袁浩1, 卢梦涵1, 刘宏伟1, 刘菊梅2, 包苏日古嘎1, 何倩1, 李海波1, 曹伟伟1, 赵吉1,3, 包智华1,3


1. 内蒙古大学生态与环境学院, 内蒙古 呼和浩特 010021;
2. 内蒙古大学生命科学学院, 内蒙古 呼和浩特 010021;
3. 内蒙古环境污染控制与废物资源化重点实验室, 内蒙古 呼和浩特 010021
收稿日期:2019-01-25;修回日期:2019-03-18;网络出版日期:2019-07-15
基金项目:国家自然科学基金(41563009);国家级大学生创新创业训练计划(20151012601);内蒙古大学高层次人才启动基金(21800-5145144)
*通信作者:包智华, E-mail:bao1016@163.com.
摘要:大量的稀土-重金属通过尾矿坝的浮尘、地表径流和渗滤液排放到周边土壤中,影响了土壤中的微生物群落结构。[目的] 分析稀土和重金属复合污染土壤真菌群落结构并分离具有同时吸附稀土和重金属的菌株。[方法] 本研究基于ITS基因,采用Illumina-Hiseq测序技术分析了包头稀土尾矿坝周边5份稀土-重金属污染土壤样品和距尾矿区20 km的1份对未受污染的土壤样品的真菌群落特征,同时采用富集培养法从污染样品中筛选出金属耐性真菌,并对其进行吸附稀土-重金属的特性分析。[结果] 群落结构为:在门水平,除了未分类门真菌(unclassified Fungi)外,子囊菌门(Ascomycota)真菌在所有土壤中占比较大(13.5%-90.5%);在纲水平上,除了未分类纲真菌外,粪壳菌纲(Sordariomycetes)真菌在B2(73.1%)、B3(28.4%)和B4(20.8%)的丰度显著高于对照样点C(7.4%),而座囊菌纲(Dothideomycetes)在B5(11.8%)的丰度明显高于B1(3.5%);在属水平,除了未分类属,足孢子虫属(Podospora)是C(0.9%)和B3(23.6%)样点的优势种。曲霉属(Aspergillus)、未分类的格孢腔菌目(unclassifiedPleosporales)和未分类的戴维迪科(unclassified Davidiellaceae)分别为B1(3.0%)、B4(10.5%)和B5(5.8%)的优势种,而蜡蚧属(Lecanicillium)真菌只在B2样点土壤存在且占优(51.6%)。Zn污染对真菌群落结构的影响大于稀土元素污染,且其浓度与优势的未分类真菌相对丰度呈负相关。从污染样品中共分离出6株真菌,它们分属于曲霉属(Aspergillus)(5株)和镰刀霉菌属(Fusarium)(1株)。所有分离菌株对镧(La3+)的吸附率均显著高于锌(Zn2+),其中Aspergillus sp.B6-3对La3+和锌Zn2+的吸附率最高,分别为19.7%和3.9%。[结论] 该研究为利用真菌去除稀土和重金属以优化生物吸附过程导向的环境生物修复和保护策略提供了机制基础。
关键词:稀土重金属分子检测真菌群落结构分离技术生物吸附过程
Fungal community structure in mining soil and its adsorption kinetics of rare earth-heavy metal ions
Hao Yuan1, Menghan Lu1, Hongwei Liu1, Jumei Liu2, Suriguga Bao1, Qian He1, Haibo Li1, Weiwei Cao1, Ji Zhao1,3, Zhihua Bao1,3


1. College of Ecology and Environment, Inner Mongolia University, Hohhot 010021, Inner Mongolia Autonomous Region, China;
2. College of Life Sciences, Inner Mongolia University, Hohhot 010021, Inner Mongolia Autonomous Region, China;
3. Inner Mongolia Key Laboratory of Environmental Pollution Prevention and Waste Recycle, Hohhot 010021, Inner Mongolia Autonomous Region, China
Received: 25 January 2019; Revised: 18 March 2019; Published online: 15 July 2019
*Corresponding author: Zhihua Bao, E-mail:bao1016@163.com.
Foundation item: Supported by the National Natural Science Foundation of China (41563009), by the National Innovation and Entrepreneurship Training Program for College Students (20151012601) and by the Starting Funding for Scientific Research from the Inner Mongolia University (21800-5145144)
Abstract: A large number of contaminants from rare earth-mines are discharged into the surrounding soil through the floating dust, surface runoff and leachate of the tailings dam, and affect particular fungal communities. [Objective] To investigate the adaption of fungal community structure to the long-term pollution stress by both rare earth and heavy metals, and to characterize the adsorption kinetics of rare earth and heavy metal by fungi isolates. [Methods] Based on the internal transcribed spacer (ITS) gene, Illumina-Hiseq sequencing technique was used to analyze the fungal community structure in five soils from B1 to B5 with increasingly higher contents of rare earth-heavy metal around Baotou rare earth tailings dam, in addition to an uncontaminated control soil about 20 km away from tailings area (sample C). Meanwhile, a total of 6 fungal isolates were obtained by culture-dependent technique, and their absorption kinetics of both rare earth and heavy metals were analyzed. [Results] At the phylum level, Ascomycota was abundant in fungal communities in all samples (13.5%-90.5%). At the class level, Sordariomycetes was apparently higher in B2 (73.1%), B3 (28.4%) and B4 (20.8%) than control C samples (7.4%); and the relative abundance of Dothideomycetes was lower in the B1 (3.5%) than B5 (11.8%). At the genus level, Podospora showed strong adaption to contaminant stress, from the low relative abundance of 0.9% in control soil, to 23.6% in B3. Meanwhile, Aspergillus, unclassified Pleosporales, and unclassified Davidiellaceae were also detected with high relative abundances in B1 (3.0%), B4 (10.5%) and B5 (5.8%), respectively. Intriguingly, Lecanicillium was detected only from the B2 sample and dominated (51.6%). The effect of Zn pollutant on fungal communities was greater than that of rare earth elements, and negative correlation was observed between soil Zn content the relative abundance of dominant unclassified fungi. Six fungal isolates were obtained from contaminated soil, and could be assigned to genera Aspergillus (five isolates) and Fusarium (one isolate). All fungal isolates showed significantly higher adsorption capacity of La3+ than that of Zn2+, and the Aspergillus sp. B6-3 had the highest adsorption rates for La3+ (19.7%) and Zn2+ (3.9%). [Conclusion] This study provided a mechanistic basis for the use of fungi to remove rare earths and heavy metals towards the optimization of biosorption process-oriented strategy for environmental bioremediation and protection.
