Mengjiao Chen, Shenghai Tuo, Yumeng Xi, Lin Zhang, Hanfang Zeng, Yeqing Li, Zhaoyu Han


Institute of Dairy Science, College of Animal Science and Technology, Nanjing Agricultural University, Nanjing 210095, Jiangsu Province, China
Received: 1 August 2017; Revised: 3 November 2017; Published online: 18 December 2017
Foundation item: Supported by the Jiangsu Provincial Cooperative Innovation Fund-Prospective Joint Research Project (By205071-07)
Corresponding author: Zhaoyu Han, Tel/Fax:+86-25-84395045;E-mail:zyhan6708@njau.edu.cn.
Abstract: Objective The aim of this experiment was to study the effects of zinc-bearing palygorskite (Zn-Pal) on rumen bacterial diversity in vitro.Methods We prepared Zn-Pal by the ion-exchange, and evaluated the compositions of bacterial communities in 60 samples based on 16S rDNA genes.Results We obtained a total of 1490959 effective sequences and 87662 OTUs. The bacterial diversity in the treatment Ⅳ group increased at 24 h, and the abundance of the treatment Ⅳ group increased at 48 h. Bacterial community composition analysis shows that the dominant phyla were Bacteroidetes, Firmicutes, Proteobacteria and Lentisphaerae. Compared with the control group, Firmicutes in treatment groups significantly increased (P < 0.05) at 24 h and 48 h, whereas Bacteroidetes in the treatment Ⅳ group was decreased (P < 0.05) at 48 h. At the genus level, the sequences could be assigned to 124 different genera. The content of Prevotella had no significant difference between the control and treatments. The relative abundance of Treponema in the treatment Ⅳ group was significantly higher at 48 h than in the control group (P < 0.05), whereas the relative abundance of Victivallis and Pseudobutyrivibrio in treatment Ⅳ was lower.Conclusion Zn-Pal may affect rumen fluid bacterial diversity in dairy cows, and the degrees of this influence varied with the dose and time of Zn-Pal.
Key words: zinc-bearing palygorskite16S rDNArumen bacterial diversityin vitrodairy cow
载锌凹凸棒石黏土对瘤胃体外发酵细菌多样性的影响
陈孟姣, 妥生海, 奚雨萌, 张林, 曾涵芳, 李烨青, 韩兆玉


南京农业大学动物科技学院, 乳牛科学研究所, 江苏 南京 210095
收稿日期:2017-08-01;修改日期:2017-11-03;网络出版日期:2017-12-18
基金项目:江苏省产学研前瞻性联合研究项目(By205071-07)
通讯作者:韩兆玉, Tel/Fax:+86-25-84395045;E-mail:zyhan6708@njau.edu.cn.
摘要:目的 研究载锌凹凸棒石黏土对瘤胃体外发酵细菌多样性的影响。方法 本试验采用离子交换法制备载锌凹凸棒石黏土,利用16S rDNA测序技术分析了载锌凹凸棒石黏土对奶牛瘤胃体外发酵细菌菌群和多样性的影响。结果 研究共获得1490959个有效序列和87662个OTUs。测序结果表明,与对照组相比,试验Ⅳ组中的细菌多样性在体外发酵24 h时提高,试验Ⅳ组中的细菌丰度在体外发酵48 h时提高。细菌菌群组成分析表明,60个样本中的优势菌门主要是厚壁菌门、拟杆菌门、变形菌门和黏胶球形菌门。与对照组相比,各试验组的厚壁菌门在体外发酵24 h和48 h时均显著增加(P < 0.05),试验Ⅳ组中拟杆菌门在发酵48 h时显著降低(P < 0.05);60个样本中共获得124个细菌菌属,其中对照组与试验组中普氏菌属含量均没有显著变化(P>0.05)。在体外发酵48 h时,试验Ⅳ组中密螺旋体属含量与对照组相比显著增加(P < 0.05),而食物谷菌属和假丁酸弧菌属含量与对照组相比均显著降低(P < 0.05)。结论 载锌凹凸棒石黏土对奶牛瘤胃体外发酵中细菌多样性产生一定影响,其影响随发酵时间和添加剂量而不同。
关键词:载锌凹凸棒石黏土16S rDNA瘤胃细菌多样性体外奶牛
The rumen of cattle is a complex ecosystem that harbors a wide variety of microorganisms, including bacteria, protozoa, archaea and fungi[1]. A principal function of the rumen microbiome is the conversion of plant materials into digestible compounds that can be used by the animal host[2]. Additionally, as the microbiome in the rumen undergoes long-term selection and evolution, the microbes and host form an interinhibitory and interdependent homeostatic relationship that has an important role in maintaining host health, improving performance, reducing environmental pollution, and ensuring food and animal product safety[3]. Therefore, studies of ruminal microbes represent a key area of nutrition research in ruminants, and it is important to improve our understanding of the complexity of microbial composition and their interactions.
Clay minerals can be used as a carrier loaded with metal ions, such as Ag+, Cu2+ and Zn2+, and are good inorganic antibacterial agents. Zinc has many physiological functions, not only is it an essential trace mineral which is required for growth, and enzyme structure and function in poultry[4], but it also can regulate microbial activity[5]. A previous study reported that dietary palygorskite inclusion would exert beneficial effects on the immunity, intestinal integrity and barrier function of broilers at early age[6]. Chalvatzi et al.[7] found that dietary inclusion of palygorskite can alter conditions within the caecum in favour of specific species. Zaid et al.[8] found that palygorskite was safe and effective in the treatment of mild-to-moderate acute diarrhea in humans. Zhang et al.[9] have reported that palygorskite was beneficial to intestinal integrity, which improved growth performance in weaned piglets. Xu et al.[10] have found that Zinc loads of 10.3% of clinoptilolite have an antibacterial rate greater than 99.9% for Escherichia coli and Staphylococcus aureus. Recently, Luo[11] prepared Zn-bearing palygorskitethrough the ion-exchange method and found that it effectively restrained the growth of E. coli in vitro and decreased its levels in the intestines of broilers, suggesting that Zn-Pal can be used as an antibiotic agent. Additionally, the presence of micropores and channels in palygorskite, together with the fine particle size and fibrous habit, accounts for its largesurface area[12]. Zn bearing zeolite and montmorillonite have been synthesized based on the high specific area and adsorption capacity of these agents[13-14]. It has also been demonstrated that dietary supplementation of these Zn-bearing nonmetallic minerals can promote growth performance, improve intestinal morphology, inhibit the growth of pathogenic bacteria and enhance intestinal antioxidant capacity[13-16]. Moreover, they could replace antibiotics widely used in poultry production due to their antimicrobial ability.
In this study, palygorskite was used to prepare Zn-Pal through the ion-exchange method, and we evaluated the composition and structure of bacterial communities in samples by Illumina MiSeq high-throughput sequencing of the V3-V4 region of the 16S rDNA genes.
