Huan Wu1#, Haiwen Wang1#, Li Zhu1, Xuyan Wei1, Bing Wang1, Chunxu Song2, Ziquan Yu1


1. State Key Laboratory of Developmental Biology of Freshwater Fish, College of Life Sciences, Hunan Normal University, Changsha 410081, Hunan Province, China;
2. Groningen Biomolecular Science and Biotechnology Institute, Department of Molecular Genetics, University of Groningen, 9747 AG Groningen, The Netherlands
Received: 12 October 2018; Revised: 13 February 2019; Published online: 5 May 2019
Foundation item: Supported by the National Basic Research Program of China (2013CB127504), by the Natural Science Foundation of Hunan Province (2015JJ2098) and by the Scientific Research Fund of Hunan Provincial Education Department (17A132)
Corresponding author: Ziquan Yu, Tel:+86-731-88872905; Fax:+86-731-88872298; E-mail:zq08yu@hunnu.edu.cn.
#These authors contributed equally to this work
Abstract: [Objective] The dlt operon in Gram-positive bacteria encodes enzymes that are necessary for the modification of D-alanylation of teichoic acids in cell wall. D-alanylation generates net positive charge on cell surface and, as a consequence, repulses the positively charged molecules, such as cationic antimicrobial peptides, thereby confers resistance to host animal. Here, we investigated the impact of dlt operon on phenotypic traits of Bacillus thuringiensis and role in virulence to insect.[Methods] We constructed the loss-of-function mutant of dlt by homologous recombination technique, and performed its morphological observation, surface charge difference analysis, stress resistance analysis and cell experiment.[Results] The results revealed that inactivation of dltA significantly decreased net negative charge of cell wall, drastically impaired the resistance of Bacillus thuringiensis to cationic antimicrobial peptides (polymyxin B and lysozyme) and alkaline. ΔdltABt mutant displayed an obviously altered profile of growth curve, irregular shape and rough surface of cell, decreased biofilm formation and increased swarming motility. Moreover, inactivation of dltA significantly decreased adhesion ability to mid-gut epithelial cell of insect, and greatly attenuated virulence to Bombyx mori.[Conclusion] These findings provide evidence that D-alanylation of TAs mediated by dlt operon is closely correlated to many phenotypic traits of Bt, and has putative roles in the pathogenicity of B. thuringiensis to insect and the protection of B. thuringiensis from insect humoral immunity.
Keywords: Bacillus thuringiensisdlt operoncationic antimicrobial peptideinsectresistancevirulence
dlt操纵子赋予苏云金芽胞杆菌对阳离子抗菌肽的抗性和对昆虫的毒力
邬欢1#, 王海文1#, 朱丽1, 魏旭艳1, 王兵1, 宋春旭2, 余子全1


1. 湖南师范大学生命科学学院, 淡水鱼类发育生物学国家重点实验室, 湖南 长沙 410081;
2. 格罗宁根大学分子遗传学系, 格罗宁根生物分子科学与生物技术研究所, 荷兰 格罗宁根 9747 AG
收稿日期:2018-10-12;修改日期:2019-02-13;网络出版日期:2019-05-05
基金项目:中国国家基础研究计划(2013CB127504);湖南省自然科学基金(2015JJ2098);湖南省教育厅科研基金(17A132)
通信作者:余子全,Tel:+86-731-88872905;Fax:+86-731-88872298;E-mail:zq08yu@hunnu.edu.cn.
#并列第一作者
摘要:[目的] 革兰氏阳性菌中的dlt操纵子编码细胞壁中磷壁酸发生D-丙氨酰化修饰所必需的酶。D-丙氨酰化使细胞表面产生正电荷,并因此排斥带正电的分子,例如阳离子抗菌肽,从而赋予对宿主的抗性。本文中,我们研究了dlt操纵子对苏云金芽胞杆菌表型性状的影响及在对昆虫毒力中发挥的作用。[方法] 通过同源重组构建了ΔdltABt基因缺失突变株,并对其进行形态学观察、表面电荷差异分析、抗逆性分析和毒力测定。[结果] 结果表明,dltA的失活显著降低了细胞表面的净负电荷,对阳离子抗菌肽(多粘菌素B和溶菌酶)的抗性和碱耐受性显著下降。同时,ΔdltABt的生长曲线发生明显改变,细胞表面粗糙且形状不规则,生物膜形成减少和群游运动能力增强。此外,dltA的失活降低了对昆虫中肠上皮细胞的粘附能力,并减弱了对家蚕的毒力。[结论] 研究结果表明,dlt操纵子介导的磷壁酸发生D-丙氨酰化修饰与苏云金芽胞杆菌的许多表型性状密切相关,并且在苏云金芽胞杆菌对昆虫的致病性及抵抗昆虫体液免疫保护中具有重要作用。
关键词:苏云金芽胞杆菌dlt操纵子阳离子抗菌肽昆虫抗性毒力
Polyanionic teichoic acid (TA), a particular and important component of cell wall in Gram-positive bacteria, acts in controlling cell shape and division, autolysis, cation homeostasis, and susceptibility to innate host defenses[1-2]. TA is composed of negatively charged glycerophosphate residues, and covalently anchors to either N-acetylmuramic acid residues of the peptidoglycan (wall-associated TAs, WTAs), or the cytoplasmic membrane via glycolipids (lipoteichoic acids, LTAs)[1]. The anionic property of TAs confers the overall negative charge of bacterial cell wall, attracting positively charged compounds, such as cationic antimicrobial peptides (CAMPs) of innate humoral immunity in higher organisms[1].
Many Gram-positive pathogenic bacteria species display resistance to CAMPs because of an increase of the positive surface charge of bacteria cell envelope[2-3]. The increase of the positive surface charge is mainly attributed to the modifications to the TAs on bacterial surface through incorporation of positively charged residues, such as D-alanine mediated by the enzymes encoded by dlt operon, and/or L-lysine mediated by the enzyme encoded by mprF[2]. The covalent modification of cationic molecules allows bacteria to adjust their net negative charge on surface, thereby decreases electrostatic interaction between the negatively charged TAs and the positively charged host immune factors, protects bacteria from host innate immune response[3]. Therefore, incorporation of D-alanine esters into TAs to partially neutralize the negative charge of cell wall is one of the most common bacterial resistance mechanisms. Nevertheless, an exceptional study has also suggested that incorporation of D-alanine into LTAs induces CAMPs resistance in group B Streptococcus sp. by modifying the rigidity and permeability of the cell wall rather than by affecting the electrostatic-driven binding of CAMPs to bacteria[4]. In addition, dlt operon has also been found in several gram-negative pathogenic bacteria, such as Erwinia carotovora, Bordetella sp., Photorhabdus sp.[5], Dickeya dadantill and Pectobacterium sp.[6], and confers resistance to CAMPs, probably by modifying the lipopolysaccharides in the cell wall[6].