Keywords: rare earth elementheavy metalmolecular surveyfungi community structureculture-dependent techniquebiosorption process
稀土元素在冶金产业、军工设备制造、高精尖材料及环保材料等领域都有广泛的应用。我国包头白云鄂博稀土矿区是国内最大的多种金属共生矿区。有调查显示包头稀土尾矿坝尾矿达到1.6亿t,稀土含量达到7%,含有大部分的稀土和重金属元素[1]。调查数据显示包头稀土尾矿区周边土壤中La、Ce、Pb、Zn的含量严重超标,稀土元素La和Ce含量分别是内蒙土壤含量均值的344倍和486倍,重金属元素Pb和Zn的含量分别是内蒙土壤含量均值的23倍和16倍,可见包头稀土尾矿坝周边环境中稀土-重金属复合污染程度严重。很多研究表明这些污染对地下水、土壤农作物、人群健康及土壤微生物群落结构造成潜在的影响[2]。
土壤微生物是土壤生态系统的重要组成部分,它通过分泌各种酶参与土壤中有机质的分解、腐殖质的形成、土壤养分循环等过程。并且土壤微生物群落结构的变化常作为评估重金属污染的生物指标[3]。
目前国内外金属矿区土壤微生物的相关研究主要集中在重金属对土壤微生物群落结构的影响[4-8],有研究发现在低浓度时重金属元素或稀土元素可以促进微生物生长[9],然而稀土-重金属复合污染对土壤真菌群落结构的影响方面鲜有报道。此外国内外关于微生物对重金属吸附能力的研究较多[10-11],但真菌同时对重金属和稀土元素吸附相关研究未见报道。
本研究选取包头稀土尾矿周边受污染土壤和相对无污染林地土壤,采用高通量技术测定土壤中真菌群落结构,同时进行真菌的分离鉴定,并对筛选菌株的稀土-重金属离子吸附特性进行测定。研究结果不仅可以填补国内外对稀土污染土壤中真菌群落结构研究的空白,还可以挖掘稀土-重金属复合污染的极端微生物资源,从而为利用真菌修复稀土污染的环境治理问题提供微生物资源和基础数据。
1 材料和方法 1.1 样品采集与分析 2015年7月在内蒙古自治区包头稀土尾矿区东南方向200 m,采用多点混合法采集深度0–20 cm的土壤样5份(B1–B5)和1份位于尾矿区向南20 km的土壤样品(C)作为对照,如图 1,每份样品都具有3个重复。采集后将样品装入无菌离心管,放入冷藏箱中运输至实验室保存。将采集土壤样品分为3份保存。1份–80 ℃低温冰箱保存,用于分子实验;1份4 ℃保存用于微生物分离;另1份室温风干过筛,用于土壤理化性质分析。测定的理化指标包括重金属和稀土元素。重金属采用微波消解法[12],稀土元素采用开放式消解法[1],消解后利用电感耦合等离子体发射光谱仪(Inductively Coupled Plasma Optical Emission Spectrometer,ICP-OES) (Icap6000,Thermo Fisher Scientific,USA)测定其含量。
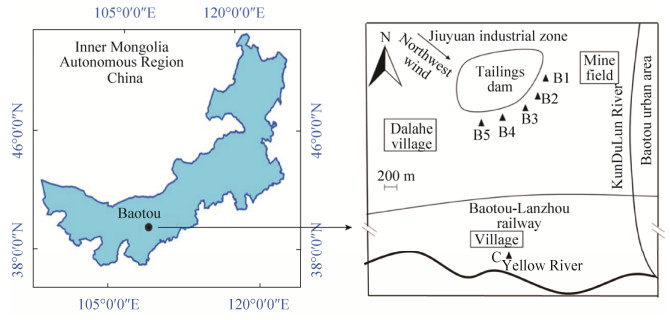 |
| 图 1 土壤样品采集位点示意图 Figure 1 Soil sample distribution map. |
| 图选项 |
1.2 真菌的分离纯化 用于真菌分离的马丁氏琼脂培养基(MD,g/L):葡萄糖10.0,蛋白胨5.0,KH2PO4 1.0,MgSO4·7H2O 0.5,1/3000孟加拉红(Rose Bengal,玫瑰红水溶液),琼脂20.0。培养基在121 ℃下灭菌30 min后,通过0.22 μm滤膜配制为过滤除菌的100 mg/L的LaCl3或ZnSO4·7H2O溶液,与培养基混合后倒平板,用于分离耐La3+和Zn2+的真菌。
1.3 土壤真菌群落高通量测序与真菌的鉴定 土壤DNA采用FastDNA? SPIN Kit for Soil (MP,Biomedicals,USA)试剂盒[13]提取。利用引物ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′)和ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′)对18S rRNA的ITS区进行PCR扩增,扩增体系见参考文献[14],并进行胶回收(GeneJET,Thermo Scientific)。基于Illumina MiSeq PE300测序平台在上海Majorbio Bio-pharm Technology Co.,Ltd上机测序。使用FLASH对每个样品的reads进行拼接,利用Qiime进行质量控制,UCLUST程序进行聚类分析以97%的一致性将序列聚类成为OTUs,用Usearch软件与Unite数据库进行物种注释分析。
采用同上试剂盒提取菌株总DNA,然后进行ITS基因的PCR扩增。利用引物ITS1 (5′-TCCGT AGGTGAACCTGCGG-3′)和ITS4 (5′-TCCTCCGC TTATTGATATGC-3′)对18S rRNA的ITS区进行PCR扩增,扩增体系见参考文献[15]。PCR产物采用1%琼脂糖凝胶电泳检测后送至上海生工公司测序。测序结果与GenBank上的已知序列进行比对,并利用MEGA 5.2软件中的邻接法构建系统发育树。将测序结果上传到NCBI数据库并获取登录号。
1.4 分离菌株耐金属离子性质的检测 利用0.22 μm的滤膜除菌,配制成含有50 mg/L的La3+和50 mg/L的Zn2+的MD固体培养基,将菌丝用无菌手术刀接种到固体培养基中观察菌株形态变化。
1.5 分离菌株对金属离子吸附能力的检测 (1) 在250 mL锥形瓶中加入100 mL含有100 mg/L的La3+或Zn2+的液体培养基MD,测定其浓度为C0。调节pH为6.8–7.0。选取代表性菌株加入到上述培养基中在30 ℃、9600 r/min的条件下进行振荡培养,每株菌株设置3个重复。
(2) 将培养后的菌液混匀倒入50 mL离心管中,4000 r/min离心15 min,每种菌液各取上清液10 mL转移至无菌的15 mL离心管中待测,测定结果为C。
(3) 向含有菌体沉淀的离心管中加入5 mL EDTA洗脱液(0.5 mol/L,pH=8),在振荡仪上振荡混匀,充分洗脱,静置20 min,4000 r/min离心15 min,取上清液5 mL转移至无菌的离心管中待测,此步骤重复3到4次以确保菌株表面金属离子彻底洗脱。EDTA洗脱下菌株表面的金属离子总质量为M。
(4) 将菌株4000 r/min离心15 min去除上清液,然后放置于80 ℃烘干12 h至恒重,称量烘干后的离心管质量,烘干后的离心管质量与空离心管的差值为菌体干重G。
(5) 将所有的待测液体通过0.45 μm的滤膜过滤处理后上ICP-OES测定。
