董明杰, 杨云娟, 唐湘华, 李俊俊, 黄遵锡


云南师范大学生命科学学院, 生物能源持续开发利用教育部工程中心, 云南省生物质能与环境生物技术重点实验室, 云南省酶工程技术研究中心, 云南 昆明 650500
收稿日期:2016-01-04;修回日期:2016-03-18;网络出版日期:2016-03-31
基金项目:国家自然科学基金(31160229);云南省教育厅科学研究基金(2015Y104)
*通信作者:Tel: +86-871-5920830; Fax: +86-871-5920952. E-mail:huangzunxi@163.com.
摘要: [目的]克隆温泉中嗜热嗜酸的脂环酸芽孢杆菌D-1(Alicyclobacillus tengchongensis CGMCC1504)的内切葡聚糖酶基因gluE1,并对该酶进行序列分析和重组酶的酶学特性分析。[方法]通过全基因组测序获得gluE1全长,并对其氨基酸序列(GluE1)进行分析。将gluE1重组到载体pEASY-E2中并转化到大肠杆菌BL21(DE3)中异源表达,利用组氨酸标签纯化GluE1并进行酶学性质分析。[结果]gluE1与NCBI数据库中GH5的内切葡聚糖酶具有较高的相似性,全长1020 bp,GC含量50.5%,编码339个氨基酸(40.45 kDa)。GluE1与数据库中序列的最高一致性为97%,与其余纤维素酶的一致性<60%。GluE1可水解CMC-Na、可溶性淀粉和大麦β-葡聚糖,表观最适pH为6.5,pH 5.0-10.0稳定并维持60%以上的酶活性。GluE1的表观最适温度为55℃,在37℃下稳定。在55℃ pH 6.5条件下,GluE1对大麦β-葡聚糖的Km、Vmax和kcat分别为8.58 mg/mL、416.67 U/mg和280.90 s-1。GluE1受Ag+、Hg2+及SDS抑制,β-巯基乙醇、Pb2+、Mg2+、Ca2+和Na+对GluE1有微弱的促进作用,NaCl对GluE1的影响不大,加入30%的NaCl,仍有64%以上的酶活性;经30%的NaCl在37℃下处理60 min,仍能保持93%以上的活性。[结论]首次报道从Alicyclobacillus属的细菌中克隆得到内切葡聚糖酶基因并对其酶学性质进行研究,GluE1具有良好的pH稳定性和有较强的耐盐性,可能具有更大应用潜力。
关键词: 纤维素酶 内切葡聚糖酶 异源表达 酶学特性
Gene cloning, heterologous expression and enzyme characterizations of halo-tolerant endoglucanase from Alicyclobacillus D-1
Dong Mingjie, Yang Yunjuan, Tang Xianghua, Li Junjun, Huang Zunxi


Engineering Research Center of Sustainable Development and Utilization of Biomass Energy, Ministry of Education, Key Laboratory of Yunnan for Biomass Energy and Biotechnology of Environment, Key Laboratory of Enzyme Engineering, College of Life Sciences, Yunnan Normal University, Kunming 650500, Yunnan Province, China
Received 04 January 2016; Revised 18 March 2016; Published online 31 March 2016
Supported by the National Natural Science Foundation of China (31160229) and by the Scientific Research Foundation of Yunnan Province (2015Y104)
Abstract: [Objective] An endoglucanase gene (gluE1) was cloned from a thermalacidophilus (Alicyclobacillus tengchongensis CGMCC1504) isolated from a hot spring, and the sequence and biochemical characterization of enzyme were analyzed. [Methods] The full-length gluE1 was obtained based on genome sequencing, analysis of amino acid sequence of GluE1. gluE1 was ligated into pEASY-E2 vector and expressed in Escherichia coli BL21 (DE3) cells. GluE1 was purified to electrophoretic homogeneity by Ni2+-NTA metal chelating affinity chromatography, and then the enzyme characterizations were determined. [Results] The 1020 bp full-length gluE1 (50.5% GC content) encodes a 339 residues polypeptide (GluE1: 40.45 kDa). GluE1 showed the highest identity of 97% with endoglucanase in public databases, and <60% identities with other endoglucanase. GluE1 efficiently hydrolyzed CMC-Na, soluble starch and barley-β-glucan, which showed apparent optimal at pH 6.5 and 55℃. GluE1 was stable and active (>60%) at pH 5.0-10.0, and had a high stability at 37℃; and it exhibited Km, Vmax and kcat values of 8.58 mg/mL, 416.67 U/mg and 280.90 s-1 respectively. GluE1 was strongly inhibited by Ag+, Hg2+ and SDS, partial promoted by β-Mercaptoethanol, Pb2+, Mg2+, Ca2+ and Na+, 30% NaCl still retains more than 64% of the activity. The residual enzyme activity kept 93% after pre-incubation of the enzyme in 30% NaCl. [Conclusion] Endoglucanase gene gluE1 from Alicyclobacillus was first reported, and GluE1 showed a good pH stability and strong halo-tolerant property. GluE1 might have greater potential applications.