1 Materials and Methods 1.1 Preparation of Zn-Pal Palygorskite was kindly provided by Jiangsu Sinitic Biotech Co., Ltd. (Xuyi, Jiangsu, P. R. China). Zn-Pal was prepared according to the method described by Yan et al.[17]. The chemical composition of Zn-Pal claymineral is SiO2, 53.07%; Al2O3, 10.43%; MgO, 6.11%; CaO, 1.85%; K2O, 1.92% and Fe2O3, 7.75% (Measured using a Minipal 4 X-ray fluorescence spectrometer, PANalytical, Netherland). The amount of Zn adsorbed by palygorskite was 24.5 mg/g with inductively coupled plasma mass spectrometry (ICP-MS, Optima 2100 DV, Perkin Elmer, USA) according to the method described by Yan et al.[17].
1.2 Characterization of Zn-Pal X-ray diffraction (XRD) patterns of palygorskite and zinc-bearing palygorskite were collected on an X'pert PRO X-ray power diffractometer, equipped with a Cu-Kα radiation source (40 kV, 40 mA) from 3 to 80° (2θ) at a scanning step time of 15.2 s, with a step interval about 0.017°, divergence slit of 0.5°. The Zeta potential of palygorskite and Zn-Pal were measured by a Malvern Zetasizer Nano system (Malvern Instruments, USA) at 25, using a folded capillary cell. The specific surface area (SBET) was determined by the Brunauer-Emmett-Teller (BET) method. Determination of cation-exchange capacity and ethylene blue absorption were performed according to the method described by Qiao et al.[18].
1.3 Experimental design The substrate used was a common total mixed ration diet (with a 50:50 forage: concentrate diet, 54.60% dry matter, 17.15% crude protein, and 34.72% neutral detergent fiber), which was dried at 65 for 48 h and broken up by passing it through a 1 mm screen. The composition and nutrient content of the basal diet are shown in Table 1.
Table 1. Composition and nutrient levels of basal diet in dairy cows after parturition (DM basis)
| Ingredients | Content/% | Nutrient levels | Content/% |
| Corn | 20.86 | ME(MJ/kg) | 10.71 |
| Barley | 1.89 | NEL(MJ/kg) | 6.89 |
| Soybean meal | 12.49 | CP | 18.32 |
| Cottonseed | 3.41 | EE | 4.33 |
| Premix① | 2.70 | Ash | 9.22 |
| DDGS | 6.24 | ADF | 22.81 |
| Wheat silage | 20.75 | NDF | 34.58 |
| Alfalfa hay | 12.13 | Ca | 0.41 |
| Chinensis wildrye | 4.04 | P | 0.38 |
| Beet meal | 12.26 | ||
| Brewers grain, wet | 3.23 | ||
| Content of per kilogram premix: 500 kIU vitamin A, 140 kIU vitamin D3, 2000 kIU vitamin E, 2200 mg Cu, 4000 mg Fe, 2400 mg Mn, 5600 mg Zn, 80 mg I, 35 mg Se and 20 mg Co. | |||
表选项
Levels of Zn-Pal in in vitro incubation fluid were 0% (Control), 0.2% (Treatment Ⅰ), 0.4% (Treatment Ⅱ), 0.6% (Treatment Ⅲ), 0.8% (Treatment Ⅳ). The Zn-Pal was mixed with the substrate before the commencement of the experiment. Ruminal fluid was collected from three healthy dairy cows (650 kg mean body weight), which were killed after a 7 day adaptation to the diet. Ruminal ?uid was collected from different locations of the rumen, then mixed and strained through four layers of cheese cloth into a pre-warmed thermos ?ask. The 100 mL of rumen fluid-buffer mixture and rumen fluid at a ratio of 4:1, was dispensed anaerobically into bottles containing 1g of TMR and added Zn-Pal. The composition and dosage of the rumen fluid-buffer mixture are performed according to the method described by Theodorou et al.[19]. The serum bottles were filled with O2-free CO2 gas, and then capped with a rubber stopper. The bottles were kept in an incubator (JSGI-250T, JSR, Gongju, Korea) at 39 ℃. At 0, 12, 24 and 48 h, fluid was sampled to determine the microbial composition using 16S rDNA amplicon pyrosequencing.
1.4 DNA extraction and 16S rDNA sequencing Genomic DNA was extracted from thawed rumen fluid samples using a stool DNA Kit (Tiangen Biotech, Beijing) following the manufacturer's procedures. In the present study, the primers B341F 5'-CCTACGGGNGGCWGCAG-3' and B785R 5'-GACTACHVGGGTATCTAATCC-3' were used for monitoring bacterial and archaeal populations in the rumen. This primer target of the V3-V4 hypervariable region can be fully covered by the Illumina MiSeq. PCR amplification was performed using a KAPA HiFi HotStart ReadyMix PCR Kit. Each reaction (25 μL) contained 12.5 μL 2×KAPA HiFi HotStart ReadyMix, 0.25 mol/L of each primer and 10 ng of DNA template. The PCR reaction was carried out at 95 for 30 s, 55 for 30 s, 72 for 30 s, and 72 for 5 min. PCR amplicon libraries were prepared by combining the PCR products for each sample. After purification, the PCR products from the different samples were quantified using the Agilent 2100 Bioanalyzer System (Santa Clara, CA, USA) and then pooled at equal concentrations. Amplicon sequencing was performed on Illumina Miseq platform at Beijing Ori-Gene Science and Technology Co., Ltd. (Beijing, China). The resulting sequences were then screened and filtered for quality and length. Sequences with a length shorter than 50 bp, having more than two primer mismatches, containing ambiguous characters or exhibiting a homopolymer run exceeding 6 bp, were removed[20].
1.5 Bioinformatics and statistical analysis Using USEARCH[21], the high-quality sequences were clustered into operational taxonomic units (OTUs) defined by 97% similarity. These OTUs were used for diversity (Shannon and Simpson), richness (Ace[22] and Chao[23]), and rarefaction curve analysis using MOTHUR[24]. Taxonomic assignments of OTUs that reached the 97% similarity level were made using RDP classifier[25] by comparison with the SILVA[26] database, classification based on Bergey's taxonomy, which is divided into kingdom, phylum, class, order, family and genus. The default threshold is 80%, and below this value is unclassified. The effect of different concentrations of zinc-bearing palygorskite on various parameters was evaluated with the one-way analysis of variance (ANOVA) protocol in SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). Means were compared by employing the LSD (least significant difference) multiple-range test at a significance level of P < 0.05. All data are presented as mean±standard error (SE).