D-alanylation of TAs is accomplished by the enzymes encoded by dlt operon consisting of four genes, dltABCD, which is highly conserved in most gram-positive bacteria[7]. dltA gene encodes a D-alanyl carrier protein ligase, which activates D-alanine for ligation to the D-alanyl carrier protein encoded by dltC. dltB and dltD encode two putative membrane proteins. DltB spans plasma membrane repeatedly, and DltD appears to be anchored to the membrane via an N-terminal hydrophobic sequence[8]. However, the exact functions of dltB and dltD are still obscure. Two models have been proposed to account for the functions of DltB and DltD: (ⅰ) DltB transfers D-alanine from DltC to undecaprenol-phosphate. The resulting lipid-linked intermediate flips across the membrane, subsequently DltD transfers D-alanine to LTAs on the trans side of the membrane[9]. In this action mode, the DltD is proposed to reside outside the cytoplasm, on the trans side of the membrane, which has been confirmed by analysis of DltD membrane topology[10]. (ⅱ) DltD facilitates transfer of D-alanine between DltA and DltC in the cytoplasm[11]; DltB translocates the alanylated DltC across the membrane and then transfer D-alanine directly onto LTAs[1]. Of note, the Dlt system in the two proposed models seems to directly D-alanylate LTAs, but not WTAs. Both in vitro and in vivo pulse-chase experiments have confirmed that the D-alanyl esters are transferred from LTAs to WTAs by transacylation[12]. If so, how the D-alanyl esters are transferred remains obscure. Intriguingly, a fifth gene, dltX, is also present in the dlt operon in some gram-positive bacteria[5]. A recently published study has confirmed that DltX is essential for D-alanylation of LTAs in Bacillus thuringiensis (Bt), despite the fact that the detailed function of DltX remains unclear[13].
Inactivation of dlt operon in many Gram-positive pathogenic bacteria species, such as Listeria monocytogenes[14], Streptococcus sp.[4-7], Enterococcus faecalis[15], B. cereus[5], Clostridium difficile[16], resulted in increased susceptibility to various CAMPs. The amphipathic CAMPs, one of the critical components of innate immunity system, exert antimicrobial activity to bacteria with a negatively charged surface by disrupting transmembrane potential and lipid symmetry, eventually resulting in cell lysis[17]. Moreover, lack of D-alanylation also affords increased susceptibility to phagocytic cell[18] and neutrophil killing[19], decreased adherence to macrophage[14] and invasion to epithelial cell[7], loss of ability to colonize cotton rat nares[20]. All these studies have suggested that D-alanylation plays a critical role in the interaction of pathogens with host immune system.
Bt is a ubiquitous gram-positive bacterium, and widely used as biological pesticide[21]. The insecticidal activity of Bt is mainly dependent on the parasporal crystal protein produced at the stationary phase[22]. Bt is able to multiply in the insect hemocoel and lead to fatal septicemia, which also makes great contribution to its insecticidal activity besides the parasporal crystal protein[23]. Upon entering the hemolymph, the entomopathogenic bacteria would confront an array of immune system mediators of both cellular and humoral reactions. The cellular reaction results in bacterial phagocytosis or encapsulation by circulating hemocytes, whereas the humoral response generates CAMPs[24]. These small and inducible CAMPs are produced by the fat body in hemolymph, and participate in insect antimicrobial defense in a systemic response[25]. As an insect pathogen, an effective manner of escaping CAMPs is critical for the survival of Bt in insect. Analyses of the published Bt genome sequences showed that the dlt operon widely distributes, and is highly conserved with the dlt operon in other gram-positive bacteria, suggesting a vital role in the interaction of Bt against insect. However, the impacts of Dlt system in Bt on virulence to host insect have never been investigated, apart from the study that dltX is necessary for D-alanylation in Bt 407 strain[13]. Therefore, this study aims to evaluate the influences of dlt operon on the phenotypic traits of Bt, resistances to insect innate immunity and virulence to insect.
For this purpose, we insertionally inactivated the dltA gene in Bt BMB171 strain to investigate the impact of D-alanylation of TAs on phenotypic traits. In particularly, we examined the role of D-alanylation of LTAs of Bt in resistance to CAMPs, and virulence to insect. Our results provided direct evidences that the dlt operon protects Bt from the insect innate immunity, and is essential for insecticidal virulence.
1 Materials and Methods 1.1 Bacterial strains, plasmids, and growth conditions The bacterial strains and plasmids used in this study are listed in Table 1. All Bt strains and E. coli strains were routinely grown in LB broth or on LB agar at 28 ℃ for Bt and 37 ℃ for E. coli. When required, the final concentrations of the antibiotics added were as follows: 100 μg/mL ampicillin (Amp), 50 μg/mL kanamycin (Kan), 100 μg/mL spectinomycin (Spc), 50 μg/mL erythromycin (Erm). These antibiotics were purchased from Sigma-Aldrich (MO, USA)[13].
Table 1. Bacterial strains and plasmids used in this study
| Strains or plasmids | Genotype or relevant characteristic | Reference or source |
| Strains | ||
| ??B. thuringiensis | ||
| ??BMB171 | Plasmid-cured, acrystalliferous mutant strain from wild-type crystalliferous strain YBT-1463 | [26] |
| ??ΔdltABt | dltA mutant of BMB171 | This study |
| ??ΔdltABt-comp | ΔdltABt complemented with the dltA gene | This study |
| ??ΔdltABt/pHT315-PlacZ | ΔdltABt harboring pHT315 cloned with a insertion of lacZ promoter | This study |
| ??E. coli | ||
| ??DH5α | F–, Φ80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rk– mk+), phoA, supE44, λ–, thi-1, gyrA96, relA1 | Laboratory collection |
| Plasmids | ||
| ??pRec-mob-Ts | Conjugative and thermosensitive cloning vector, Spcr | Presented by Professor Ming Sun |
| ??pUC19 | cloning vector in E. coli (GenBank accession No. L09137), Ampr | [27] |
| ??pHT315 | E. coli-Bt shuttle vector, Ampr, Ermr | [28] |
| ??pRec-mob-Ts-up-down | pRec-mob-Ts harbors the USH and DSH, for allelic-exchange recombination of dltA, Spcr | This study |
| ??pHT315-PlacZ-dltA | pHT315 harboring dltA under the control of lacZ promoter, for complementation strain of dltA in ΔdltABt, Ampr, Ermr | This study |
| ??pHT315-PlacZ | pHT315 harboring the promoter of lacZ, Ampr, Ermr | This study |
表选项
1.2 Construction of dltA mutant in Bt BMB171 strain The thermosensitive vector pRec-mob-Ts was used to inactive the dltA gene in Bt BMB171 strain[26] by allelic replacement. A 577-bp BamH Ⅰ- Sac Ⅰ fragment as upstream homologous arm (USH) was amplified by PCR, using the primer pair: dltA-up-F (ACGCGGATCCAAGAAACCTTGGAAAATACG) and dltA-up-R (TACCGAGCTCCCGCATATGGATCATTTTAT), with the restriction sites (underlined). Likewise, a 571-bp Kpn Ⅰ-Sal Ⅰ fragment as downstream homologous arm (DSH) was generated by PCR, using the primer pair: dltA-down-F (ACGGGGTACCAACGTTCCCACCCTAGAATTC) and dltA-down-R (ACGCGTCGACCGGATACTTATGGAAGTGCC), with the restriction sites (underlined). The USH and DSH fragments were inserted into the upstream and downstream of spectinomycin resistance cassette in the pRec-mob-Ts vector, respectively, therefore resulting in the recombinant plasmid pRec-mob-Ts-up-down for allelic exchange recombination. In the recombinant plasmid, the USH and DSH were flanked by a spectinomycin resistance cassette. The plasmid was verified by restriction mapping, and subsequently transferred into BMB171 by electroporation[29]. Subsequent steps in mutagenesis for dltA were exactly performed as described previously[29]. The chromosomal allelic replacement was checked by PCR, using the appropriate primer pair of dltA-up-F and dltA-down-R. The resultant mutant of dltA was termed ΔdltABt.