Key words: cellulose endoglucanase heterologous expression enzyme characterizations
纤维素是地球上最丰富的可再生资源,如果能充分利用纤维素资源,将对缓解全球的能源危机,食品和饲料资源紧张以及环境污染有重大意义[1]。纤维素酶是指所有参与降解纤维素的各种酶的总称,广泛存在于多种微生物中,由3种酶组成:内切-1,4-β-D-葡聚糖酶(endo-1,4-β-D-glucanases,EC 3.2.1.4,EG);1,4-β-D-纤维二糖水解酶(1,4-β-D-cellobiohydro-lases,EC 3.2.1.91,CBH);1,4-β-D-葡萄糖苷酶(1,4-β-D-glucosidases,EC 3.2.1.21,BGL)。纤维素通过3种酶的协同作用,最终降解为葡萄糖[2]。其中内切-1,4-β-D-葡聚糖酶是纤维素酶系最主要的成分,它首先作用于纤维素,随机水解纤维素分子内部的β-1,4-糖苷键,将纤维素链打断,对整个纤维素的降解起到至关重要的作用。内切葡聚糖酶无处不在,广泛地被细菌,真菌,植物和昆虫等生物所产生[3-4]。然而较多内切葡聚糖酶来源于微生物,目前已经有很多内切葡聚糖酶在细菌和丝状真菌中被发现[5]。同时,内切葡聚糖酶在纤维素酶家族中还是最多样的一类酶,分布于11个糖苷水解酶家族中,即GH5、6、7、8、9、12、44、45、48、51和74家族[6],其中大多数的内切葡聚糖酶分布于GH6、7、8、9、12和45家族[7]。GH5家族的内切葡聚糖酶已有很多报道,如第1个内切葡聚糖酶在P. janthine llum中发现[8];2004年Posta等在Thermobifida fusca发现1个较新的GH5家族的内切葡聚糖酶[9];2009年Watsan等在海洋细菌Saccharophagus degradans中发现GH5家族的内切葡聚糖酶,并对纤维素有较好的降解作用[10]。
近年的研究报告显示,内切葡聚糖酶在食品、动物饲料、洗涤剂,纺织工业和造纸等工业上有潜在的应用[11-13]。这些潜在的应用使得内切葡聚糖酶的需求大大增加,这使得研究出性质更优的,特别是在pH和温度上有更大突破的内切葡聚糖酶越来越多[14-15]。除此之外,碱性多种纤维素酶,特别是碱性内切葡聚糖酶在洗涤剂方面的应用也被广泛研究,在1988年,一种碱性的内切葡聚糖酶第1次被应用于洗衣粉中[16],之后,越来越多来源于细菌的碱性纤维素酶被广泛用于世界洗涤行业。与碱性纤维素酶相比,仅仅有少数耐盐或嗜盐的纤维素酶被报道[17-18]。Hirasawa等从1株耐盐和嗜碱的杆菌Bacillus agaradhaerens发现了耐盐的内切葡聚糖酶[17],Wang等从1株嗜盐的杆菌Salinivibrio sp. strain NTU-05研究得到活性较高的内切葡聚糖酶,并对其性质和特征进行研究,提出了耐盐的纤维素酶能够治理农业废弃物和纤维素类的生物材料的修复[19]。
综上所述,鉴于内切葡聚糖酶在现代工业生产中的重要地位,本课题首次开展从脂环酸芽孢杆菌D-1中克隆GH5家族的内切葡聚糖酶基因,异源表达并分析其酶学性质,旨在获得性质优良的新型耐盐内切葡聚糖酶,这对挖掘云南温泉环境中蕴藏的具有应用潜力的新型酶制剂具有重要意义。
1 材料和方法 1.1 主要试剂和仪器 基因组提取试剂盒及质粒小提试剂盒购于天根生化科技(北京)有限公司;DNA聚合酶和dNTPs购于日本TaKaRa公司;pEASY-E2表达载体购于北京全式金生物技术有限公司;Nickel-NTA树脂购于美国Qiagen公司;大麦β-葡聚糖购于美国Sigma公司;昆布多糖、燕麦木聚糖和微晶纤维素购于北京百灵威科技有限公司;其余试剂皆为国产分析纯。细胞超声波破碎仪购于宁波新芝生物科技股份有限公司;离心机购于德国Eppendorf公司;PCR仪购于北京东胜创新生物科技有限公司;核酸电泳仪、蛋白电泳仪及凝胶成像仪购于美国BIO-RAD公司。