2 Results 2.1 Characterization of Zn-Pal It can be seen from Table 2 that the BET surface area of Pal is 164.29 m2/g, the cation exchange capacity of Pal is 28.01 mmol/100 g, the Zeta potential of Pal is -13.60 mV, and the BET surface area of Zn-Pal is 136.60 m2/g, the cation exchange capacity of Zn-Pal is 35.52 mmol/100 g, the Zeta potential of Zn-Pal is -15.5 mV.
Table 2. Physico-chemical properties of the palygorskites
| Items | Palygorskite | Zinc-bearing palygorskite |
| BET surface area/(m2/g) | 155.74 | 136.60 |
| Cation exchange capacity/ (mmol/100 g) | 28.01 | 35.52 |
| Ethylene blue absorption/ (mmol/100 g) | 15.00 | 15.00 |
| Zeta potential/(mV) | -13.60 | -15.50 |
| These data were measured by Qiao et al. (2015) and the determinations of cation exchange capacity, ethylene blue absorption, specific surface area (BET method) were performed according to the method described by Yongduo and Gaoxiang (2004). Zeta potential was measured by a Malvern Zetasizer Nano system (Malvern Instruments, USA). | ||
表选项
2.2 OTU statistics and analysis of alpha diversity The effect of Zn-Pal on OTUs and alpha diversity after different times of in vitro incubation is shown in Table 3. Indices of bacterial richness based on OTUs were estimated by the method of Ace and Chao, and indices of bacterial diversity were determined using the method of Simpson and Shannon. A total of 1490959 quality effective sequences and 1425181 high-quality sequences were obtained from the 60 samples. These sequences included an average of 23753 reads per rumen sample. Among the 60 samples, the total number of OTUs detected by our analysis was 87662 with an average of 1461 OTUs per sample. Simpson in the treatment Ⅳ was lower compared with control at 24 h (P < 0.05), and OTUs in the treatment Ⅳ became higher than control at 48 h (P < 0.05), while alpha diversity indexs in other treatments has no significant differences compared with control (P > 0.05). Compared with treatment Ⅱ group, OUTs and Chao in treatment Ⅳ group were higher at 48 h (P < 0.01). Most rarefaction curves for each sample approached the saturation plateau, which indicated that the sampling effort had sufficient sequence coverage to accurately describe the bacterial composition of each group.
Table 3. Influence of zinc-bearing palygorskite on the Alpha diversity
| t/h | Item | Control | TreatmentⅠ | TreatmentⅡ | Treatment Ⅲ | Treatment Ⅳ | SEM | P |
| 0 | Reads | 24721.33±3275.87 | 24319.00±4182.84 | 25155.67±2301.31 | 25028.33±5272.50 | 14230.00±1165.33 | 1752.81 | 0.21 |
| OTUs | 1402.08±6.88 | 1402.22±20.69 | 1406.59±17.18 | 1384.00±11.73 | 1383.60±13.52 | 6.17 | 0.70 | |
| Chao | 1954.03±16.95 | 1959.01±69.40 | 1979.60±30.82 | 1854.73±42.47 | 1866.60±31.38 | 21.04 | 0.19 | |
| Ace | 1973.69±25.86 | 1970.73±71.48 | 1997.06±45.25 | 1877.97±31.69 | 1879.43±28.82 | 21.44 | 0.23 | |
| Coverage | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | - | 0.24 | |
| Shannon | 6.08±0.01 | 6.07±0.02 | 6.09±0.01 | 6.04±0.02 | 6.05±0.01 | 0.01 | 0.25 | |
| Simpson | 0.01000±0.00008 | 0.01±0.00 | 0.01±0.00 | 0.0100±0.0041 | 0.01±0.00 | 0.00 | 0.02 | |
| 12 | Reads | 15745.67±1593.64 | 18127.67±1174.31 | 17844.33±2533.88 | 16130.67±2968.67 | 17468.67±2302.93 | 873.62 | 0.91 |
| OTUs | 1096.78±22.64 | 1108.78±15.98 | 1108.93±13.75 | 1082.10±8.33 | 1125.56±9.40 | 6.84 | 0.38 | |
| Chao | 1560.89±63.47 | 1622.42±25.51 | 1596.18±9.60 | 1601.57±33.62 | 1649.23±54.77 | 17.77 | 0.67 | |
| Ace | 1579.29±65.76 | 1730.55±77.05 | 1612.13±16.53 | 1618.62±46.70 | 1716.89±71.66 | 27.79 | 0.34 | |
| Coverage | 0.97±0.00 | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | - | 0.47 | |
| Shannon | 4.86±0.03 | 4.86±0.06 | 4.89±0.06 | 4.73±0.02 | 4.94±0.03 | 0.02 | 0.05 | |
| Simpson | 0.05±0.00 | 0.05±0.00 | 0.05±0.00 | 0.05±0.00 | 0.04±0.00 | - | 0.10 | |
| 24 | Reads | 19488.33±5732.20 | 24783.67±1075.16 | 21021.33±3104.38 | 16567.00±2996.16 | 22184.00±8126.50 | 1633.37 | 0.65 |
| OTUs | 1140.43±12.71 | 1119.77±16.43 | 1109.15±10.15 | 1114.16±6.35 | 1110.84±7.04 | 5.20 | 0.34 | |
| Chao | 1643.39±30.46 | 1615.66±33.99 | 1616.99±18.76 | 1657.90±15.52 | 1582.07±28.28 | 12.16 | 0.36 | |
| Ace | 1690.34±60.73 | 1689.97±66.01 | 1662.54±50.13 | 1848.33±101.47 | 1673.73±34.04 | 31.05 | 0.32 | |
| Coverage | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | 0.96±0.00 | - | 0.47 | |
| Shannon | 5.09±0.03ab | 5.07±0.02ab | 5.05±0.09ab | 5.00±0.05b | 5.20±0.01a | 0.03 | 0.13 | |