(6) 吸附率计算公式Q=(C0–C)/C0×100%,Q为吸附率,C0为未加菌株配制的金属离子培养液浓度,C为加菌株培养后培养液金属离子的浓度[16]。吸附能力计算公式F=M/G,F为菌株吸附能力,M为EDTA洗脱下菌株表面的金属离子质量,G为菌株的干重(DW)。
1.6 土壤污染评价 采用土壤污染负荷指数法(Pollution loading index,PLI)[17](公式1,2)。
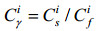 | 公式(1) |
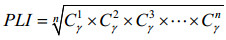 | 公式(2) |
2 结果和分析 2.1 土壤理化分析与污染评价 由表 1可知土壤污染负荷指数B4>B5>B3>B1>B2>C,6个样品中对照样C无污染,其他5个样点均受稀土元素污染,还伴随着不同程度重金属污染,且B4和B5的污染程度显著高于B1、B2、B3。B4、B5样点受到严重稀土-重金属复合污染,B1、B2、B3样点受到严重的稀土元素污染。其中B4样点受到最为严重的稀土元素污染,La、Ce、Nd、Pr分别是内蒙土壤均值的66倍、77倍、71倍和72倍。B5样点受到最为严重重金属元素污染,Cr、Pb、Zn分别是内蒙土壤均值的13倍、9倍、3倍。
表 1. 土壤稀土和重金属浓度及污染指数 Table 1. Concentration of rare earth element and heavy metal and pollution index in soil
| Samples | Heavy metal/(mg/kg) | Rare earth/(mg/kg) | PLI | ||||||||
| Cr | Cd | Pb | Zn | Sm | Nd | Pr | Ce | La | |||
| C | 36.31±2.84c | 0.01±0.01d | 15.70±3.14b | 46.88±9.53d | 4.66±1.31e | 6.90±0.38e | 5.77±3.39e | 8.47±1.85e | 4.85±0.24c | 0.41±0.04e | |
| B1 | 86.31±9.18c | 0.07±0.02b | 15.78±3.55b | 40.16±9.63d | 55.84±8.86c | 697.50±107.34c | 206.48±32.70c | 1863.74±282.57c | 1093.53±221.91b | 7.70±0.85c | |
| B2 | 46.20±4.39c | 0.04±0.02cd | 9.76±9.65b | 461.75±58.75a | 23.82±9.52d | 210.54±5.79d | 117.63±12.61d | 612.24±71.53d | 338.21±46.72c | 4.70±1.12d | |
| B3 | 39.52±2.81c | 0.085±0.01ab | 25.09±5.31b | 267.96±40.13b | 86.85±3.31b | 1017.51±49.40b | 299.25±13.22b | 2504.90±343.14b | 1080.60±577.11b | 10.98±0.93b | |
| B4 | 182.53±22.03b | 0.10±0.02a | 27.21±3.03b | 132.00±9.07c | 108.38±12.47a | 1381.89±161.88a | 411.75±48.51a | 3823.75±478.79a | 2167.28±310.84a | 14.98±2.74a | |
| B5 | 506.12±92.17a | 0.06±0.00bc | 142.00±8.52a | 183.50±2.32c | 63.36±2.63c | 796.94±44.69c | 236.28±10.85c | 2142.83±72.82bc | 1169.72±42.58b | 14.98±0.42a | |
| Background value | 36.5 | 0.037 | 15.0 | 48.6 | 3.81 | 19.2 | 5.68 | 49.1 | 32.8 | – | |
| In bold showed that the metal contents of the sample were more than three times of the background value of Inner Mongolia. PLI was calculated based on the background value of Inner Mongolia[18]. Different letters indicate significant differences between different sites, the test level P<0.05. | |||||||||||
表选项
2.2 土壤真菌群落分析
2.2.1 土壤真菌群落多样性: C、B1、B2、B3、B4、B5高通量测序结果显示,划分的OTUs数C>B2>B1>B5>B3>B4,Shannon多样性指数C>B4>B3>B1>B5>B2 (表 2)。由Shannon多样性指数可知对照样C与重度污染样点B5的α-多样性指数有显著差异,稀土-重金属复合污染对真菌群落α-多样性有影响。
表 2. 土壤真菌群落多样性指数 Table 2. Diversity indices of soil fungal communities
| Sample | Taxon tags | OTUs | Diversity index | |
| Shannon | Simpson | |||
| C | 43276–65927 | 633±45.76a | 4.04±0.24a | 0.07±0.02b |
| B1 | 38982–60855 | 184±36.29bc | 3.11±0.91ab | 0.14±0.01ab |
| B2 | 50441–71687 | 250±13.52b | 2.64±0.24b | 0.22±0.05a |
| B3 | 41658–62554 | 140±26.08cd | 3.23±0.12ab | 0.10±0.02b |
| B4 | 40802–59203 | 73±15.31d | 3.33±0.57ab | 0.06±0.03b |
| B5 | 51258–57470 | 143±64.63cd | 2.97±0.65b | 0.13±0.09ab |