1.2 样品、菌株和载体 大肠杆菌(Escherichia coli) Trans-T1,BL21 (DE3)和载体pEASY-E2购于北京全式金公司,菌株A. tengchongensis CGMCC1504来自于本实验室筛选。
1.3 序列分析 根据A. tengchongensis CGMCC1504基因组功能注释,对注释纤维素酶基因于NCBI数据库里进行序列比对分析;DNA之间和蛋白质之间的在线比对分别使用BLASTn和BLASTp (http://blast.ncbi. nlm.nih.gov/Blast.cgi);信号肽的预测使用SignalP 4.1 (http://www.cbs.dtu.dk/serv-ices/SignalP/);糖苷水解酶的分类使用dbCAN (http://csbl.bmb.uga.edu/ dbCAN/annotate.php);多序列比对使用MEGA 6[20]软件。
1.4 获得重组菌株BL21(DE3)/gluE1 根据A.tengchongensis CGMCC1504基因组功能注释、gluE1基因序列分析和载体信息设计引物Dgh5F/Dgh5R,由昆明擎科生物公司合成内切葡聚糖酶GluE1引物:Dgh5F:5′-ATGTCTGGCGTCAACCTTGG-3′;Dgh5R:5-GCGGGCGCTTACGATGCGAACTAATTC-3′。
利用基因组提取试剂盒提取菌株A.tengchon- gensis CGMCC1504的基因组(方法参照试剂盒说明书)。以A. tengchongensis CGMCC1504基因组为模板,用引物Dgh5F/Dgh5R扩增,扩增条件为:94 ℃ 5 min;95 ℃ 30 s,70-56 ℃ 30 s (每个循环降0.5 ℃),72 ℃ 2 min,28个循环;95 ℃ 30 s,56 ℃ 30 s,72 ℃ 2 min,7个循环;72 ℃ 10 min。将PCR产物连接pEASY-E2载体,转化大肠杆菌Trans1-T1后(方法参照试剂盒说明书),提取所有阳性克隆子的混合质粒(方法参照试剂盒说明书),获得含有gluE1的重组质粒pEASY-E2-gluE1。将该质粒转化大肠杆菌BL21(DE3)后,获得重组大肠杆菌菌株BL21(DE3)/gluE1,并送到捷瑞基因组测序中心测序以进行验证。
1.5 蛋白表达与纯化 取重组大肠杆菌菌株BL21(DE3)/gluE1,以0.1%的接种量接种于LB(含0.1 mg/mL Amp)培养液中,37 ℃快速振荡16 h。然后将此活化的菌液以1%接种量接种到新鲜的LB(含0.1 mg/mL Amp)培养液中,快速振荡培养约2-3 h (OD600达到0.6-1.0)后,加入终浓度0.7 mmol/L的IPTG进行诱导,于20 ℃继续振荡培养约20 h。8500 r/min离心5 min,收集菌体。用适量的pH 7.0 McIlvaine buffer悬浮菌体后,于低温水浴下超声波破碎菌体。以上胞内浓缩的初酶液经12000 r/min离心10 min,吸取上清并用Nickel-NTA树脂纯化目的蛋白。纯化的蛋白进行12%的SDS-聚丙烯酰胺凝胶电泳(SDS-PAGE)分析和Bradford法进行蛋白定量。