| Simpson | 0.04±0.00b | 0.04±0.00b | 0.04±0.00b | 0.04±0.00b | 0.03±0.00a | - | 0.04 | |
| 48 | Reads | 20921.33±3534.80ab | 25927.67±2781.81a | 16930.67±1224.23b | 29444.33±2443.74a | 28355.67±3333.49a | 1642.80 | 0.04 |
| OTUs | 1155.65±13.01B | 1222.97±41.64AB | 1161.95±12.85B | 1244.42±22.34AB | 1296.55±33.16A | 17.42 | 0.02 | |
| Chao | 1623.53±28.87Bbc | 1792.55±66.42ABab | 1579.31±27.03Bc | 1778.65±41.18ABab | 1868.33±82.88Aa | 35.56 | 0.02 | |
| Ace | 1634.97±23.82Bb | 1968.60±130.21Aba | 1618.91±16.63Bb | 1882.83±70.62ABab | 2089.41±144.00Aa | 60.77 | 0.02 | |
| Coverage | 0.97±0.00Aa | 0.96±0.00ABb | 0.97±0.00Aa | 0.96±0.00ABb | 0.96±0.00Bb | - | 0.02 | |
| Shannon | 5.56±0.05 | 5.56±0.05 | 5.56±0.07 | 5.58±0.07 | 5.69±0.02 | 0.02 | 0.41 | |
| Simpson | 0.02±0.00 | 0.02±0.00 | 0.02±0.00 | 0.02±0.00 | 0.01±0.00 | - | 0.62 | |
| With the same row different lowercase letters mean significant at P < 0.05 and different capital letters mean significant at P < 0.01. | ||||||||
表选项
2.3 Relative abundance of Bacterial Phyla In this study, the composition of the bacterial community in rumen fluids was examined to determine the effect of Zn-Pal on microbiota in dairy cattle. Twenty-five different phyla were detected in these samples (including unclassified). The relative abundances of the 10 most abundant phyla are presented in Figure 1 and the relative abundances of the most abundant phyla are presented in Table 4. The five groups showed very similar taxonomic compositions at the phylum-level, even at different time points. At 0 h, Bacteroidetes (range 39%-41%) and Firmicutes (range 30%-32%) were the dominant phyla in the five groups (P > 0.05). At 12 h, Bacteroidetes, Firmicutes and Proteobacteria were the most common groups and accounted for 35.33%-37.00%, 31.33%-33.00% and 16.67%-19.00% of the reads, respectively. There were no significant differences between groups (P > 0.05) except for Proteobacteria which were higher in the four treatment groups compared with controls (P < 0.05). At 24 h, the five groups were dominated by Bacteroidetes, Firmicutes and Proteobacteria, which represented 32.00%-36.33%, 27.00%-29.33% and 15.00%-20.00% of the reads, respectively. And the three phyla in all treatment groups had no significant differences compared with controls except Firmicutes in the treatment Ⅳ group, which was higher than in controls, and Proteobacteria in the treatment Ⅳ group was lower than in the controls (P < 0.05). At 48 h, Firmicutes (37%-39%), Bacteroidetes (32.33%-36.00%), Proteobacteria (10.67%-12.33%) and Lentisphaerae (5.00%-6.33%) were the dominant bacterial phyla in cattle rumen fluid. Firmicutes numbers in the treatment Ⅱ group and in the treatment Ⅳ group were increased compared with controls (P < 0.05) while Bacteroidetes in the treatment Ⅳ group was decreased compared with controls (P < 0.05).
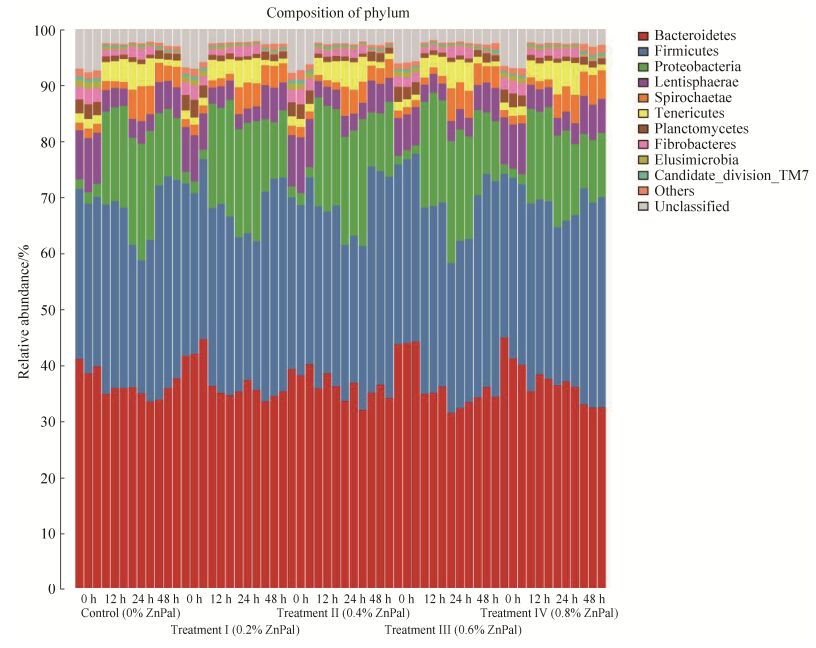 |
| Figure 1 Phylum-level composition of the rumen microbiome. The relative abundances of the 10 most abundant phylum. |
| 图选项 |
Table 4. Influence of zinc-bearing palygorskite on the relative abundance (%) of bacterial groups (phylum level)
| t/h | Item | Control | TreatmentⅠ | TreatmentⅡ | Treatment Ⅲ | Treatment Ⅳ | SEM | P |
| 0 | Bacteroidetes | 39.67±0.88 | 40.07±0.67 | 39.70±0.58 | 40.33±0.88 | 40.80±0.58 | 0.59 | 0.14 |
| Firmicutes | 30.00±0.00 | 30.67±0.88 | 31.33±0.88 | 32.00±0.58 | 31.00±1.00 | 0.38 | 0.20 | |
| Lentisphaerae | 8.83±0.33 | 8.67±0.67 | 8.93±0.33 | 8.67±0.33 | 8.93±0.33 | 0.30 | 0.24 | |
| Proteobacteria | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | - | - | |
| 12 | Bacteroidetes | 35.67±0.33 | 35.33±0.33 | 37.00±1.00 | 35.33±0.33 | 36.67±0.88 | 0.32 | 0.29 |
| Firmicutes | 33.00±0.58 | 32.67±0.67 | 31.33±1.20 | 33.00±0.00 | 32.33±0.88 | 0.33 | 0.55 | |
| Lentisphaerae | 3.33±0.33a | 3.33±0.33a | 3.00±0.00a | 3.00±0.00a | 4.00±0.00b | 0.10 | 0.04 | |
| Proteobacteria | 17.33±0.33ab | 19.00±1.16a | 18.33±0.67ab | 19.00±0.58a | 16.67±0.33b | 0.38 | 0.13 | |
| 24 | Bacteroidetes | 34.67±0.88ab | 36.00±0.58a | 34.33±1.45ab | 32.00±0.58b | 36.33±0.33a | 0.50 | 0.03 |
| Firmicutes | 26.00±1.53a | 27.00±0.58ab | 27.67±0.88ab | 28.67±0.88ab | 29.33±0.88b | 0.48 | 0.22 | |
| Lentisphaerae | 3.33±0.33 | 3.00±0.00 | 3.33±0.33 | 3.67±0.33 | 3.33±0.33 | 0.12 | 0.65 | |
| Proteobacteria | 19.67±0.67a | 20.00±0.58a | 20.33±1.33a | 20.00±1.16a | 15.00±1.00b | 0.65 | 0.02 | |