| Different letters indicate significant differences between different sites, the test level P<0.05. | ||||
表选项
2.2.2 土壤真菌群落组成: 通过土样中OTU数值排名前10的菌株进行门、纲和属水平的群落结构分析,重复样点取平均值,其他物种划分为Others。如图 2所示未分类的真菌(unclassified Fungi)在包头尾矿区样点呈现一定的丰度梯度B2<B3<B4<B5。在门水平上(图 2-A),除了未分类的门外,6个样品中均是子囊菌门(Ascomycota)真菌相对丰度较高,且在污染样点B1 (31.50%)、B2 (90.48%)、B3 (43.53%)、B4 (56.77%)和B5 (26.30%)土壤的相对丰度高于对照样点土壤C (13.48%);担子菌门(Basidiomycota)真菌在尾矿区除去B2 (1.04%)样点土壤,其相对丰度在B1 (12.16%)、B3 (12.61%)、B4 (6.54%)和B5 (3.26%)样品高于无污染的C (2.34%)土样;罗茨菌门(Rozellomycota)真菌只在无污染样品存在。在纲水平上(图 2-B),除了未分类的,粪壳菌纲(Sordariomycetes)真菌的相对丰度在C (7.44%)样品中低于B2、B3、B4、B5受污染样品;座囊菌纲(Dothideomycetes)和散囊菌纲(Eurotiomycetes)真菌在污染样点相对丰度高于对照样C;伞菌纲(Agaricomycetes)真菌在B3 (10.52%)样点相对丰度最高,在对照样点C (6.92%)要高于其他污染样点。在属水平上(图 2-C),除了未分类的属,曲霉属(Aspergillus)真菌在污染样点B1 (2.99%)、B2 (1.29%)、B3 (1.32%)、B4 (8.33%)和B5 (3.52%)的相对丰度高于对照样点C (0.06%);蜡蚧属(Lecanicillium)、足孢子虫属(Podospora)、丛枝菌根真菌属(Rhizophagus)、未分类的格孢腔菌目(unclassified Pleosporales)、未分类的伞菌目(unclassified Agaricales)、未分类的戴维迪科(unclassified Davidiellaceae)和未分类虫草科(unclassified Cordycipitaceae)真菌仅在污染样点中存在。B4、B5污染程度严重,且真菌群落组成更多样,后续用这2个受污染严重土壤进行优势菌的分离。
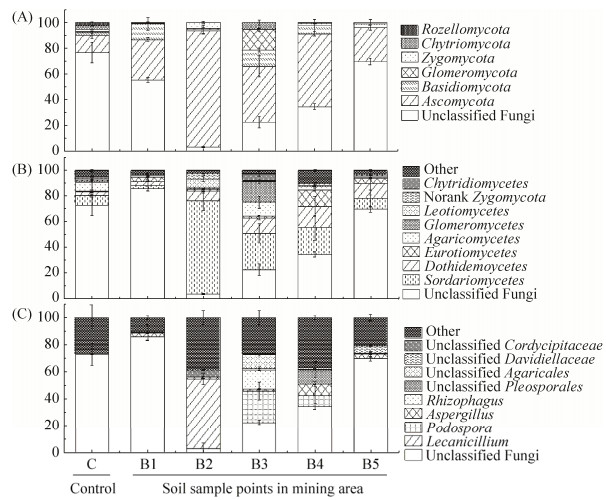 |
| 图 2 土壤样品的真菌群落结构 Figure 2 Fungal community structures of soil samples. A: Phylum; B: Class; C: Genus. |
| 图选项 |
2.2.3 土壤真菌群落、样点和土壤理化因子间的冗余分析(RDA): 将样品中丰度>1%的真菌群落、样点和理化因子进行冗余分析,结果表明(图 3)第1轴和第2轴的累计解释变异量达到67.9%,金属Zn对真菌群落结构影响最大。无Zn污染的B1样点与对照样点C群落结构相近,Zn污染较重的B2样点单独形成cluster,而稀土污染相对较重的样点B3、B4和B5群落结构较相似。蜡蚧属(Lecanicillium)和unclassified Cordycipitaceae真菌与Zn呈正相关。足孢子虫属、丛枝菌根真菌属、unclassified Davidiellaceae、unclassified Pleosporales和unclassified Agaricales真菌与金属元素La、Ce、Nd、Sm、Pr、Cr、Cd、Pb呈正相关。
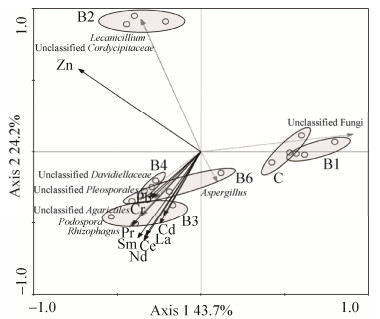 |
| 图 3 土壤真菌优势类群和土壤环境因子间的冗余分析 Figure 3 Redundancy analysis for soil fungal communities and soil physicochemical factors. |
| 图选项 |
2.2.4 金属与属水平OTU数值排名前十的菌株相关性分析: 属水平上OTU数值排名前10的菌株与土壤中稀土和重金属相关性分析结果显示(表 3),unclassified Fungi和Lecanicillium与金属Zn呈显著负相关,unclassified Cordycipitaceae与金属Zn呈显著正相关,Podospora与金属Sm呈正相关,Aspergillus与5种稀土元素都呈正相关,unclassified Pleosporales与5种稀土元素和Cd都呈正相关,unclassified Davidiellaceae与重金属Cr和Pb呈显著正相关,unclassified Cordycipitaceae与Zn浓度正相关。
表 3. 土壤稀土和重金属浓度与属水平优势物种相关性分析 Table 3. Correlation coefficient between concentrations of metal and genus-level dominant group
| Fungus | Heavy metal/(mg/kg) | Rare earth/(mg/kg) | ||||||||
| Cr | Cd | Pb | Zn | Sm | Nd | Pr | Ce | La | ||
| unclassified Fungi | –0.376 | –0.254 | –0.354 | –0.579* | –0.414 | –0.388 | –0.440 | –0.378 | –0.301 | |
| Lecanicillium | –0.271 | –0.323 | –0.274 | 0.827** | –0.414 | –0.451 | –0.325 | –0.428 | –0.398 | |
| Podospora | –0.249 | 0.333 | –0.223 | 0.171 | 0.512* | 0.456 | 0.438 | 0.386 | 0.203 | |
| Aspergillus | 0.111 | 0.427 | 0.048 | –0.332 | 0.504* | 0.547* | 0.541* | 0.576* | 0.604** | |
| Rhizophagus | –0.290 | 0.280 | –0.130 | 0.238 | 0.378 | 0.324 | 0.304 | 0.250 | 0.080 | |