1.6 酶学特性分析 重组内切葡聚糖酶GluE1的活性测定方法采用DNS定糖法:将大麦β-葡聚糖溶于缓冲液中,使其终浓度为0.7% (W/V);反应体系含100 μL稀释酶液,900 μL的底物;底物在反应温度下预热5 min后,加入酶液反应10 min,然后加1.5 mL DNS终止反应,冷却至室温后在540 nm波长下测定OD值;1个酶活单位(U)定义为在给定条件下每分钟分解底物产生1 μmol还原糖所需的酶量。缓冲液为:0.2 mol/L McIlvaine buffer (pH 2.0-8.0)、0.1 mol/L Tris-HCl (pH 8.0-9.0)和0.2 mol/L glycine-NaOH (pH 9.0-12.0)。
酶的底物特异性:将纯化的GluE1在50 ℃下和pH 5.0的含0.7%不同底物缓冲液中进行酶促反应。酶的最适pH测定:将纯化的GluE1在50 ℃下和pH 2.0-12.0的缓冲液中进行酶促反应。酶的最适温度测定:在pH 6.5的缓冲液中,于10-80 ℃下进行酶促反应。酶的pH稳定性测定:将纯化的酶液置于pH 2.0-12.0的缓冲液中,在37 ℃下处理1 h,然后在pH 6.5及55 ℃下进行酶促反应,以未处理的酶液作为对照。酶的热稳定性测定:将同样酶量的酶液置于37、55、60 ℃中,处理0-60 min后,在pH 6.5及55 ℃下进行酶促反应,以未处理的酶液作为对照。酶的动力学参数测定:用0.05%-1.5%大麦β-葡聚糖为底物,在pH 6.5和55 ℃下,根据Lineweaver-Burk方法测定Km、Vmax和kcat。酶的金属离子和有机试剂抗性测定:在酶促反应体系中加入终浓度为1 mmol/L和10 mmol/L的CaCl2、CuSO4、NiSO4、CoCl2、MgSO4、KCl、ZnSO4、FeCl3、Pb(CH3COO)2、MnSO4、FeSO4、HgCl2、AgNO3、NaCl、EDTA、β-Mercaptoethanol、SDS,以及终浓度分别为1.0% (V/V)和0.5% (V/V)的Tween-80和Triton X-100,在pH 6.5和55 ℃条件下测定酶活性,以不含金属离子和有机试剂的反应体系作为对照。酶的NaCl抗性测定:在酶促反应体系中加入终浓度为3%-30%的NaCl,在pH 6.5和55 ℃条件下测定酶活性,以不含NaCl的反应体系作为对照。酶的NaCl稳定性测定:将纯化的酶液置于3%-30% 的NaCl水溶液中,在37 ℃下处理60 min,然后在pH 6.5及55 ℃下进行酶促反应,以未加NaCl但在37 ℃下保温60 min的酶液作为对照。
1.7 酶解产物分析 产物分析反应体系含4.5 mL 0.7% (W/V)的大麦β-葡聚糖,0.5 mL适当稀释酶液。在pH 6.5及55 ℃下,依次在酶促反应的1 h、3 h内终止反应并分析水解产物,其中对照为大麦β-葡聚糖和失活的稀释酶液(100 ℃下处理5 min)。产物分析采用薄层层析法[21]。
1.8 菌株序列号 菌株D-1的16S rRNA基因和内切葡聚糖酶基因gluE1收录于GenBank数据库,登录号分别为DQ351931和KP289284。
2 结果和分析 2.1 基因克隆和序列分析 利用设计的引物PCR,获得D-1的内切葡聚糖酶基因全长(gluE1),共1020 bp。起始密码子为ATG,终止密码子为TAA,GC含量为50.5%,编码339个氨基酸组成的多肽,GluE1不含信号肽,理论分子量为40.45 kDa,理论等电点为6.14。BLASTp结果表明:GluE1与NCBI数据库中GH5的内切葡聚糖酶的最高一致性为97%,与其余纤维素酶的一致性<60%;与已报道的GH5家族的内切葡聚糖酶的多序列比对如图 1,预测Glu136为GluE1的催化保守残基[22-23]。
 |