| 48 | Bacteroidetes | 36.00±1.16a | 34.33±0.33ab | 35.00±0.58a | 34.67±0.67a | 32.33±0.33b | 0.39 | 0.04 |
| Firmicutes | 37.00±1.00a | 38.00±0.58ab | 39.33±0.67b | 37.33±0.67ab | 38.00±0.58b | 0.32 | 0.26 | |
| Lentisphaerae | 5.33±0.33 | 5.67±0.33 | 5.00±0.58 | 5.00±0.58 | 6.33±0.33 | 0.18 | 0.26 | |
| Proteobacteria | 12.00±0.58 | 11.67±0.88 | 11.00±1.00 | 12.33±1.33 | 10.67±0.33 | 0.40 | 0.67 | |
| With the same row different lowercase letters mean significant at P < 0.05 and different capital letters mean significant at P < 0.01. | ||||||||
表选项
At the genus level, the sequences could be assigned to 124 different genera. The relative abundances of the 14 most abundant genera are presented in Figure 2 and Table 5. At 0 h, RC9_gut_group was predominant with an abundance of 8.09%-9.54%, followed by Prevotella, Victivallis, Fibrobacter, Anaeroplasma, Oligosphaera and Incertae_Sedis in all five groups. At 12 h, the most abundant genera were Prevotella, RC9_gut_group, Anaeroplasma, Succinivibrio, Incertae-Sedis and Fibrobacter, which together accounted for 30.85%, 29.99%, 32.26%, 30.94% and 31.36% of the reads, respectively. The relative abundances of RC9_gut_group and Succiniclaticum in treatment Ⅳ significantly increased; however, the relative abundance of Fibrobacter in treatment group Ⅲ significantly declined compared with controls (P < 0.05). At 24 h, the most abundant sequences were those related to Prevotella, Treponema, RF9_gut_group, Anaeroplasma, Succinivibrio, Fibrobacter and Incertae_Sedis in all groups. The Treponema genus was significantly lower in treatment Ⅱ and treatment Ⅳ groups than that of the controls (P < 0.05). Also, in comparison with controls, Succiniclasticum in the treatment Ⅱ group became lower, however levels of Succiniclasticum in treatment Ⅳ were higher (P < 0.05). At 48 h, the genera were numerically dominated by sequences related to Prevotella, RC9_gut_group, Succiniclasticum, Incertae_Sedis, Treponema, Pseudobutyrivibrio and Victivallis in all groups. The relative abundances of RC9_gut_group in all treatment groups were lower than in controls (P < 0.05). The relative abundances of Succiniclasticum and Pseudobutyrivibrio were decreased, while the relative abundance of Incertae_sedis was increased in the treatment Ⅱ group compared with controls (P < 0.05). The relative abundance of Treponema in the treatment Ⅳ group was significantly higher, however the relative abundance of Victivallis and Pseudobutyrivibrio in treatment Ⅳ was significantly lower than in controls (P < 0.05).
 |
| Figure 2 Genera-level composition of the rumen microbiome. The relative abundances of the 12 most abundant genera. |
| 图选项 |
Table 5. Influence of zinc-bearing palygorskite on the relative abundance (%) of bacterial groups (genus level)
| t/h | Item | Control | TreatmentⅠ | TreatmentⅡ | Treatment Ⅲ | Treatment Ⅳ | SEM | P |
| 0 | RC9_gut_group | 8.09±0.20 | 8.53±0.22 | 8.16±0.18 | 8.54±0.22 | 8.45±0.64 | 0.20 | 0.15 |
| Prevotella | 5.20±0.06 | 5.67±0.38 | 4.70±0.26 | 5.92±0.28 | 5.20±0.22 | 0.17 | 0.18 | |
| Victivallis | 2.57±0.10 | 2.23±0.02 | 2.68±0.23 | 2.48±0.14 | 2.30±0.06 | 0.08 | 0.13 | |
| Fibrobacter | 2.51±0.02 | 2.17±0.06 | 2.36±0.05 | 1.78±0.17 | 2.19±0.08 | 0.09 | 0.09 | |
| Succiniclasticum | 2.18±0.03 | 2.39±0.07 | 2.00±0.04 | 2.39±0.04 | 2.24±0.02 | 0.06 | 0.26 | |
| Anaeroplasma | 1.38±0.25 | 1.17±0.09 | 1.25±0.12 | 1.07±0.06 | 1.20±0.02 | 0.04 | 0.25 | |
| Oligosphaera | 1.38±0.15 | 1.52±0.26 | 1.56±0.20 | 1.07±0.02 | 1.36±0.04 | 0.07 | 0.17 | |
| Incertae_Sedis | 1.37±0.03 | 1.37±0.13 | 1.47±0.02 | 1.44±0.05 | 1.36±0.04 | 0.04 | 0.09 | |
| Ruminococcus | 0.77±0.04 | 0.74±0.03 | 0.68±0.02 | 0.80±0.10 | 0.57±0.05 | 0.03 | 0.20 | |
| Butyrivibrio | 0.56±0.03 | 0.66±0.02 | 0.55±0.05 | 0.66±0.03 | 0.63±0.04 | 0.02 | 0.53 | |
| Treponema | 0.44±0.06 | 0.41±0.04 | 0.45±0.07 | 0.30±0.11 | 0.36±0.02 | 0.02 | 0.10 | |
| Pseudobutyrivibrio | 0.22±0.12 | 0.30±0.16 | 0.33±0.12 | 0.37±0.11 | 0.24±0.15 | 0.20 | 0.07 | |
| 12 | Prevotella | 20.33±0.62ab | 19.38±0.49b | 21.64±0.35a | 21.15±0.51a | 20.36±0.59ab | 0.28 | 0.09 |
| RC9_gut_group | 4.11±0.10bc | 4.41±0.08ab | 4.20±0.06abc | 3.86±0.12c | 4.53±0.16a | 0.75 | 0.01 | |
| Anaeroplasma | 2.53±0.12 | 2.16±0.16 | 2.39±0.05 | 2.16±0.08 | 2.29±0.11 | 0.09 | 0.71 | |
| Succiniclasticum | 1.38±0.13a | 1.63±0.21ab | 1.54±0.20ab | 1.66±0.35ab | 1.75±0.06b | 0.05 | 0.24 | |