1.3 Construction of complementation strain for ΔdltABt For complementation analysis, the promoter for lacZ was amplified from pUC19 vector[28] using the primer pair: PlacZ-F (AAAACTGCAGGCCCAATACGCAAACCGCCTC) and PlacZ-R (ACGCGTCGACTGGCGTAATCATGGTCATAGC), with the restriction sites for Pst Ⅰ and Sal Ⅰ (underlined), respectively. The amplified PlacZ fragment was subcloned into E. coli-Bt shuttle vector pHT315[29], resulting in recombinant plasmid pHT315-PlacZ. The entire dltA ORF (open reading frame) from BMB171 was amplified using the forward primer E-dltA-F (ACGCGTCGACTTCTAGGGTGGGAACGTTATG) and reverse primer E-dltA-R (ACGCGGATCCCATATGCGGTCATGCTGTAAC), with the restriction sites (underlined) for Sal Ⅰ and BamH Ⅰ, respectively. The amplified ORF of dltA was sequenced, and inserted into the downstream of PlacZ in above constructed plasmid pHT315-PlacZ, yielding recombinant plasmid pHT315-PlacZ-dltA. In this plasmid, the dltA gene is under the control of lacZ promoter, which is constitutively active in LB medium[30]. The resultant plasmid pHT315-PlacZ-dltA was verified by PCR amplification using the two primer pairs mentioned above, and subsequently electroported into ΔdltABt strain, resulting complementation strain ΔdltABt-comp for complementation analysis.
1.4 Growth curve Fresh overnight inocula of Bt strains were adjusted to OD600 (optical density at 600 nm) value of 1.0 with fresh LB broth. A volume of 0.5 mL of each inoculum was inoculated to 50 mL of fresh LB broth supplemented with appropriate antibiotic. Strains were grown at 28 ℃ with agitation of 200 r/min. Cultures were sampled at an interval of 2 h for optical measurement on a DU 800 spectrophotometer (Beckman Coulter, Brea, CA, USA). The OD600 value and growth time of each strain were plotted into non-linear regression curve. Each point represents the mean and standard deviation from five replicates. The assays were employed in triplicate.
1.5 Scanning electron microscopy (SEM) Exponential phase cultures of various Bt strains were harvested by centrifugation, and the pellet was washed with 0.5 mol/L NaCl for three times. Cells were subsequently fixed with 4% gluteraldehyde at room temperature for 2 h. They were then washed three times for 5 min with a solution of 0.1 mol/L NaCl. All samples were progressively dehydrated with increasing concentration ethanol (30%-50%- 70%-80%-90%-95%-100%) at room temperature for 10 min in each bath. The samples were then subjected to freeze drying and imaged with a SU8010 ultra-high resolution scanning electron microscope (Hitachi, Japan).
1.6 Alcian blue binding assay Alcian blue binding assay was carried out as described previously[31]. Bt cells grown in LB medium were harvested at mid-exponential phase by centrifugation (2000×g for 3 min), and washed once with 20 mmol/L morhpolinepropanesulfonic acid (MOPS) buffer (pH 7.0). The pellets were resuspended in MOPS buffer to a final OD600 of 0.5. Subsequently, cationic dye alcian blue 8GX (Sigma-Aldrich, MO, USA) was added to a final concentration of 65 μg/mL. Samples were rotated with 3 r/min at room temperature for 10 min, and then the mixtures were centrifuged to pellet the complex of bacterial cells and bound cationic dye. The supernatant fluid was measured at 650 nm using a DU 800 spectrophotometer to quantify unbound alcian blue. For control, tubes containing equivalent amount of alcian blue in MOPS buffer without Bt cell were treated in parallel. The percentage of alcian blue binding to Bt cells were calculated as (A650 of supernatant without Bt cells–A650 of supernatant with Bt cells)/A650 of supernatant without Bt cells×100%. Five replicates were measured for each strain, and the experiment was performed in triplicate.
1.7 Susceptibility to CAMPs In vitro susceptibilities to CAMPs were evaluated by determining the MICs (minimum inhibitory concentrations) of Bt strains to polymyxin B sulfate and lysozyme (Sigma-Aldrich)[5], respectively. Each CAMP was diluted to concentrations ranging from 20 μg/mL to 1280 μg/mL by two-fold dilution, and the inhibition assays were performed in 96-well plates inoculated with about 5×104 CFU (colony-forming unit) of mid- exponential phase Bt strain per well (a final volume of 200 μL). After incubation at 28 ℃ for 24 h, growth was scored by measuring the OD600 of each well in microtiter plates with a multi-mode microplate reader SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA). MIC was recorded as the lowest concentration that completely inhibited growth of Bt. Three replicates were set for each strain, and all the assays were performed in triplicate.
1.8 Autolysis assay The autolysis assay was carried out as previously described[5], with some modifications. Briefly, Bt strains were grown in LB medium at 28 ℃ with shaking of 200 r/min. Cells at exponential phase were harvested by centrifugation, and washed twice with ice-cold sodium phosphate buffer (10 mmol/L, pH 7.0), then resuspended in the same buffer supplemented in 0.05% Triton X-100 with OD600 value of about 0.8. Five replicates were set for each Bt strain. Bacterial cells were incubated at 28 ℃ without shaking, and the autolysis was monitored by measuring the decrease of OD600 value at an internal of 30 min on a DU 800 spectrophotometer. The changes of OD600 over 4–6 h were recorded. All the assays were performed in triplicate.
1.9 pH sensitivity assay The pH sensitivities of WT (wild-type) and mutant strains were determined by comparing their survival ability in sodium phosphate buffer (20 mmol/L Na2HPO4/NaH2PO4, 1 mmol/L MgCl2, 10 mmol/L L-arginine)[7] with pH ranging from 7.0 to 10.0. Bt at exponential phase was suspended with OD600 value of 0.8 in phosphate buffer with specific pH value (1 mL). Aliquots were removed at specified time points, and plated on LB agar to enumerate surviving CFU. All the assays were performed in triplicate.
1.10 Biofilm microtiter plate assay Biofilm formation was evaluated in 96-well polystyrene microtiter plates (Corning, NY, USA) as described previously[32], with some modifications. 200 μL of fresh overnight bacterial culture with OD600 value of about 1.2 was placed in each well. After 48 h of stationary incubation at 28 ℃, bacterial cells were removed and the wells were gently washed three times with double-distilled H2O without disturbing the biofilm on the bottom of the wells. Bound cells per well were stained with 200 μL of a 0.1% (W/V) aqueous solution of crystal violet. The microtiter plates were incubated at room temperature for 30 min. After three gentle washings with double-distilled H2O, the microtiter plates were dried upside down for 1 h. Crystal violet per well was solubilized with 200 μL of absolute ethanol. The absorbance at 595 nm was quantified by microplate reader SpectraMax M5. Five replicate wells were used for each strain. All the assays were performed in triplicate.
1.11 Swarming assay Bacterial swarming motility assays were performed in 9-cm petri dishes prepared with 25-mL of LB medium solidified with 1.0% (W/V) agar[33]. Swarming plates were inoculated with 2 μL of bacterial cultures with an OD600 of approximately 0.5, and incubated at 28 ℃. Bacterial swarming diameters were measured at 24 h, 48 h, and 72 h after inoculation. Swarming assays were repeated three times with three replicates for each strain.