| 图 1. GluE1与糖苷水解酶第5家族内切葡聚糖酶序列的部分比对分析 Figure 1. Partial amino acid sequence alignment of GluE1 with glycosyl hydrolase family 5 endoglucanase. Sequence names are shown with accession numbers except GluE1,as follows: AB055433,Aspergillus kawachii endoglucanase[24]; COG2730,Peptoclostridium difficile BI1 endoglucanase[25]; EU379561,Penicillium brasilianumendoglucanase[26]; HQ706361,uncultured bacterium endoglucanase[27]; NUC00762,Neurospora crassa endoglucanase[28]; The asterisks show the putative catalytic residues. The symbols above the sequences indicate the secondary structure. |
| 图选项 |
2.2 gluE1的异源表达 PCR扩增得到的gluE1经连接后得到含内切葡聚糖酶基因gluE1的重组质粒pEASY-E2-gluE1,重组蛋白理论分子量为40.45 kDa,理论等电点为6.14。重组质粒pEASY-E2-gluE1成功转化大肠杆菌BL21(DE3)。阳性克隆子经测序验证正确并在超声波破碎的细胞上清液中检测到内切葡聚糖酶酶活,初步于pH 7.0、50 ℃条件下测定粗酶的比活为50.82 U/mg。经Nickel-NTA树脂纯化,去除杂蛋白,蛋白浓度为0.62 mg/mL,同样于pH 7.0、50 ℃条件下测定纯酶的比活为106.20 U/mg。纯化的蛋白进行12%的SDS-聚丙烯酰胺凝胶电泳(SDS-PAGE)分析,结果显示条带大小与理论分子量相一致(图 2)。再次经MALDI-TOP/MS分析,该蛋白条带的质谱与GluE1的13条肽段分子量相匹配,说明该蛋白条带即为GluE1 (图 3)。
 |
| 图 2. 已纯化GluE1的SDS-PAGE分析 Figure 2. SDS-PAGE analyses of GluE1. Lane 1: the crude pEASY-E2; Lane 2: the crude GluE1; Lane 3: low-molecular weight markers; Lane 4: GluE1 purified by Ni2+-NTA chelating affinity chromatography. |
| 图选项 |
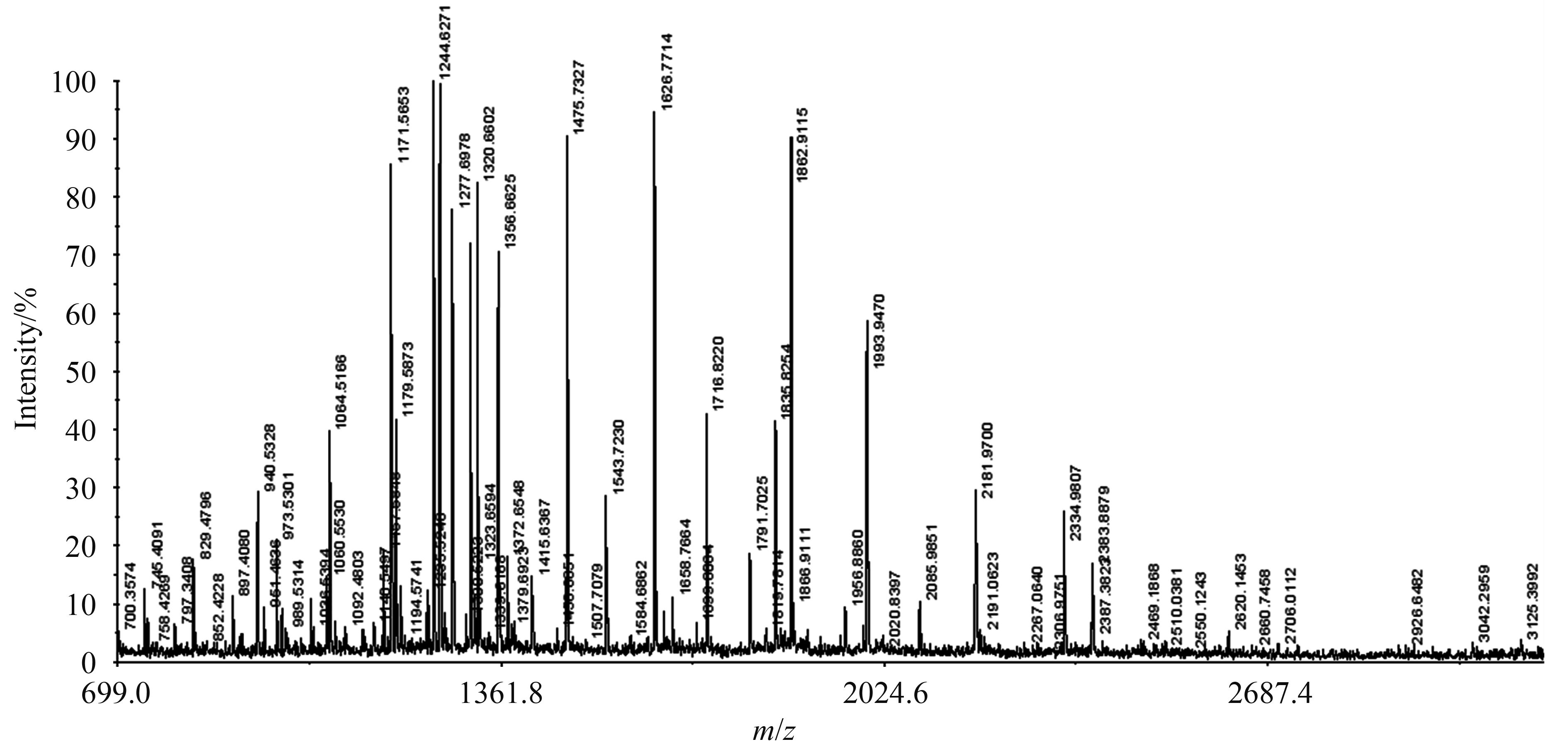 |
| 图 3. 已纯化GluE1的质谱分析 Figure 3. Identification of the band cut from the SDS-PAGE gel of the purified enzyme by mass spectrometry analysis. Mass spectrogram results of GluE1,have 13 matched peptides. |
| 图选项 |
2.3 GluE1的酶学性质 2.3.1 底物特异性:在pH 5.0和50 ℃条件下,GluE1对0.7% (W/V)CMC-Na、可溶性淀粉、大麦β-葡聚糖的比活分别为(5.90±0.33) U/mg,(6.36±0.33) U/mg和(130.66±0.69) U/mg,对昆布多糖、燕麦木聚糖和微晶纤维素均无酶活。
2.3.2 最适反应pH及pH稳定性:GluE1的表观最适pH是6.5 (图 4-A);经pH 5.0-10.0的缓冲液处理1 h后,仍能保持60%以上的活性(图 4-B)。