| Incertae_Sedis | 1.38±0.07 | 1.35±0.04 | 1.39±0.05 | 1.21±0.01 | 1.16±0.04 | 0.04 | 0.32 | |
| Fibrobacter | 1.12±0.11a | 1.05±0.07ab | 1.10±0.05ab | 0.89±0.01b | 1.26±0.04a | 0.41 | 0.05 | |
| Victivallis | 0.85±0.11 | 0.90±0.07 | 0.87±0.05 | 0.94±0.03 | 1.04±0.05 | 0.03 | 0.37 | |
| Oligosphaera | 0.59±0.07 | 0.52±0.02 | 0.47±0.09 | 0.47±0.05 | 0.60±0.09 | 0.02 | 0.05 | |
| Butyrivibrio | 0.47±0.02 | 0.41±0.14 | 0.41±0.12 | 0.47±0.06 | 0.48±0.05 | 0.02 | 0.55 | |
| Treponema | 0.42±0.05 | 0.32±0.04 | 0.35±0.02 | 0.38±0.09 | 0.49±0.04 | 0.02 | 0.14 | |
| Pseudobutyrivibrio | 0.37±0.01 | 0.36±0.04 | 0.42±0.01 | 0.29±0.04 | 0.39±0.04 | 0.02 | 0.53 | |
| Ruminococcus | 0.30±0.03 | 0.31±0.06 | 0.29±0.02 | 0.32±0.04 | 0.26±0.01 | 0.01 | 0.70 | |
| 24 | Prevotella | 19.65±0.56ab | 21.58±0.34a | 19.87±1.34ab | 18.20±0.65b | 20.85±0.63a | 0.43 | 0.09 |
| Treponema | 4.26±0.12a | 3.56±0.29ab | 3.26±0.41b | 4.03±0.34ab | 3.47±0.14b | 0.14 | 0.09 | |
| RC9_gut_group | 3.73±0.02 | 3.41±0.07 | 3.62±0.06 | 3.45±0.10 | 3.92±0.09 | 0.12 | 0.71 | |
| Anaeroplasma | 3.59±0.28 | 3.43±0.21 | 3.64±0.10 | 3.61±0.28 | 4.14±0.37 | 0.12 | 0.44 | |
| Succiniclasticum | 2.09±0.24b | 1.92±0.17bc | 1.82±0.31c | 1.97±0.28bc | 2.38±0.23a | 0.06 | 0.02 | |
| Fibrobacter | 1.79±0.04ab | 1.75±0.03ab | 1.53±0.07b | 1.85±0.08a | 1.69±0.04ab | 0.05 | 0.26 | |
| Incertae_Sedis | 1.52±0.15 | 1.54±0.09 | 1.63±0.03 | 1.69±0.08 | 1.48±0.10 | 0.04 | 0.49 | |
| Victivallis | 0.88±0.14 | 0.67±0.05 | 0.88±0.07 | 0.86±0.12 | 1.00±0.01 | 0.03 | 0.02 | |
| Butyrivibrio | 0.77±0.10 | 0.86±0.06 | 0.79±0.14 | 0.67±0.06 | 0.82±0.06 | 0.04 | 0.63 | |
| Ruminococcus | 0.52±0.10 | 0.59±0.03 | 0.61±0.04 | 0.55±0.04 | 0.44±0.04 | 0.03 | 0.25 | |
| Pseudobutyrivibrio | 0.43±0.06 | 0.55±0.03 | 0.46±0.04 | 0.44±0.08 | 0.55±0.05 | 0.03 | 0.34 | |
| Oligosphaera | 0.30±0.06 | 0.20±0.03 | 0.28±0.01 | 0.33±0.04 | 0.28±0.03 | 0.02 | 0.17 | |
| 48 | Prevotella | 11.67±0.83ab | 11.49±0.71ab | 12.80±0.62a | 12.00±0.89ab | 10.36±0.13b | 0.34 | 0.24 |
| RC9_gut_group | 9.59±0.21a | 8.84±0.32b | 8.71±0.18bc | 8.85±0.16b | 8.10±0.20c | 0.15 | 0.01 | |
| Succiniclasticum | 3.02±0.19a | 2.96±0.24b | 2.40±0.08b | 2.72±0.14ab | 3.24±0.23a | 0.10 | 0.07 | |
| Incertae_Sedis | 2.05±0.10b | 2.11±0.01b | 2.36±0.07a | 2.01±0.11b | 2.08±0.05b | 0.04 | 0.01 | |
| Treponema | 1.97±0.21a | 2.11±0.12a | 1.68±0.20a | 2.02±0.21a | 3.04±0.33b | 0.15 | 0.02 | |
| Pseudobutyrivibrio | 1.75±0.10a | 1.66±0.22ab | 1.47±0.13b | 1.71±0.11a | 1.64±0.04b | 0.04 | 0.14 | |
| Victivallis | 1.58±0.02a | 1.38±0.01ab | 1.38±0.02ab | 1.34±0.04ab | 1.14±0.02b | 0.06 | 0.31 | |
| Butyrivibrio | 1.32±0.04 | 1.44±0.07 | 1.45±0.07 | 1.41±0.06 | 1.31±0.05 | 0.04 | 0.67 | |
| Ruminococcus | 1.29±0.06 | 1.23±0.16 | 1.31±0.06 | 1.15±0.05 | 1.22±0.06 | 0.03 | 0.31 | |
| Anaeroplasma | 0.49±0.10 | 0.46±0.04 | 0.50±0.01 | 0.46±0.07 | 0.59±0.08 | 0.03 | 0.72 | |
| Oligosphaera | 0.27±0.08 | 0.31±0.05 | 0.27±0.05 | 0.30±0.04 | 0.39±0.02 | 0.02 | 0.11 | |
| Fibrobacter | 0.13±0.01 | 0.14±0.04 | 0.15±0.05 | 0.17±0.01 | 0.15±0.02 | 0.01 | 0.78 | |
| With the same row different lowercase letters mean significant at P < 0.05 and different capital letters mean significant at P < 0.01. | ||||||||
表选项
3 Discussion Zinc-bearing palygorskite (Zn-Pal) could replace antibiotics widely used in poultry production due to their antimicrobial activity. Here we tested the effects of Zn-Pal on microbial composition from dairy cattle, in order to evaluate its potential application in this setting. The change of BET surface area and cation exchange capacity is due to the process of zinc-bearing. The change of zeta potential is due to the dissociation and dispersion of the palygorskite crystal beam and the exchange of sodium ions with metal ions on the surface of palygorskite. In our study, the Zeta potentials of palygorskite were negative, and Zn-Pal was more negative that palygorskite alone. The palygorskite particle can be considered as an anion with large size and high charge density, which attracts the ions of opposite sign (counterions) and repels the ions of the same sign (co-ions)[27]. While at the same ion valency, the thickness of the diffuse layer might reduce due to increase in the concentration of electrolyte[28]. Therefore, the difference in the surface structures between palygorskite and Zn-Pal might indicate that they would have differential effects on microbial composition.