1.12 Cell binding assay An in vitro fluid-phase assay was used to investigate the adherence of Bt cells to a continuous mid-gut epithelia cell line CF-203.3 of spruce budworm Choristoneura fumiferana[34]. CF-203.3 cells were grown at 28 ℃ in 50-mL flasks with SF900 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5% Fetal Bovine Serum (FBS) (Invitrogen)[34]. Meanwhile, the zebrafish (Brachydanio rerio) embryonic fibroblast ZF4[35] was used as control to test the binding specificity of Bt. ZF4 cells were cultured at 28 ℃ in cell culture plates with DMEF/F-12 medium (Invitrogen) containing penicillin (60 μg/mL), streptomycin (100 μg/mL) and 10% FBS[35]. The binding assay was performed as previously described[31]. Cells were washed thrice with phosphate-buffer saline (PBS) (pH 7.0), and then resuspended in the same PBS buffer. Early exponential-phase Bt cells grown in LB medium were harvested, and washed as above. Cells and bacteria were mixed with a ratio of 1:100, and rotated at 400×g for 5 min to place bacteria on the monolayers. Mixed samples were incubated at 28 ℃ for 1 h, and centrifuged at 1000×g for 5 min. The pellets were washed thrice with LB to remove non-cell-associated bacteria, and resuspended in 1 mL fresh LB broth. The pellet was sonicated for 5 seconds at amplitude of 10 (Sonics, Newtown, CT, USA) to lyse cells but not bacteria. The resuspensions were serially diluted and seeded on selective LB agar plates. All plates were incubated at 28 ℃ for 24 h, and colonies were enumerated. Five replicates were set for each strain. The assays were performed in triplicate.
1.13 In vivo pathogenicity assay Silkworm larvae (Bombyx mori) were examined as a host model of Bt infection to assess the biofunction of dlt operon in the virulence of Bt to insect. Silkworms were raised from fertilized eggs at 27 ℃, which were kindly presented by Doctor Hongying Zhou, Hubei Academy of Agricultural Science (Wuhan, China). Hatched larvae were fed an artificial diet Silkmate 2S (Nosan Corp., Kanagawa, Japan) until they developed to the fourth molted larva. On the first day of fifth-instar larvae, silkworms were fed for one day an antibiotic-free artificial diet, Silkmate (Katakura Industries Co., Ltd., Tokyo, Japan), and then were used pathogenicity assays. The surfaces of fifth-instar larvae were sterilized with 70% (V/V) ethanol. Appropriate dilutions of bacteria were then directly injected into the hemolymph of fifth-instar larvae through the dorsal surface[36], using a 1-mL microsyringe equipped with a 27 G needle. A silkworm larva was injected with about 1×104 CFU. Bacteria concentration was determined by counting the number of CFU formed after plating of dilutions on LB agar. Groups of 20 larvae were injected with vegetative bacterial suspension, or PBS buffer as control. At least three groups were performed for each strain. The mortality was recorded for up to 5 d of incubation at 30 ℃. We recorded the time of insect death during the whole pathogenicity assay to establish the LT50 (the time by which 50% of the insects die). All the pathogenicity assays were performed in triplicate.
1.14 Statistical analysis The data concerning alcian blue binding assay, cell binding assay, pH tolerance assay, biofilm formation assay, and in vivo pathogenicity assays were analyzed by the unpaired two-tailed Student's t-test. The statistical significance of the swarming motility data was determined using the one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Differences were considered significant at P < 0.05.
2 Results 2.1 Analysis of dlt operon in Bt BMB171 and construction of dltA mutant In silico analysis showed that the genome of Bt BMB171 (GenBank accession No. CP001903) harbors a dlt operon comprising dltA, dltB, dltC, and dltD. All the four genes are highly conserved with those in other Gram-positive bacteria. Biofunctional annotation suggested that dltA encodes a D-alanine-poly (phosphoribitol) ligase, dltB encodes a D-alanyl-lipoteichoic acid biosynthesis protein, dltC encodes a D-alanine-poly (phosphoribitol) ligase subunit 2, and dltD encodes a D-alanyl- lipoteichoic acid biosynthesis protein. Unlike other Bt strains such as 97-27 (AE017355) and 407 (NC_018877), a fifth gene dltX is lacking in the dlt operon of BMB171. In order to investigate the role of dlt operon in Bt, inactivation of dltA in BMB171 was achieved by precise, allelic replacement, resulting in mutant ΔdltABt. For complementation test, the entire ORF (open reading frame) of dltA driven by lacZ promoter (PlacZ) was cloned into E. coli-Bt shuttle vector pHT315, yielding recombinant plasmid pHT315-PlacZ-dltA. The recombinant plasmid was transformed to ΔdltABt, yielding complementation strain ΔdltABt-comp. As control, the empty vector pHT315 harboring PlacZ (pHT315-PlacZ) was also transformed to ΔdltABt to exclude the effect of vector on the complementation strain.
2.2 Inactivation of dltA alters the surface charge of Bt cell To confirm the effect of D-alanylation of TAs on surface charge, the capacities of WT and ΔdltABt binding cationic dye alcian blue were compared. The result showed that ΔdltABt mutant bound (65.2±3.4)% of the alcian blue in comparison to a (44.4±1.7)% binding by the WT strain (Figure 1), suggesting that inactivation of dltA causes decrease of D-alanylation of TAs (P < 0.01). The complementation strain ΔdltABt-comp exhibited similar binding capacity to the WT strain, and the binding capacity of ΔdltABt/pHT315-PlacZ is similar to that of ΔdltABt mutant (Figure 1). This result was reminiscent of a significant increase of surface net negative charge in ΔdltABt mutant.
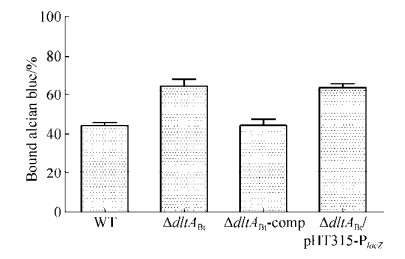 |
| Figure 1 Effects of dlt operon on the binding capacity of the WT, ΔdltABt, ΔdltABt-comp, and ΔdltABt/pHT315-PlacZ strains to cationic dye alcian blue. Samples were run in triplicate. Data are shown as x±s from three biological replicates. |
| 图选项 |
2.3 Effects of dlt operon on growth kinetics, lysis, and cell morphology We next investigated the effects of dlt operon on Bt growth, autolysis, and cell morphology. In general, the ΔdltABt and ΔdltABt/pHT315-PlacZ exhibited different growth characteristics in comparison to WT strain. The ΔdltABt and ΔdltABt/ pHT315-PlacZ showed greatly lower growth rate during late exponential phase, and an approximately two-fold-lower of OD600 at the entry of the stationary phase (Figure 2-A). During the whole stationary phase, the values of OD600 of ΔdltABt and ΔdltABt/pHT315-PlacZ showed a slight decline (Figure 2-A). While, the OD600 of ΔdltABt-comp in the stationary phase was just about 57% compared to WT (Figure 2-A). To confirm the lytic process in ΔdltABt mutant, the autolysis rates between ΔdltABt mutant and WT strain in Triton X-100 were compared. As expected, the ΔdltABt exhibited a sharp autolysis rate, and complementation strain ΔdltABt- comp displayed an approximately similar autolysis rate to WT (Figure 2-B). The higher rate of autolysis in ΔdltABt might explain the steady decline of OD600 of the ΔdltABt mutant at the stationary phase.
