high-throughput phenotyping and multiomics analyses. FEMS Yeast Research, 2018, 18(8): foy106. |
| [58] | 张黎杰.酿酒酵母JJJ1基因敲除提高乙酸耐性和纤维乙醇发性能.浙江大学硕士学位论文, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10335-1016280038.htm |
| [59] | Dong J, Chen DD, Wang GL, Zhang CY, Du LP, Liu SS, Zhao Y, Xiao DG. Improving freeze-tolerance of baker's yeast through seamless gene deletion of NTH1 and PUT1. Journal of Industrial Microbiology & Biotechnology, 2016, 43(6): 817-828. |
| [60] | Z?hringer H, Thevelein JM, Nwaka S. Induction of neutral trehalase Nth1 by heat and osmotic stress is controlled by STRE elements and Msn2/Msn4 transcription factors: variations of PKA effect during stress and growth. Molecular Microbiology, 2010, 35(2): 397-406. |