The ruminant rumen has abundant bacterial population and is similar to other vertebrate digestive tract microflora. Bacteria account for about 50% to 80% of the rumen biomass. Bacteroides and Firmicutes are the two major categories of rumen bacteria, accounting for about 80% of the total, in addition to the less abundant Spirochaetes, Actinobacteria and Proteobacteria[29-30]. Bacteroides plays an important role in the degradation of non-fibrous materials, and the thick-walled bacteria are mainly decomposing Firmicutes[31]. In our study, the Bacteroidetes and Firmicutes also were the most abundant in samples obtained from all treatment groups and this dominant position is not altered with changes of Zn-Pal levels in the diet. Proteobacteria is a gram-negative bacterium whose outer membrane is mainly composed of LPS. Many bacterial belong to the micro-aerobic and facultative anaerobic types. There are five main categories of which Proteobacteria contains a variety of pathogens, such as E. coli, Salmonella. Li[32] have reported that zinc-bearing palygorskite (ZnBP) has an antibacterial activity on E. coli K88 in an artificial gastroinestinal pH environment from piglets. Xu et al.[10]proved the antibacterial rates of the coatings for E. coli and Staphylococcus aureus exceeded 99.0% when the content of zinc-typed antibacterial agent was at 3% in coatings. Wang et al.[33] suggested that the addition of zinc-bearing clinoptilolite (ZnCP) to feed exerted protective effects on performance and gut health of broilers against S. pullorum infection. Moreover, Tang et al.[15] indicated that zinc-bearing clinoptilolite (ZnCP) supplementation may modulate digestive enzyme activities and intestinal structure and function of broiler chickens. In our study, Proteobacteria in treatment Ⅳ group was lower than control at all times, while Proteobacteria in treatment groups Ⅰ, Ⅱ and Ⅲ became higher than in the control. This can be partially explained by the fact that a low dose of Zn-Pal may benefit the growth of beneficial bacteria, and a high dose of Zn-Pal may inhibit the propagation of harmful bacteria. This could be caused by fact that the Zn2+ content on the surface of the embankment is higher than that of the bacteria, and therefore the antibacterial activity is improved. The slow release of Zn2+ penetrates the negatively charged microbial cell membrane, destroys the activity of the cell synthetic machinery, interferes with DNA synthesis, and blocks division and proliferation [16, 34].
Prevotella degrades starch and is the major flora of proteolytic bacteria in the rumen[35]. This study also detected that Prevotella content was the highest in the rumen of all treatment groups, and it was confirmed that Prevotella was the dominant flora of the rumen based on traditional culture and various molecular biology methods[36-37].In our study, the number of Prevotella increased gradually with the increase in the amount of Zn-Pal. Fibrobacter, Ruminococcus and Butyrivibrio are the major fiber degrading bacteria. In our study, compared with control group, in all treatment groups the three types of bacteria are reduced. We speculated that this change could be due to the inhibitory effect of Zn on the digestion of fiber by rumen microbes[38]. Arelovich et al.[39] have proved that Zn tends to selectively inhibit growth, or metabolic activities, of rumen microbes. Vázquezarmijo et al.[40] have found that Zn can reduce the cellulolytic activity of bacteria in the inoculation medium. In conclusion, Zn-Pal affected rumen fluid bacterial diversity in vitro, and its impact varied with the application dose and time. Zn-Pal might be a good alternative to antibotics in dairy cattle.
References
| [1] | Hespell RB, Akin DE, Dehority BA. Bacteria, fungi, and protozoa of the rumen//Mackie RI, White BA, Isaacson RE. Gastrointestinal Microbiology, vol 2. Gastrointestinal Microbes and Host Interactions. New York, NY: Chapman & Hall, 1997. |
| [2] | Mackie RI. Mutualistic fermentative digestion in the gastrointestinal tract:diversity and evolution. Integrative and Comparative Biology, 2002, 42(2): 319-326. DOI:10.1093/icb/42.2.319 |
| [3] | Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(13): 4596-4601. DOI:10.1073/pnas.0400706101 |
| [4] | Walker CF, Black RE. Zinc and the risk for infectious disease. Annual Review of Nutrition, 2004, 24: 255-275. DOI:10.1146/annurev.nutr.23.011702.073054 |
| [5] | Kennedy DW, Bunting LD. Alterations in ruminal utilization of magnesium and zinc in lambs fed different ratios of concentrate:forage. International Journal for Vitamin and Nutrition Research, 1991, 61(1): 67-71. |
| [6] | Chen YP, Cheng YF, Li XH, Zhang H, Yang WL, Wen C, Zhou YM. Dietary palygorskite supplementation improves immunity, oxidative status, intestinal integrity, and barrier function of broilers at early age. Animal Feed Science and Technology, 2016, 219: 200-209. DOI:10.1016/j.anifeedsci.2016.06.013 |
| [7] | Chalvatzi S, Kalamaki MS, Arsenos G, Fortomaris P. Dietary supplementation with the clay mineral palygorskite affects performance and beneficially modulates caecal microbiota in laying pullets. Journal of Applied Microbiology, 2016, 120(4): 1033-1040. DOI:10.1111/jam.13041 |
| [8] | Zaid MRB, Hasan M, Khan AA. Attapulgite in the treatment of acute diarrhoea:a double-blind placebo-controlled study. Journal of Diarrhoeal Diseases Research, 1995, 13(1): 44-46. |
| [9] | Zhang JM, Lü YF, Tang CH, Wang XQ. Effects of dietary supplementation with palygorskite on intestinal integrity in weaned piglets. Applied Clay Science, 2013, 86: 185-189. DOI:10.1016/j.clay.2013.10.009 |
| [10] | Xu GL, Dong FQ, Zhu GP, Deng YQ, Feng QM, Li GW. Study on zinc-type antibacterial element material and its application in coating. Journal of Sichuan University (Engineering Science Edition), 2005, 37(1): 52-56. (in Chinese) 徐光亮, 董发勤, 朱桂平, 邓跃全, 冯启明, 李国武. 锌型抗菌基元材料及其在涂料中的应用研究. 四川大学学报(工程科学版), 2005, 37(1): 52-56. |
| [11] | Luo YW, Wang LC, Guo F, Zhou YM, Wang T. Effects of Zinc-bearing attapulgite on growth performance, immunity and serum biochemical indexes of broilers. Journal of the Chinese Cereals and Oils Association, 2008, 23(3): 131-135. (in Chinese) 罗有文, 王龙昌, 郭芳, 周岩民, 王恬. 载Zn2+凹凸棒石黏土对肉鸡生长、免疫和血液生化指标的影响. 中国粮油学报, 2008, 23(3): 131-135. |