 |
| Figure 2 Impacts of the inactivation of dltA on the growth and autolysis of Bt. A: Growth curves of the WT, ΔdltABt, ΔdltABt-comp, and ΔdltABt/pHT315-PlacZ strain, in LB medium at 28 ℃ with shaking of 200 r/min. OD600 was scored by a spectrophotometer. The experiment was carried out in triplicate. Data points are shown as x±s from three biological replicates. B: Autolysis rates of Bt strains in the presence of Triton X-100. Autolysis rates were determined by monitoring the decreases of OD600 over a period of 4 h in PBS at room temperature. The results are shown as x±s from three biological replicates. |
| 图选项 |
Moreover, the phenotypic differences between WT and ΔdltABt were examined by SEM. The SEM observation showed that deletion of dltA significantly affected cell surface morphology. WT cell was plump with a regular and smooth surface (Figure 3-A). In sharp contrast, ΔdltABt and ΔdltABt/pHT315-PlacZ were wizened with an irregular and wrinkled surface (Figure 3-B and D). The complementation strain cells partially restored the cell phenotype of WT strain (Figure 3-C). These data agree well with a recent published study in which a dltX mutant exhibited the same phenotype as the ΔdltABt[13]. Therefore, this result further confirms that dlt operon is closely related to the phenotype of Bt cell.
 |
| Figure 3 Effects of dltA inactivation on Bt BMB171 cell phenotype. Scanning electron micrographs shown exponential phase cells of WT (A), ΔdltABt (B), ΔdltABt-comp (C) and ΔdltABt/pHT315-PlacZ (D). Bar represents 0.2 μm. |
| 图选项 |
2.4 dlt operon is required for resistance to CAMPs In order to investigate the role of dlt operon in the resistance to CAMPs, we compared the resistance of WT and ΔdltABt to CAMPs by determining MICs. When challenged with polymyxin B and lysozyme, the MICs obtained were lower for ΔdltABt than for WT by factors of 32 and 4, respectively (Table 2). ΔdltABt/ pHT315-PlacZ exhibited the same sensitivity with the ΔdltABt to polymyxin B and lysozyme. Full resistance was obtained in the complementation strain ΔdltABt- comp (Table 2), demonstrating that dltA deletion is responsible for the resistance defect. Therefore, the dlt operon of Bt is likely to be an important component of the intrinsic resistance of Bt to CAMPs.
Table 2. MICsa of CAMPs for WT strain BMB171, ΔdltABt, ΔdltABt-comp and ΔdltABt/ pHT315-PlacZ
| Strains | MIC of CAMP/(μg/mL) | |
| Polymyxin B | Lysozyme | |
| WT | 640 | > 1280 |
| ΔdltABt | 20 | 320 |
| ΔdltABt-comp | 640 | > 1280 |
| ΔdltABt/pHT315-PlacZ | 20 | 320 |
| aAs determined by the LB dilution method (three replicates). MICs were scored after 24 h of incubation at 28 ℃. | ||
表选项
2.5 Inactivation of dltA impairs the tolerance of Bt to alkaline In consideration of alkaline environment in host insect intestine, we examined the tolerance of WT and ΔdltABt to increasing pH by assessing their survival ability in alkaline buffer with a pH value ranging from 7.0 to 10.0. Our result showed that WT and ΔdltABt exhibited a slight decline of survival ratio in sodium phosphate buffer at pH 7.0 and 8.0, and no obvious difference between them was observed (Figure 4-A and B). Comparatively, the ΔdltABt and ΔdltABt/pHT315-PlacZ exhibited a significantly accelerated rate of death in alkaline buffer (pH 9.0, 10.0) (Figure 4-C and D), suggesting the mutant is more sensitive to alkaline than WT. In addition, ΔdltABt and ΔdltABt/pHT315-PlacZ obviously exhibited alkalinity-dependent rate of death (Figure 4-B–D). The complementation strain ΔdltABt-comp exhibited similar tolerance to alkaline with WT (Figure 4-B–D). These data suggest that D-alanylation of TAs could provide Bt relative protection in alkaline environment of insect intestine.
 |
| Figure 4 Effect of D-alanylation of LTAs on the tolerance of Bt to alkaline. Exponential phase bacteria were incubated up to 4 h in sodium phosphate buffer containing L-arginine buffered to pH 7.0 (A), pH 8.0 (B), pH 9.0 (C), and pH 10.0 (D). The tolerance to alkaline was determined by monitoring the decreases of OD600. Data are shown as x±s from three biological replicates. |
| 图选项 |
2.6 D-alanylation of TAs is involved in biofilm formation To evaluate the impact of dlt operon on biofilm formation, WT and mutant strains were tested for their ability to adhere to abiotic polystyrene surface. Biofilm formation was quantified by crystal violet staining. On the basis of OD595 of solubilized dye, ΔdltABt and ΔdltABt/pHT315-PlacZ produced significantly less biofilm than WT (P < 0.05) (Figure 5). The complementation strain ΔdltABt-comp completely restored the ability of biofilm formation (Figure 5). This result suggests that D-alanylation of LTAs in Bt is closely correlated with biofilm formation.
 |
| Figure 5 Biofilm formation of WT, ΔdltABt, ΔdltABt-comp, and ΔdltABt/pHT315-PlacZ. Biofilm quantification was determined by the crystal violet staining method using measurement of the absorbance at 595 nm. Data are shown as x±s from five replicates. |
| 图选项 |
2.7 D-alanylation of LTAs has a negative impact on swarming motility To determine whether Bt swarming motility is affected by D-alanylation of LTAs, the swarming motilities of WT and ΔdltABt on 1% agar plate were tested. In general, the swarming diameters of ΔdltABt and ΔdltABt/pHT315-PlacZ were significantly larger than that of WT (Figure 6-A). The average swarming diameters of WT and the mutant strains were shown in Figure 6-B. At 24 h of incubation, the swarming diameter of WT showed no difference with those of the mutant strains (Figure 6-B). At 48 h and 72 h, the swarming diameters of ΔdltABt and ΔdltABt/pHT315- PlacZ were significantly larger than that of WT (Figure 6-B). Swarming motility of the complementation strain ΔdltABt-comp is similar to the WT (Figure 6-B). All these results indicated that D-alanylation of LTAs in Bt exerts negative influence on the swarming motility.