| unclassified Pleosporales | 0.096 | 0.498* | –0.187 | –0.123 | 0.585* | 0.596** | 0.607** | 0.623** | 0.642** | |
| unclassified Agaricales | –0.293 | 0.272 | –0.137 | 0.226 | 0.373 | 0.319 | 0.298 | 0.244 | 0.073 | |
| unclassified Davidiellaceae | 0.820** | 0.088 | 0.752** | –0.001 | 0.110 | 0.130 | 0.110 | 0.136 | 0.143 | |
| Unclassified Cordycipitaceae | –0.271 | –0.308 | –0.270 | 0.837** | –0.409 | –0.451 | –0.323 | –0.427 | –0.397 | |
| * and ** indicate significantly different at the 0.05 and 0.01 probability levels, respectively. | ||||||||||
表选项
2.3 分离菌株的ITS基因系统发育分析 通过利用含100 mg/L的La和Zn的MD培养基对B4、B5土壤样品进行菌株的筛选,得到6株真菌,其中5株属于曲霉菌属真菌,1株属于镰刀菌属真菌,将菌株ITS序列与数据库对比,建立系统发育树(图 4)显示菌株B6-4、B6-2、B6-6、B6-5与Aspergillus flavus最近缘(相似度范围99%–100%),B6-3和B7-1分别与Aspergillus nidulans CMXY2059 (MG991576)和Fusarium verticillioides HS17 (KY426418)最近缘(99%–100%)。
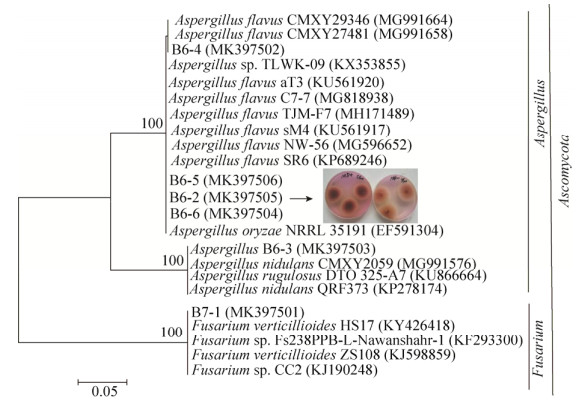 |
| 图 4 真菌分离菌株的ITS基因系统发育树 Figure 4 Phylogenetic tree of fungal isolates based on ITS gene. The numbers in the brackets are gene sequence accession numbers in GenBank; The numbers of nodes indicate bootstrap values and represent the percentages of 1000 bootstrap replications in which the taxa to the right are placed together; The scale bar represents the horizontal branch lengths and the number of substitutions per nucleotide position. |
| 图选项 |
2.4 分离菌株的金属吸附能力测定 4株代表性真菌菌株对La3+和Zn2+的吸附实验结果表明,经过3 d培养后培养液金属离子浓度明显降低,对于La3+吸附率最高的是B6-3达到19.73%,而后依次为B6-2 (12.88%)>B7-1 (8.52%)>B6-4 (7.09%),对于Zn2+吸附率最高的是B7-1达到4.75%,而后依次为B6-3 (3.92%)>B6-2 (3.13%)>B6-4 (1.91%)。吸附能力测定结果(图 5)显示B6-2菌株对La3+、Zn2+的吸附能力最强分别达到8.41 mg/g和0.89 mg/g。其他菌株对金属La3+吸附能力依次为B6-3>B7-1>B6-4,对金属Zn吸附能力依次为B7-1>B6-3>B6-4。
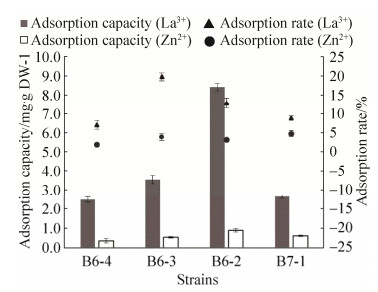 |
| 图 5 4株分离菌株对镧离子和锌离子的吸附能力和吸附率 Figure 5 Adsorption capacity/rate of La3+ and Zn2+ for four representative strains. |
| 图选项 |
3 讨论 金属污染会导致土壤微生物多样性及群落结构的变化[19],从而影响土壤生态系统稳定性。本研究采集稀土尾矿区周边重金属和稀土元素双重污染土壤,分析真菌群落多样性与金属浓度的关系,并对分离菌株进行了吸附特性研究。本研究中重金属和稀土污染样点的Shannon指数及OTUs数量均小于无污染对照样点C (表 1,表 2),该结果与前人研究结果一致[20]。重金属和稀土元素污染会抑制不具有耐性真菌的生长,从而稀土-重金属污染土壤中真菌群落结构多样性降低,但耐性菌株由于其适应性将大量繁殖生长,使稀土-重金属污染土壤中真菌OTUs表现增多。真菌群落结构分析结果表明,unclassified Fungi在未污染样点的丰度最高,而在污染样点呈现一定的丰度梯度(图 2),其与土壤样点Zn污染程度成负相关(表 3),Zn污染越严重的土壤中unclassified Fungi丰度越低,反之Zn未污染样点与稀土元素污染样点(C和B1)群落结构相似(图 3)。因此,Zn污染是影响群落结构的重要因素,暗示重金属污染程度对土壤真菌群落结构的影响可能比稀土元素污染大。其他物种如Ascomycota真菌存在于所有样点,但在Zn污染较重的土壤相对丰度最高,说明其对Zn耐性较强;Rozellomycota真菌只在无污染土壤中出现,该门菌可能不具有金属污染耐性。在纲水平,Sordariomycetes真菌在B2–B5样点相对丰度普遍高于对照样,暗示该类群对金属污染土壤有适应能力;Dothidemoycetes和Eurotiomycetes真菌在污染样点相对丰度高于对照样,该类群可能具有金属污染耐性。在属水平,Lecanicillium真菌仅在B2样点存在且占比高,且与金属锌浓度显示正相关,可能其对金属锌耐性高,并且有研究[21]发现金属元素Zn2+促进该属菌株酶活性;在属水平上,Podospora、Aspergillus、Rhizophagus、unclassified Pleosporales、unclassified Agaricales、unclassified Davidiellaceae、unclassified Cordycipitaceae和Crustoderma真菌仅在污染样点存在并与稀土元素浓度呈正相关。有研究表明,在金属污染土壤中Aspergillu真菌的相对丰度较高,且具有金属耐性[22]。而Rhizophagu真菌也能在重金属污染土壤中生存[23]。本研究结果暗示,以上菌群能适应稀土和重金属复合污染环境。