| [12] | Zhang ZF, Wang WB, Wang AQ. Highly effective removal of Methylene Blue using functionalized attapulgite via hydrothermal process. Journal of Environmental Sciences, 2015, 33(7): 106-115. |
| [13] | Hu CH, Gu LY, Luan ZS, Song J, Zhu K. Effects of montmorillonite-zinc oxide hybrid on performance, diarrhea, intestinal permeability and morphology of weanling pigs. Animal Feed Science and Technology, 2012, 177(1/2): 108-115. |
| [14] | Hu CH, Qian ZC, Song J, Luan ZS, Zuo AY. Effects of zinc oxide-montmorillonite hybrid on growth performance, intestinal structure, and function of broiler chicken. Poultry Science, 2013, 92(1): 143-150. DOI:10.3382/ps.2012-02250 |
| [15] | Tang ZG, Wen C, Li P, Wang T, Zhou YM. Effect of zinc-bearing zeolite clinoptilolite on growth performance, nutrient retention, digestive enzyme activities, and intestinal function of broiler chickens. Biological Trace Element Research, 2014, 158(1): 51-57. DOI:10.1007/s12011-014-9900-3 |
| [16] | Tang ZG, Wen C, Wang LC, Wang T, Zhou YM. Effects of zinc-bearing clinoptilolite on growth performance, cecal microflora and intestinal mucosal function of broiler chickens. Animal Feed Science and Technology, 2014, 189: 98-106. DOI:10.1016/j.anifeedsci.2013.12.014 |
| [17] | Yan R, Zhang L, Yang X, Wen C, Zhou YM. Bioavailability evaluation of zinc-bearing palygorskite as a zinc source for broiler chickens. Applied Clay Science, 2015, 119: 155-160. |
| [18] | Qiao LH, Chen YP, Wen C, Zhou YM. Effects of natural and heat modified palygorskite supplementation on the laying performance, egg quality, intestinal morphology, digestive enzyme activity and pancreatic enzyme mRNA expression of laying hens. Applied Clay Science, 2015, 104: 303-308. DOI:10.1016/j.clay.2014.12.010 |
| [19] | Theodorou MK, Williams BA, Dhanoa MS, Mcallan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science and Technology, 1994, 48(3/4): 185-197. |
| [20] | Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nature Methods, 2008, 5(3): 235-237. DOI:10.1038/nmeth.1184 |
| [21] | Edgar RC. UPARSE:highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 2013, 10(10): 996-998. DOI:10.1038/nmeth.2604 |
| [22] | Chao A. Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics, 1984, 11(4): 265-270. |
| [23] | Chao A, Lee SM. Estimating the number of classes via sample coverage. Journal of the American Statistical Association, 1992, 87(417): 210-217. DOI:10.1080/01621459.1992.10475194 |
| [24] | Brown KR. Diagnosis and management of anaerobic infections. Tropical Doctor, 1977, 7(3): 111-114. DOI:10.1177/004947557700700308 |
| [25] | Wang Q, Garrity GM, Tiedje JM, Cole JR. Na?ve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 2007, 73(16): 5261-5267. DOI:10.1128/AEM.00062-07 |
| [26] | Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Gl?ckner FO. The SILVA ribosomal RNA gene database project:improved data processing and web-based tools. Nucleic Acids Research, 2012, 41(D1): D590-D596. DOI:10.1093/nar/gks1219 |
| [27] | Marchuk A, Rengasamy P. Clay behaviour in suspension is related to the ionicity of clay-cation bonds. Applied Clay Science, 2011, 53(4): 754-759. DOI:10.1016/j.clay.2011.05.019 |
| [28] | Rusmin R, Sarkar B, Biswas B, Churchman J, Liu YJ, Naidu R. Structural, electrokinetic and surface properties of activated palygorskite for environmental application. Applied Clay Science, 2016, 134: 95-102. DOI:10.1016/j.clay.2016.07.012 |
| [29] | Kittelmann S, Seedorf H, Walters WA, Clemente JC, Knight R, Gordon JI, Janssen PH. Simultaneous amplicon sequencing to explore co-occurrence patterns of bacterial, archaeal and eukaryotic microorganisms in rumen microbial communities. PLoS One, 2013, 8(2): e47879. DOI:10.1371/journal.pone.0047879 |
| [30] | Tajima K, Nonaka I, Higuchi K, Takusari N, Kurihara M, Takenaka A, Mitsumori M, Kajikawa H, Aminov RI. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe, 2007, 13(2): 57-64. DOI:10.1016/j.anaerobe.2006.12.001 |
| [31] | Evans NJ, Brown JM, Murray RD, Getty B, Birtles RJ, Hart CA, Carter SD. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Applied and Environmental Microbiology, 2011, 77(1): 138-147. DOI:10.1128/AEM.00993-10 |
| [32] | Li XH, Zhu YP, Wen C, Zhou YM. Zinc desorption and antibacterial activity on E. coli K88 of Zinc-bearing Palygorskite. Non-Metallic Mines, 2015, 38(3): 9-12. (in Chinese) 李晓晗, 朱玉萍, 温超, 周岩民. 载锌凹凸棒石黏土中锌的解吸及抑菌作用研究. 非金属矿, 2015, 38(3): 9-12. |
| [33] | Wang LC, Zhang TT, Wen C, Jiang ZY, Wang T, Zhou YM. Protective effects of zinc-bearing clinoptilolite on broilers challenged with Salmonella pullorum. Poultry Science, 2012, 91(8): 1838-1845. DOI:10.3382/ps.2012-02284 |
| [34] | Xia JL, Wang C, Liu XX. Research on antimicrobial agents and their mechanisms of actions. Journal of Central South University (Natural Science), 2004, 35(1): 31-38. (in Chinese) 夏金兰, 王春, 刘新星. 抗菌剂及其抗菌机理. 中南大学学报(自然科学版), 2004, 35(1): 31-38. |
| [35] | Wallace RJ, Onodera R, Cotta MA. Metabolism of nitrogen-containing compounds//Hobson PN, Stewart CS. The Rumen Microbial Ecosystem. Dordrecht, Netherlands: Springer, 1997: 283-328. |
| [36] | Bekele AZ, Koike S, Kobayashi Y. Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiology Letters, 2010, 305(1): 49-57. DOI:10.1111/fml.2010.305.issue-1 |
| [37] | Jami E, Mizrahi I. Similarity of the ruminal bacteria across individual lactating cows. Anaerobe, 2012, 18(3): 338-343. DOI:10.1016/j.anaerobe.2012.04.003 |
| [38] | Eryavuz A, Dehority BA. Effects of supplemental zinc concentration on cellulose digestion and cellulolytic and total bacterial numbers in vitro. Animal Feed Science and Technology, 2009, 151(3/4): 175-183. |
| [39] | Arelovich HM, Owens FN, Horn GW, Vizcarra JA. Effects of supplemental zinc and manganese on ruminal fermentation, forage intake, and digestion by cattle fed prairie hay and urea. Journal of Animal Science, 2000, 78(11): 2972-2979. DOI:10.2527/2000.78112972x |
| [40] | Vázquez-Armijo JF, Martínez-Tinajero JJ, López D, Salem AFZM, Rojo R. In vitro gas production and dry matter degradability of diets consumed by goats with or without copper and zinc supplementation. Biological Trace Element Research, 2011, 144(1/3): 580-587. |