 |
| Figure 6 Swarming motility assay in LB medium solidified with 1.0% agar. Swarming motility plates were inoculated with WT, ΔdltABt, ΔdltABt-comp, and ΔdltABt/pHT315-PlacZ. A: Bacterial swarming motility at 24, 48, and 72 h of incubation. One representative phenotype out of three independent experiments is shown; B: Mean values for swarming motility diameters at 24, 48 and 72 h. Error bars indicate standard deviation values of 9 measurements from three independent experiments. Means with different letters (a, b, c) indicate significant differences among the data for each experimental setup (Turkey's test, P < 0.05). |
| 图选项 |
2.8 dlt operon is crucial for special binding to mid-gut epithelial cell of insect To assess the impact of D-alanyl modification of TAs to binding ability, we compared the capacities of WT and the mutants binding to insect mid-gut epithelial cell CF203.3. Meanwhile, non-target host cell ZF4 was used as control to check binding specificity of Bt. In general, both WT and mutant strains displayed greatly stronger binding capacities to CF203.3 than to ZF4 (Figure 7-A and B), suggesting specificity of Bt binding to target host cell. For CF203.3 cells, a remarkable decrease in the total number of cell-associated ΔdltABt bacteria was observed compared to WT (52% reduction, P < 0.01) (Figure 7-A). The ΔdltABt/pHT315-PlacZ mutant exhibited similar adherence ability to WT strain; and the complementation strain ΔdltABt- comp completely restored the binding ability to CF203.3 cells (Figure 7-A). This result reveals that D-alanylation of TAs plays a role in facilitating Bt attach to mid-gut epithelial cell of insect.
 |
| Figure 7 Impact of dlt operon on the adhesion ability of Bt to insect mid-gut epithelial cell line CF203.3 (A) and zebrafish embryonic fibroblast ZF4 (B). The experiments were performed in triplicate. Data are shown as x±s from three biological replicates. |
| 图选项 |
2.9 Inactivation of dltA greatly attenuates virulence to insect All the above findings suggest that dlt operon plays an essential role in the interaction of Bt and insect, which lead us to investigate the effect of dlt operon on insecticidal virulence. Equal amount of cells of WT and mutants were directly injected into the hemocoels of silkworm larvae, respectively. About 40% mortality was observed with ΔdltABt and ΔdltABt/pHT315-PlacZ after 24 h of injection, while the mortality of WT is up to 80% (Figure 8). The complementation strain ΔdltABt-comp partially restored virulence and its mortality reached up to about 59% after 24 h of injection. Interestingly, all the mortality virtually occurs within the first 24 hours after injection, agreeing well with a previous study in which a Δdlt mutant of B. cereus exhibited toxicity to Spodoptera littoralis and Galleria mellonella within 20 h post-injection[5]. The values of LT50 with a dose of 2×104 CFU per larva were 16 h for the WT strain, 20 h for the complementation strain ΔdltABt-comp, and more than 120 h for the ΔdltABt and ΔdltABt/pHT315- PlacZ mutant. This result indicates that D-alanylation of LTAs is crucial to the virulence of Bt to host insect.
 |
| Figure 8 In vivo virulence of WT, ΔdltABt, ΔdltABt- comp, and ΔdltABt/pHT315-PlacZ to silkworm larva. Bacteria at the end of the exponential phase were collected, and about 1×104 CFU of each strain were injected per fifth-instar larva. 20 larvae were injected for each strain. All experiments were performed in triplicate, and data are shown as x±s from one representative experiment of three independent experiments with similar results. |
| 图选项 |
3 Discussion The widespread distribution of dlt operon among gram-positive pathogenic bacteria suggests that D-alanylation of TAs is biologically important in various microbial habitats, including affording resistance to CAMPs of host. Here, we investigated the role of dlt operon in Bt, which is very critical for understanding and overcoming the potential resistance of Bt when long-term and large-scale application as insecticide. We firstly inactivated dltA, an essential gene in dlt operon, by allelic replacement. By contrast, the resulting mutant ΔdltABt exhibited a series of changes, including surface net charge, bacterial cell morphology, growth and autolysis rate, biofilm production, swarming motility, susceptibility to CAMPs, adhesion ability to insect mid-gut epithelial cell, and attenuated virulence. These observations further illustrate the potential significance of dlt operon in adaption and survival of Bt in host. It should be noted that several phenotypic traits of the complementation strain ΔdltABt-comp did not fully restored to the level of WT, including growth rate at exponential phase (Figure 2-A), swarming motility (Figure 6-B), and virulence (Figure 8). In order to account for this discrepancy, we checked the expression of dltA by quantitative real time PCR in the complementation strain. Our result showed that the transcription of dltA in the complementation strain is about 35% of that in WT (data not shown). The lower transcription of dltA is likely attributed to the low activity of promoter PlacZ in Bt, resulting in the phenotypes were not fully restored.
ΔdltABt mutant displayed a slower growth rate at late exponential phase and a rapider lysis process at stationary phase, which were also confirmed in other Gram-positive bacteria, such as Lactobacillus plantarum[37], and B. cereus[5]. However, an exceptional example showed that depletion of D-alanine does not affect global autolysis in C. butyricum[38]. Moreover, disruption of dltA strongly alter the morphology of Bt cell since the ΔdltABt displayed irregular shape and rough surface which were greatly different from WT (Figure 3). Such drastic changes in cell morphology have also been observed in a ΔdltX mutant derived from Bt 407 strain[13]. In L. plantarum, deletion of dlt resulted in cell perforations of cell envelope[37]. In ΔdltABt, the cell surface was more negatively charged than that of WT (Figure 1), which is attributed to the absence of positively charged D-alanyl esters that can neutralize the negative charge. The contribution of dlt operon to cell wall charge has been confirmed in many Gram-positive bacteria, except for Lactococcus lactis[39]. In which, the D-alanylation of TAs in the cell wall does not significantly affect the surface charge[39]. The authors proposed that the incorporated D-alanyl esters of TAs are located inside the cell wall, and are not exposed at the cell surface, thereby do not affect the surface charge[39]. Taken together, inactivation of dlt operon can result in a wide range of physiological consequence in different Gram-positive bacteria, suggesting functional diversity of dlt operon.
The biofunction of dlt operon is responsible for the modification to the TAs on bacterial surface through incorporation of D-alanine, and results in the increase of negative change of cell surface[3]. In the current study, alcian binding assay confirmed that inactivation of dltA in BMB171 obviously increased net negative charge of Bt surface (Figure 1). Meanwhile, the increase of net negative charge on Bt cell surface could enhance the electrostatic interaction with CAMPs, thereby lead to hypersensibility to CAMPs (Table 1). In many Gram-positive bacteria, inactivations of dlt operon were observed to increase negative charge on cell surface, and resulted in decrease of bacteria to CAMPs[4-5, 7, 11, 31]. In addition, inactivation of dltA in Bt also led to decrease of tolerance ability to alkaline, which is likely attributed to the change of cell wall, such as perforation, and increased permeability of cell. In this study, although significant change of morphology of ΔdltABt cell was observed, there is no evidence to support the change of cell wall permeability. The mechanism of altered tolerance ability to alkaline in ΔdltABt should be investigated in the following study.
Inactivation of dlt operon in Bt reduced biofilm formation (Figure 5), promoted swarming motility (Figure 5), which are two life traits involved in bacterial behaviors. In B. subtilis, the two life traits have been confirmed to be associated with the bacterium's ability to differentiate into distinct coexisting cell types, including competent cells, cannibal, motile, matrix-producing, sufactin- producing, and sporulating cells[40-41]. In this study, swarming motility is significantly enhanced in ΔdltABt, suggesting that, indeed, D-alanine incorporation affords other ecological characteristics, rather than merely conferring bacterial immunity to CAMPs. Swarming motility is a collective bacterial phenomenon[42], and requires the presence of flagellum in cell surface[43]. The ability of swarming motility for survival of pathogens is important since it is helpful for the movement of pathogens to a suitable environment where they can utilize nutrients and proliferate rapidly. In this study, the significantly increased swarming motility in ΔdltABt is probably attributed to the altered flagella expression caused by the lack of D-alanylation of TAs. However, there is no any experimental evidence available for supporting this postulate. The detailed mechanism of how D-alanylation of TAs negatively controls the flagellum expression is beyond the scope of this study but worthwhile for further investigation.