在重金属废水处理中生物吸附法应用广泛,它具有高效廉价的特点,其通过1种或几种菌株制成的微生物联合制剂,利用微生物本身的化学结构及成分特点,来去除水体中金属离子[24]。微生物金属污染修复技术的原理主要分为3种方式,(1)吸附作用:微生物细胞表面蛋白可以吸附重金属离子或者细胞壁带有的负离子可将金属阳离子集于细胞表面;(2)转化作用:微生物可以促进金属元素(离子)甲基化与氧化还原作用;(3)代谢作用:通过微生物新陈代谢产物进行修复,其中利用微生物吸附技术去除水体中重金属离子,由于其经济且有效而备受关注[25]。国内外利用曲霉菌,尤其是黑曲霉菌来去除环境中金属离子的研究较多[26-27],而曲霉菌株常被制作成生物吸附剂。近些年相继出现矿区地下水稀土污染问题[28],但曲霉菌对稀土元素吸附作用等相关研究未见报道。所以,本研究从稀土和重金属复合污染土壤占比较大的曲霉菌(图 2-A)中分离出5株曲霉菌(Aspergillus)(图 4),除了unclassified Fungi外,其占比在B4与B5样点最大(图 2-C),可见分离出了高通量测序中的比较重要的类群。而这些菌株在稀土-重金属元素胁迫下颜色变黑是因为其具有吸附金属离子的能力(图 5)从而导致其颜色变化。这些曲霉菌菌株均在100 mg/L的Zn2+溶液中生长良好,且都可以吸附Zn2+,这与Thippeswamy (2012)[27]等的造纸厂废水中的实验结果类似。此外,本研究首次测定曲霉菌和镰刀霉菌对稀土元素La3+的吸附率(7.09%–19.74%)和吸附能力(2.49–8.41 mg/g),菌株间吸附能力有差异,同一菌株对La3+的吸附能力(>2.49 mg/g)均高于Zn2+(<0.89 mg/g)(图 5)。菌株B6-2对La3+的吸附能力最高(8.41 mg/g)。但是分离菌株对Zn2+的吸附能力均低于已知鲁氏毛霉菌(Mucor rouxii,6.28 mg/g)和酿酒酵母菌(Saccharomyces cervisiae,11.8 mg/g)的相关研究[25],而真菌对La3+吸附能力未见报道。本研究首次分离出具有La和Zn耐性且具有吸附La3+和Zn2+的真菌,故稀土尾矿区将是一个良好的重金属和稀土吸附菌种的资源库。
本研究分离出来的曲霉菌(B6-2、B6-4和B6-3)和镰刀霉菌(B7-1)对La3+和Zn2+有一定的吸附能力,且对La3+的吸附能力均高于Zn2+。但复合菌的吸附能力以及实际污染水体中的作用如何?对其他稀土和重金属的吸附能力、环境因素对吸附能力的影响以及吸附La3+的机制等在今后工作中有必要作更进一步研究。相较其他吸附菌株,以往研究多集中在对重金属离子污染的吸附去除,而本研究所分离出来的菌株不仅可以有效吸附稀土La3+还可以吸附重金属Zn2+,对稀土-重金属复合污染的水体,尤其对稀土La3+污染有较好的应用潜力。
References
| [1] | Guo W, Fu RY, Zhao RX, Zhao WJ, Guo JY, Zhang J. Distribution characteristic and current situation of soil rare earth contamination in the Bayan Obo Mining Area and Baotou tailing reservoir in Inner Mongolia. Environmental Science, 2013, 34(5): 1895-1900. (in Chinese) 郭伟, 付瑞英, 赵仁鑫, 赵文静, 郭江源, 张君. 内蒙古包头白云鄂博矿区及尾矿区周围土壤稀土污染现状和分布特征. 环境科学, 2013, 34(5): 1895-1900. |
| [2] | Jin SL, Huang YZ. A review on ecological toxicity of rare earth elements in soil. Asian Journal of Ecotoxicology, 2014, 9(2): 213-223. (in Chinese) 金姝兰, 黄益宗. 土壤中稀土元素的生态毒性研究进展. 生态毒理学报, 2014, 9(2): 213-223. |
| [3] | Liu JM, Cao WW, Jiang HM, Cui J, Shi CF, Qiao XH, Zhao J, Si WT. Impact of heavy metal pollution on ammonia oxidizers in soils in the vicinity of a tailings dam, Baotou, China. Bulletin of Environmental Contamination and Toxicology, 2018, 101(1): 110-116. |
| [4] | Liu JX, Li C, Jing JH, Jia T, Liu XG, Wang XY, Chai BF. Composition and environmental adaptation of microbial community in Shibahe copper tailing in Zhongtiao Mountain in Shanxi. Environmental Science, 2017, 38(1): 318-326. (in Chinese) 刘晋仙, 李毳, 景炬辉, 贾彤, 刘兴港, 王小云, 柴宝峰. 中条山十八河铜尾矿库微生物群落组成与环境适应性. 环境科学, 2017, 38(1): 318-326. |
| [5] | Sun WM, Xiao EZ, Krumins V, Dong YR, Xiao TF, Ning ZP, Chen HY, Xiao QX. Characterization of the microbial community composition and the distribution of Fe-metabolizing bacteria in a creek contaminated by acid mine drainage. Applied Microbiology and Biotechnology, 2016, 100(19): 8523-8535. DOI:10.1007/s00253-016-7653-y |
| [6] | de Beeck MO, Lievens B, Busschaert P, Rineau F, Smits M, Vangronsveld J, Colpaert JV. Impact of metal pollution on fungal diversity and community structures. Environmental Microbiology, 2015, 17(6): 2035-2047. DOI:10.1111/1462-2920.12547 |