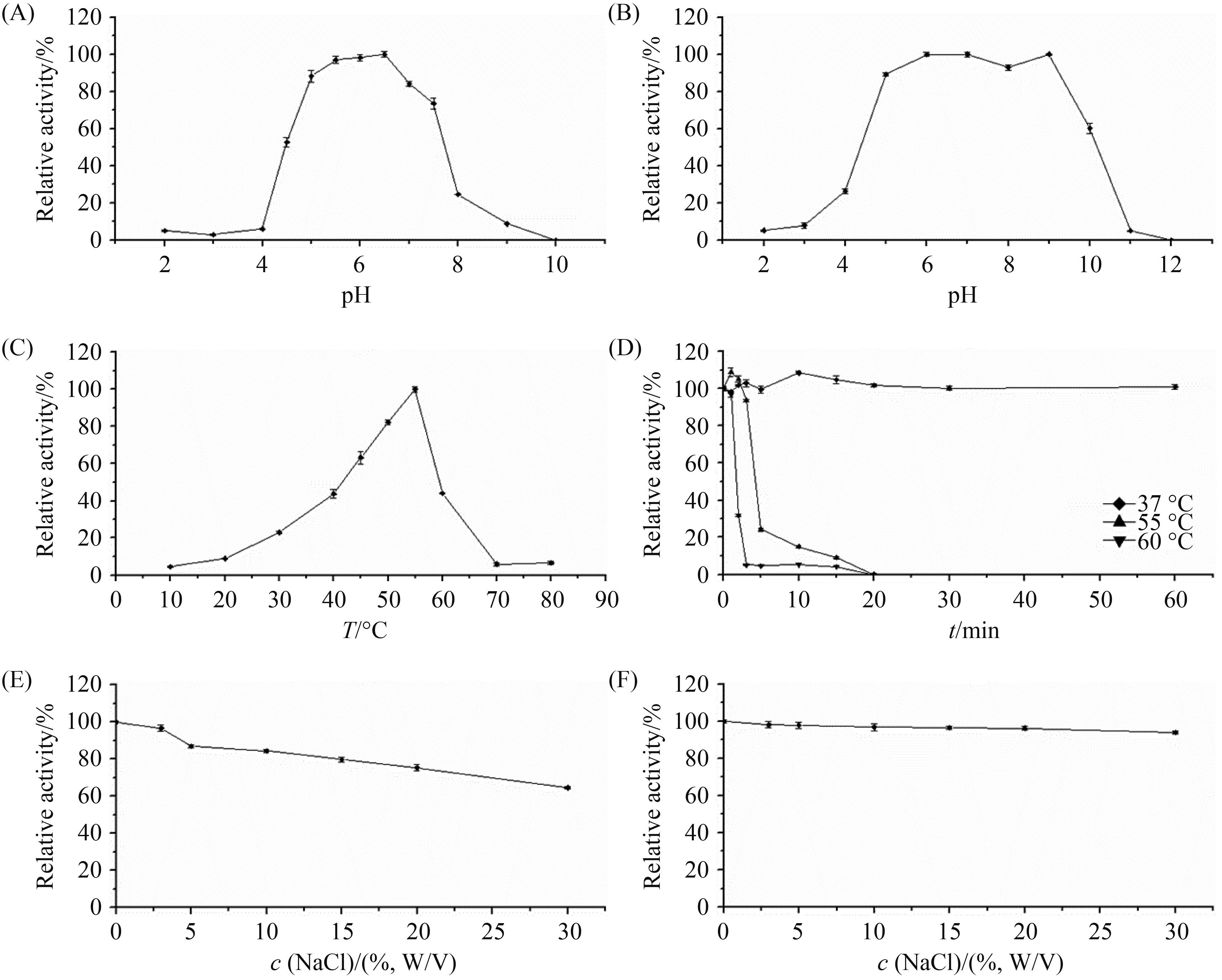 |
| 图 4. 已纯化GluE1的酶学特性 Figure 4. Characterization of the purified GluE1. A: effect of pH on GluE1. B: pH stability of GluE1; C: effect of temperature on GluE1; D: thermostability of GluE1. E: the effect of NaCl on the purified GluE1 determined at pH 6.5 and 55 ℃. F: stability of in NaCl. After pre-incubation of the enzyme in 3%–30% (W/V) NaCl at 37 ℃ for 60 min, the residual enzyme activity more than 93%. See materials and methods, enzyme characterization for details. |
| 图选项 |
2.3.3 最适温度及热稳定性:GluE1的表观最适温度为55 ℃ (图 4-C);在37 ℃下稳定,在55 ℃和60 ℃下半衰期均分别为4 min和90 s (图 4-D);
2.3.4 NaCl抗性及NaCl稳定性:3%-30%的NaCl对GluE1的影响不大,加入30%的NaCl,仍有64%以上的酶活性,体现了较强的NaCl抗性(图 4-E);经3%-30%的NaCl在37 ℃下处理60 min,仍能保持93%以上的活性,体现了较强的耐盐性(图 4-F)。
2.3.5 动力学参数:在55 ℃、pH 6.5条件下,GluE1对大麦β-葡聚糖的Km、Vmax和kcat分别为8.58 mg/mL、416.66 U/mg和280.90 s-1。
2.3.6 化学试剂的影响:1 mmol/L和10 mmol/L的Hg2+、Ag+和SDS可以完全抑制GluE1活性;1 mmol/L和10 mmol/L的β-巯基乙醇、Pb2+、Mg2+、Ca2+和Na+ 对GluE1有微弱的促进作用;其余金属离子和有机试剂对GluE1的影响较小(剩余酶活大于71%)(表 1)。
表 1. 金属离子及化学试剂对GluE1活力的影响 Table 1. Effect of metal ions and organic reagents on the activity of purified GluE1
| Reagents | Relative activity/%a | |
| 10 mmol/L | 1 mmol/L | |
| None | 100.0±0.7 | 100.0±1.1 |
| β-Mercaptoethanol | 109.9±0.8 | 103.1±0.9 |
| CaCl2 | 108.0±1.0 | 100.4±0.5 |
| PbAc | 104.4±1.1 | 102.3±2.1 |
| MgSO4 | 101.8±1.9 | 100.9±1.3 |
| NaCl | 100.7±1.3 | 100.4±1.0 |
| Tween-80 | 99.4±0.9b | 97.9±0.3c |
| ZnSO4 | 99.2±1.8 | 97.3±0.1 |
| Triton-100 | 98.9±1.0b | 88.3±1.3c |
| FeCl3 | 95.9±1.5 | 98.0±1.2 |
| KCl | 95.7±2.2 | 99.5±1.6 |
| NiSO4 | 94.7±1.6 | 96.5±0.9 |