Production of biofilm is recognized as a virulence factor in pathogens[44]. Previous studies have described the effect of D-alanylation of TAs on biofilm production in S. aureus[45], E. faecalis[15], L. reuteri[46], and Streptococcus sp.[47], which all showed that lack of D-alanylation of TAs significantly decreases the ability of biofilm formation. Especially in S. aureus, D-alanine incorporation of TAs has been confirmed to be necessary for biofilm formation[45]. Here, ΔdltABt mutant displayed significantly decreased biofilm formation, suggesting that dlt operon also plays a key role for biofilm formation in Bt. Biofilm formation is thought to be a two-step process that requires the primary adhesion of bacteria to a target surface followed by the formation of multiple cell layers[48]. The charge of TAs was confirmed to play a pivotal role in the initial step of biofilm formation in S.aureus[45]. Hence, the positive impact of D-alanylation of TAs on biofilm formation in Bt can be explained by two possibilities: (ⅰ) the much stronger negative charge in the ΔdltABt mutant probably leads to a pronounced increase in the repulsive force, thereby disabling the adherence of bacteria to artificial surface; (ⅱ) low growth rate during the exponential phase and high autolysis rate during the stationary phases in ΔdltABt (Figure 2-A) decrease the density and, thus, the number of adhering cells necessary during the first stage of biofilm formation.
ΔdltABt mutant displayed altered adherence to the mid-gut epithelial cell of insect in this study (Figure 7-A). Previous studies have also demonstrated that dlt mutant displays altered adherence to host cells. For example, dltA mutant of L. monocytogenes exhibited decreased adherence to murine bone marrow-derived macrophages[14]; dltA mutant of S. gordonii displayed a significantly lower level of binding to dendritic cells than its parent[31]; dltA mutant of Streptococcus sp. exhibited weak abilities of adherence and invasion to human pharyngeal epithelial cell line Hep-2[7]. In this study, the decreased binding capacity of ΔdltABt to mid-gut epithelial cell of insect suggests that D-alanylation plays an important role in modulating the binding of Bt to host insect cell. The impaired ability of adherence could be attributed to an increase of negative charge in the ΔdltABt surface causing a decreased electrostatic interaction with the negatively charged host cells.
We also took advantage of the silkworm infection model to investigate the prominent role of dlt operon in the virulence of Bt to host insect. Inactivation of dltA significantly attenuates the in vivo virulence of Bt to silkworm by injection (Figure 8). Traditionally, the insecticidal activity of Bt is thought to be mainly dependent on the crystal proteins, which can be activated by protease and then perforate the membrane of mid-gut epithelial cells, ultimately lead to lysis of intestine cells and mid-gut disarrangements[23]. While, Bt spores may colonize, germinate, and multiply in the hemocoel of insect, eventually killing larvae by septicemia[21, 49]. In spite of the main contribution of insecticidal crystal proteins, spores have also been shown to contribute to overall entomopathogenicity[49]. The current study further illustrates the contribution of the vegetative cell of Bt modified by D-alanylation to insecticidal activity.
In conclusion, this study confirmed that the dlt resistance system of the entomopathogenic bacterium Bt is effective against the humoral immune system of insect, and contributes to the virulence. Moreover, the impact of this system on cellular responses to Bt infection in insect is currently being studied, and these results will increase our understanding of the mutual antagonism mechanism between insect and its pathogenic bacterium.
References
| [1] | Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiology and Molecular Biology Reviews, 2003, 67(4): 686-723. DOI:10.1128/MMBR.67.4.686-723.2003 |
| [2] | Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in gram-positive physiology and host interactions. Nature Reviews Microbiology, 2008, 6(4): 276-287. DOI:10.1038/nrmicro1861 |
| [3] | Peschel A. How do bacteria resist human antimicrobial peptides?. Trends in Microbiology, 2002, 10(4): 179-186. DOI:10.1016/S0966-842X(02)02333-8 |
| [4] | Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, Shai Y. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B Streptococcus by increasing the cell wall density. PLoS Pathogens, 2012, 8(9): e1002891. DOI:10.1371/journal.ppat.1002891 |
| [5] | Abi Khattar Z, Rejasse A, Destoumieux-Garzón D, Escoubas JM, Sanchis V, Lereclus D, Givaudan A, Kallassy M, Nielsen-Leroux C, Gaudriault S. The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. Journal of Bacteriology, 2009, 191(22): 7063-7073. DOI:10.1128/JB.00892-09 |
| [6] | Pandin C, Caroff M, Condemine G. Antimicrobial peptide resistance genes in the plant pathogen Dickeya dadantii. Applied and Environmental Microbiology, 2016, 82(21): 6423-6430. DOI:10.1128/AEM.01757-16 |
| [7] | Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-alanylation of teichoic acids promotes group A Streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. Journal of Bacteriology, 2005, 187(19): 6719-6725. DOI:10.1128/JB.187.19.6719-6725.2005 |
| [8] | Schneewind O, Missiakas D. Lipoteichoic acids, phosphate-containing polymers in the envelope of gram-positive bacteria. Journal of Bacteriology, 2014, 196(6): 1133-1142. DOI:10.1128/JB.01155-13 |
| [9] | Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. Journal of Biological Chemistry, 1995, 270(26): 15598-15606. DOI:10.1074/jbc.270.26.15598 |
| [10] | Reichmann NT, Cassona CP, Gründling A. Revised mechanism of D-alanine incorporation into cell wall polymers in gram-positive bacteria. Microbiology, 2013, 159(9): 1868-1877. |
| [11] | Debabov DV, Kiriukhin MY, Neuhaus FC. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in D-alanylation. Journal of Bacteriology, 2000, 182(10): 2855-2864. DOI:10.1128/JB.182.10.2855-2864.2000 |
| [12] | Koch HU, D?ker R, Fischer W. Maintenance of D-alanine ester substitution of lipoteichoic acid by reesterification in Staphylococcus aureus. Journal of Bacteriology, 1985, 164(3): 1211-1217. |
| [13] | Kamar R, Réjasse A, Jéhanno I, Attieh Z, Courtin P, Chapot-Chartier MP, Nielsen-Leroux C, Lereclus D, El Chamy L, Kallassy M, Sanchis-Borja V. DltX of Bacillus thuringiensis is essential for D-alanylation of teichoic acids and resistance to antimicrobial response in insects. Frontiers in Microbiology, 2017, 8: 1437. DOI:10.3389/fmicb.2017.01437 |
| [14] | Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, Trieu-Cuot P. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Molecular Microbiology, 2002, 43(1): 1-14. |
| [15] | Fabretti F, Theilacker C, Baldassarri L, Kaczynski Z, Kropec A, Holst O, Huebner J. Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides. Infection and Immunity, 2006, 74(7): 4164-4171. DOI:10.1128/IAI.00111-06 |