| [7] | Deng LJ, Zeng GM, Fan CZ, Lu LH, Chen XF, Chen M, Wu HP, He XX, He Y. Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Applied Microbiology and Biotechnology, 2015, 99(19): 8259-8269. DOI:10.1007/s00253-015-6662-6 |
| [8] | Jia T, Cao MW, Jin JH, Liu JX, Chai BF. Endophytic fungi and soil microbial community characteristics over different years of phytoremediation in a copper tailings dam of Shanxi, China. Science of The Total Environment, 2017, 574: 881-888. DOI:10.1016/j.scitotenv.2016.09.161 |
| [9] | Kapoor A, Viraraghavan T, Cullimore DR. Removal of heavy metals using the fungus Aspergillus niger. Bioresource Technology, 1999, 70(1): 95-104. DOI:10.1016/S0960-8524(98)00192-8 |
| [10] | Gururajan K, Belur PD. Screening and selection of indigenous metal tolerant fungal isolates for heavy metal removal. Environmental Technology & Innovation, 2018, 9: 91-99. |
| [11] | Bao ZH, Sato Y, Kubota M, Ohta H. Isolation and characterization of thallium-tolerant bacteria from heavy metal-polluted river sediment and non-polluted soils. Microbes and Environments, 2006, 21(4): 251-260. DOI:10.1264/jsme2.21.251 |
| [12] | Liu L, Yang F, Liu ZG, Huang DG, Huang JM, Fang HY, Yang GH. Determination of heavy metals in soils and plants with microwave digestion and ICP-AES. Environmental Chemistry, 2008, 27(4): 511-514. (in Chinese) 刘雷, 杨帆, 刘足根, 黄冬根, 黄精明, 方红亚, 杨国华. 微波消解ICP-AES法测定土壤及植物中的重金属. 环境化学, 2008, 27(4): 511-514. DOI:10.3321/j.issn:0254-6108.2008.04.022 |
| [13] | Jin ZW, Zhong WH, Wu SS, Han C. Effect of vegetation degradation on microbial communities in alpine grassland soils in Northwest Yunnan. Acta Microbiologica Sinica, 2018, 58(12): 2174-2185. (in Chinese) 金志薇, 钟文辉, 吴少松, 韩成. 植被退化对滇西北高寒草地土壤微生物群落的影响. 微生物学报, 2018, 58(12): 2174-2185. |
| [14] | Chen ZJ, Yuan J, Sun F, Zhang F, Chen Y, Ding CY, Shi JW, Li YY, Yao LG. Planktonic fungal community structures and their relationship to water quality in the Danjiangkou Reservoir, China. Scientific Reports, 2018, 8: 10596. DOI:10.1038/s41598-018-28903-y |
| [15] | Long MR, Xie XL, Feng GD, Yao Q, Zhu HH. Isolation and identification of cadmium-tolerant filamentous fungi from lead-zinc tailings. Microbiology China, 2013, 40(12): 2203-2216. (in Chinese) 隆美容, 谢小林, 冯广达, 姚青, 朱红惠. 铅锌尾矿中耐重金属镉的丝状真菌的分离鉴定. 微生物学通报, 2013, 40(12): 2203-2216. |
| [16] | Velmurugan P, Shim J, You Y, Choi S, Kamala-Kannan S, Lee KJ, Kim HJ, Oh BT. Removal of zinc by live, dead, and dried biomass of Fusarium spp. isolated from the abandoned-metal mine in South Korea and its perspective of producing nanocrystals. Journal of Hazardous Materials, 2010, 182(1/3): 317-324. |
| [17] | Si WT, Liu JM, Cai L, Jiang HM, Zheng CL, He XY, Wang JY, Zhang XF. Health risks of metals in contaminated farmland soils and spring wheat irrigated with Yellow River water in Baotou, China. Bulletin of Environmental Contamination and Toxicology, 2015, 94(2): 214-219. |