| EDTA | 93.7±1.8 | 99.8±2.0 |
| CoCl2 | 85.3±2.3 | 99.3±0.6 |
| CuSO4 | 80.8±1.1 | 85.8±0.6 |
| FeSO4 | 72.9±0.6 | 89.8±1.4 |
| MnSO4 | 71.4±0.8 | 99.8±0.8 |
| HgCl2 | 0.0 | 0.0 |
| AgNO3 | 0.0 | 0.0 |
| SDS | 0.0 | 0.0 |
| a: values represent the means±SD (n=3) relative to the untreated control samples. b: the final concentration of Triton X-100 or Tween 80 is 1.0% (V/V). c: the final concentration of Triton X-100 or Tween 80 is 0.5% (V/V). | ||
表选项
2.3.7 TLC法分析水解产物:结果表明,GluE1可将大麦β-葡聚糖水解为纤维二糖、三糖、四糖和五糖等。说明GluE1为内切型的酶(图 5)。
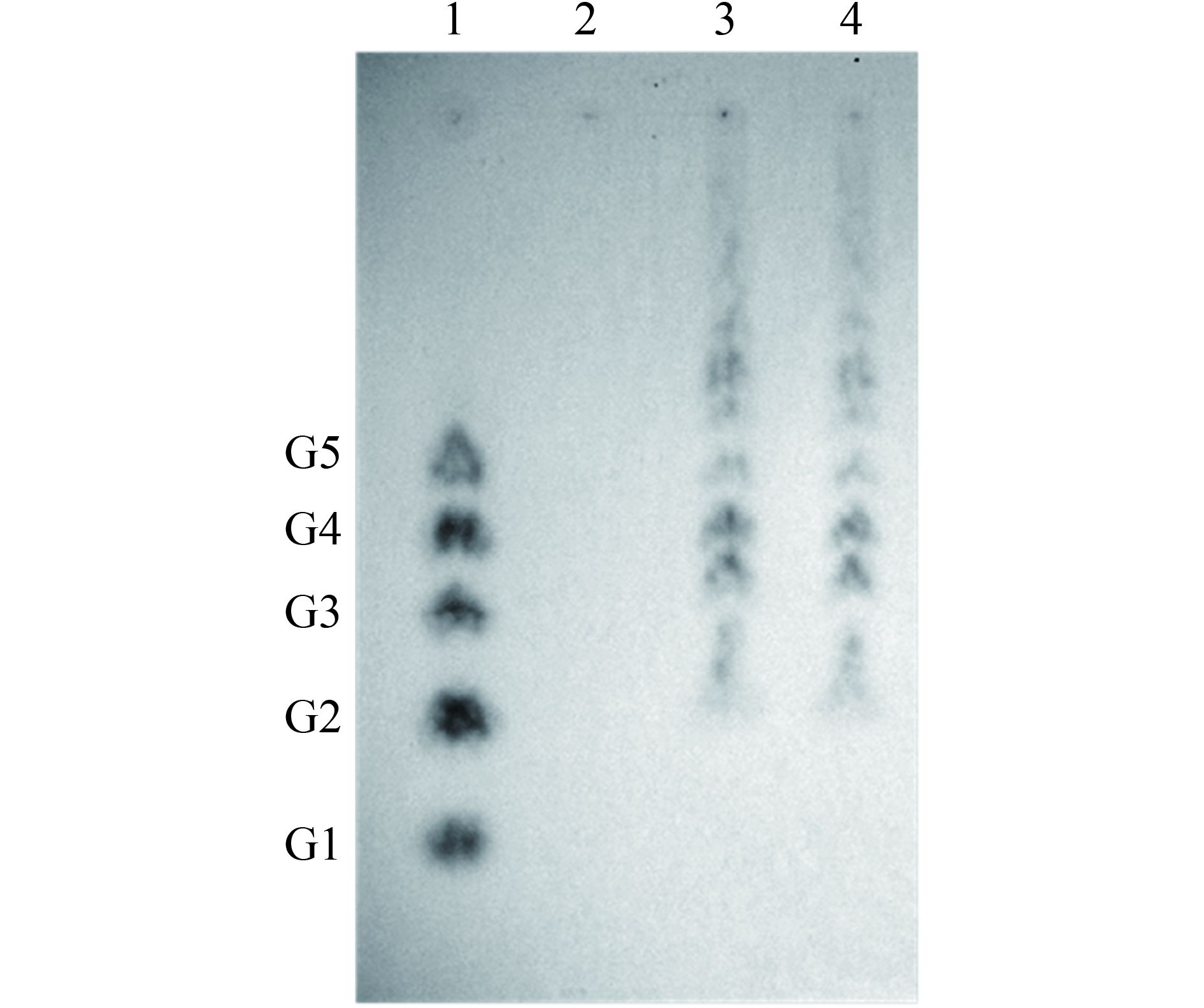 |
| 图 5. GluE1纯酶水解产物 Figure 5. Thin layer chromatography (TLC) of the hydrolysis products of 0.7% (W/V) barley-β-glucan. Lane 1: glucose (G1), cellobiose (G2), cellotriose (G3), cellotetraose (G4) and cellopentaose (G5) marker; Lane 2: barley-β-glucan with inactivated (100 ℃ for 5 min) purified GluE1; Lane 3: barley-β-glucan hydrolyzed by the purified GluE1 for 1 h (1.0 U/mL reaction system, 55 ℃,> pH 6.5, 1 h); Lane 4: barley-β-glucan hydrolyzed by the purified GluE1 for 3 h (1.0 U/mL reaction system, 55 ℃,pH 6.5, 3 h). |
| 图选项 |
3 讨论 脂环酸芽孢杆菌D-1是筛选自云南腾冲温泉中的1株嗜热嗜酸菌,属于极端微生物中的1种。然而极端微生物通常在极端环境下生存能进化出独特的适应机制,因此这些微生物在物种、基因组成和生态功能上具备有多样性,也很可能具有一些不同的代谢途径和遗传背景,这些对基因研究和生物技术应用都具有很大的价值,并且将成为新型酶资源开发的一个潜在的微生物来源。本研究的GluE1虽然没有表现出耐高温的性质,但其表现出的耐盐性这体现了极端环境来源的微生物能进化出独特的资源。
实验结果显示,GluE1对大麦β-葡聚糖(通过β-1,4和β-1,3-葡糖苷键聚合)有更高的底物特异性与羧甲基纤维素钠(通过β-1,4-葡糖苷键聚合)相比,而对昆布多糖(通过β-1,6和β-1,3-葡糖苷键聚合)没有活性,此结果与来源于Rhodothermus marinus[29],Rhizopus oryzae[30]和Bacillus sphaericus JS1[31]能水解β-1,4-葡糖苷键的内切葡聚糖酶类似,同时,这与已报道的纤维素酶Cel48Y的性质类似(对大麦β-葡聚糖有更高的活性与羧甲基纤维素钠相比)[32],这进一步说明GluE1是通过β-1,4-葡糖苷键聚合在一起的,除此之外,GluE1能降解多种β-葡聚糖,这使之成为非常有用的生产生物燃料的材料成为可能[33]。