| [16] | McBride SM, Sonenshein AL. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology, 2011, 157(5): 1457-1465. DOI:10.1099/mic.0.045997-0 |
| [17] | Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacological Reviews, 2003, 55(3): 27-55. |
| [18] | Poyart C, Pellegrini E, Marceau M, Baptista M, Jaubert F, Lamy MC, Trieu-Cuot P. Attenuated virulence of Streptococcus agalactiae deficient in D-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Molecular Microbiology, 2003, 49(6): 1615-1625. DOI:10.1046/j.1365-2958.2003.03655.x |
| [19] | Kristian SA, Lauth X, Nizet V, Goetz F, Neumeister B, Peschel A, Landmann R. Alanylation of teichoic acids protects Staphylococcus aureus against toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. The Journal of Infectious Diseases, 2003, 188(3): 414-423. DOI:10.1086/376533 |
| [20] | Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nature Medicine, 2004, 10(3): 243-245. DOI:10.1038/nm991 |
| [21] | Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Reviews, 1998, 62(3): 775-806. |
| [22] | Pardo-López L, Soberón M, Bravo A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiology Reviews, 2013, 37(1): 3-22. DOI:10.1111/j.1574-6976.2012.00341.x |
| [23] | Vachon V, Laprade R, Schwartz JL. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. Journal of Invertebrate Pathology, 2012, 111(1): 1-12. DOI:10.1016/j.jip.2012.05.001 |
| [24] | Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual Review of Immunology, 2007, 25: 697-743. DOI:10.1146/annurev.immunol.25.022106.141615 |
| [25] | Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Developmental & Comparative Immunology, 1999, 23(4-5): 329-344. |
| [26] | He J, Shao XH, Zheng HJ, Li MS, Wang JP, Zhang QY, Li L, Liu ZD, Sun M, Wang SY, Yu ZN. Complete genome sequence of Bacillus thuringiensis mutant strain BMB171. Journal of Bacteriology, 2010, 192(15): 4074-4075. DOI:10.1128/JB.00562-10 |
| [27] | Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene, 1985, 33(1): 103-119. |
| [28] | Arantes O, Lereclus D. Construction of cloning vectors for Bacillus thuringiensis. Gene, 1991, 108(1): 115-119. DOI:10.1016/0378-1119(91)90495-W |
| [29] | Yu ZQ, Bai PS, Ye WX, Zhang FJ, Ruan LF, Yu ZN, Sun M. A novel negative regulatory factor for nematicidal cry protein gene expression in Bacillus thuringiensis. Journal of Microbiology and Biotechnology, 2008, 18(6): 1033-1039. |
| [30] | de Souza MT, Lecadet MM, Lereclus D. Full expression of the cryⅢA toxin gene of Bacillus thuringiensis requires a distant upstream DNA sequence affecting transcription. Journal of Bacteriology, 1993, 175(10): 2952-2960. DOI:10.1128/jb.175.10.2952-2960.1993 |
| [31] | Chan KG, Mayer M, Davis EM, Halperin SA, Lin TJ, Lee SF. Role of D-alanylation of Streptococcus gordonii lipoteichoic acid in innate and adaptive immunity. Infection and Immunity, 2007, 75(6): 3033-3042. DOI:10.1128/IAI.01549-06 |
| [32] | O'Toole GA. Microtiter dish biofilm formation assay. Journal of Visualized Experiments, 2011(47): 2437. |
| [33] | Gills A, Dupres V, Mahillon J, Dufrêne YF. Atomic force microscopy: a powerful tool for studying bacterial swarming motility. Micron, 2012, 43(12): 1304-1311. DOI:10.1016/j.micron.2012.01.014 |
| [34] | Zhang DY, Ampasala DR, Zheng SC, Cusson M, Cheng XW, Krell PJ, Feng QL. Molecular cloning and characterization of a putative nuclear DEAD box RNA helicase in the spruce budworm, Choristoneura fumiferana. Archives of Insect Biochemistry and Physiology, 2006, 61(4): 209-219. DOI:10.1002/arch.20105 |
| [35] | Driever W, Rangini Z. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In vitro Cellular & Developmental Biology-Animal, 1993, 29A(9): 749-754. |
| [36] | Hamamoto H, Kurokawa K, Kaito C, Kamura K, Razanajatovo IM, Kusuhara H, Santa T, Sekimizu K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrobial Agents and Chemotherapy, 2004, 48(3): 774-779. DOI:10.1128/AAC.48.3.774-779.2004 |
| [37] | Palumbo E, Deghorain M, Cocconcelli PS, Kleerebezem M, Geyer A, Hartung T, Morath S, Hols P. D-alanyl ester depletion of teichoic acids in Lactobacillus plantarum results in a major modification of lipoteichoic acid composition and cell wall perforations at the septum mediated by the Acm2 autolysin. Journal of Bacteriology, 2006, 188(10): 3709-3715. DOI:10.1128/JB.188.10.3709-3715.2006 |
| [38] | Wydau-Dematteis S, Louis M, Zahr N, Lai-Kuen R, Saubaméa B, Butel MJ, Pons JL. The functional dlt operon of Clostridium butyricum controls the D-alanylation of cell wall components and influences cell septation and vancomycin-induced lysis. Anaerobe, 2015, 35(Part B): 105-114. |
| [39] | Giaouris E, Briandet R, Meyrand M, Courtin P, Chapot-Chartier MP. Variations in the degree of D-alanylation of teichoic acids in Lactococcus lactis alter resistance to cationic antimicrobials but have no effect on bacterial surface hydrophobicity and charge. Applied and Environmental Microbiology, 2008, 74(15): 4764-4767. DOI:10.1128/AEM.00078-08 |
| [40] | Lopez D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiology Reviews, 2009, 33(1): 152-163. DOI:10.1111/j.1574-6976.2008.00148.x |
| [41] | López D, Kolter R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiology Reviews, 2010, 34(2): 134-149. DOI:10.1111/j.1574-6976.2009.00199.x |
| [42] | Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J, Michiels J. Living on a surface: swarming and biofilm formation. Trends in Microbiology, 2008, 16(10): 496-506. DOI:10.1016/j.tim.2008.07.004 |
| [43] | Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annual Review of Microbiology, 2003, 57: 249-273. DOI:10.1146/annurev.micro.57.030502.091014 |
| [44] | O'Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annual Review of Microbiology, 2000, 54: 49-79. DOI:10.1146/annurev.micro.54.1.49 |
| [45] | Gross M, Cramton SE, G?tz F, Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infection and Immunity, 2001, 69(5): 3423-3426. DOI:10.1128/IAI.69.5.3423-3426.2001 |
| [46] | Walter J, Loach DM, Alqumber M, Rockel C, Hermann C, Pfitzenmaier M, Tannock GW. D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100-23 results in impaired colonization of the mouse gastrointestinal tract. Environmental Microbiology, 2007, 9(7): 1750-1760. DOI:10.1111/j.1462-2920.2007.01292.x |
| [47] | Nilsson M, Rybtke M, Givskov M, H?iby N, Twetman S, Tolker-Nielsen T. The dlt genes play a role in antimicrobial tolerance of Streptococcus mutans biofilms. International Journal of Antimicrobial Agents, 2016, 48(3): 298-304. DOI:10.1016/j.ijantimicag.2016.06.019 |
| [48] | Von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. European Journal of Clinical Microbiology and Infectious Diseases, 1999, 18(12): 843-846. DOI:10.1007/s100960050417 |
| [49] | Salamitou S, Ramisse F, Brehélin M, Bourguet D, Gilois N, Gominet M, Hernandez E, Lereclus D. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology, 2000, 146(11): 2825-2832. DOI:10.1099/00221287-146-11-2825 |