| [18] | State Environmental Protection Agency, China National Environmental Monitoring Centre. Background values of soil elements in China. Beijing: China Environmental Science Press, 1990: 2-3. (in Chinese) 国家环境保护局, 中国环境监测总站. 中国土壤元素背景值. 北京: 中国环境科学出版社, 1990: 2-3. |
| [19] | Narendrula-Kotha R, Nkongolo KK. Bacterial and fungal community structure and diversity in a mining region under long-term metal exposure revealed by metagenomics sequencing. Ecological Genetics and Genomics, 2017, 2: 13-24. DOI:10.1016/j.egg.2016.11.001 |
| [20] | Zhang ZD, Gu MY, Wang W, Zhu J, Tang QY, Song SQ, Xie YQ, Zhang LJ. Analysis of bacterial community in radiation polluted soils by high-throughput sequencing. Microbiology China, 2016, 43(6): 1218-1226. (in Chinese) 张志东, 顾美英, 王玮, 朱静, 唐琦勇, 宋素琴, 谢玉清, 张丽娟. 基于高通量测序的辐射污染区细菌群落特征分析. 微生物学通报, 2016, 43(6): 1218-1226. |
| [21] | Qiu JZ, Qiu ZX, Guan X. Effects of metal ions on the activity of Chitinase from Lecanicillium lecanii. Acta Laser Biology Sinica, 2009, 18(1): 83-86. (in Chinese) 邱君志, 邱振兴, 关雄. 金属离子对蜡蚧菌几丁质酶活力的影响. 激光生物学报, 2009, 18(1): 83-86. DOI:10.3969/j.issn.1007-7146.2009.01.017 |
| [22] | Valix M, Loon LO. Adaptive tolerance behaviour of fungi in heavy metals. Minerals Engineering, 2003, 16(3): 193-198. DOI:10.1016/S0892-6875(03)00004-9 |
| [23] | Fuentes A, Almonacid L, Ocampo JA, Arriagada C. Synergistic interactions between a saprophytic fungal consortium and Rhizophagus irregularis alleviate oxidative stress in plants grown in heavy metal contaminated soil. Plant and Soil, 2016, 407(1/2): 355-366. |
| [24] | Wang JL, Chen C. Research advances in heavy metal removal by biosorption. Acta Scientiae Circumstantiae, 2010, 30(4): 673-701. (in Chinese) 王建龙, 陈灿. 生物吸附法去除重金属离子的研究进展. 环境科学学报, 2010, 30(4): 673-701. |
| [25] | Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH. Biosorption of heavy metals: a review. Journal of Chemical Science and Technology, 2014, 3(4): 74-102. |
| [26] | Akar T, Tunali S. Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(Ⅱ) and Cu(Ⅱ) ions from an aqueous solution. Bioresource Technology, 2006, 97(15): 1780-1787. DOI:10.1016/j.biortech.2005.09.009 |
| [27] | Thippeswamy B, Shivakumar CK, Krishnappa M. Bioaccumulation potential of Aspergillus niger and Aspergillus flavus for removal of heavy metals from paper mill effluent. Journal of Environmental Biology, 2012, 33(6): 1063-1068. |
| [28] | Tu T, Wang Y, An D, Li J, Yang YM, Tang J, Xi BD. Present situation, hazard and treatment technology of groundwater pollution in rare earth mining area of southern Jiangxi. Journal of Environmental Engineering Technology, 2017, 7(6): 691-699. (in Chinese) 涂婷, 王月, 安达, 李娟, 杨延梅, 唐军, 席北斗. 赣南稀土矿区地下水污染现状、危害及处理技术与展望. 环境工程技术学报, 2017, 7(6): 691-699. DOI:10.3969/j.issn.1674-991X.2017.06.095 |