GluE1对大麦β-葡聚糖的Km、Vmax和kcat分别为8.58 mg/mL、416.66 U/mg 和280.90 s-1,展现了一个比较高的酶活力和亲和力,这与大多数GH5家族的酶特性不同,已报道的内切葡聚糖酶大多对羧甲基纤维素钠有更高的活性(73-256 U/mg)[34-36]。GluE1可将大麦β-葡聚糖水解为纤维二糖、三糖、四糖和五糖等低聚糖,说明GluE1为内切型的酶[29, 37],同时也是1种不同的内切葡聚糖酶,这一性质使GluE1比较适合于三维结构测定等应用程序,特别是应用于生物燃料方面[38]。
GluE1最适作用温度和pH分别为55 ℃和6.5,这与已报道的大多数该类酶的性质相符,如Acidothermus cellulolyticus[39],Rhodothermus marinus[22],Thermobifida fusca[40]和Caldibacillus cellulovorans[41]来源的内切葡聚糖酶的最适温度和最适pH分别是55-70 ℃和pH 5.0-7.0。GluE1在pH为6.0-7.0酶活稳定,是1种中性的内切纤维素酶,在纺织工业上,用中性内切纤维素酶处理后的棉织物效果好,对织物损伤小,防沾色效果优良。同时,如果能将中性内切葡聚糖酶应用于洗涤行业将能大幅度的减小洗涤污染物对环境的影响。因此中性内切型纤维素酶在纺织可能有重要的应用价值。GluE1在pH 5.0-10.0的缓冲液中比较稳定,与目前研究的内切葡聚糖酶相比较,来自于厌氧细菌的为pH 6-12.0[42],Lepista flaccida的为pH 2.0-9.0[43],D. eschscholzii的为pH 4.0-7.0[44]和A. niger的为pH 2.0-7.0[45],GluE1有较宽泛的pH稳定性,同时,也说明GluE1是一种耐碱性的酶,这大大增加了其在洗涤剂方面的应用。
众所周知,金属离子作为一种辅酶因子或抑制因子对酶的水解有很大的影响[46]。1 mmol/L和10 mmol/L的 Hg2+、Ag+和SDS可以完全抑制GluE1活性,类似的影响已经被报道,来自于Arthrobotrys oligospora,Chalara paradoxa 和D. eschscholzii的内切葡聚糖酶[44, 47-48];1 mmol/L和10 mmol/L的β-巯基乙醇、Pb2+、Mg2+、Ca2+和Na+对GluE1有微弱的促进作用;β-巯基乙醇对该酶活力的促进作用相对明显,因此,GluE5有可能属于SH类酶,β-巯基乙醇能使这类酶回到还原态从而增加酶的活性[49]。其余金属离子和有机试剂对GluE1的影响较小(剩余酶活大于71%),这使得GluE1可能具有更大应用潜力。
值得关注的是,这是首次报道来源于脂环酸芽孢杆菌的耐盐内切葡聚糖酶。尽管GluE1来源于非嗜盐菌株,但是经3%-30%的NaCl在37 ℃下处理60 min,仍能保持93%以上的活性,这体现了较强的耐盐性。大多数文献表明,耐盐性较高的原因是酸性氨基酸所占的比例较大,嗜盐性蛋白质需要通过增加盐浓度来补偿固有的电负性[50-51]。但是,GluE1的酸性氨基酸占15.90%,理论等电点为6.14,这是与上述耐盐机理不相符,因此,还需进一步的研究。由于GluE1能适应高盐环境,这使之可能成为一种筛选和鉴别对离子液体有抵抗性纤维素的良好材料[52]。纤维素是植物细胞壁的主要组成部分[53],利用纤维素酶能有效的应用于植物中有益营养成分的提取,特别是内切葡聚糖酶能应用于从盐含量较高的植物中提取有用物质,这将在工业生产中有很大的应用价值。同时,Wang提出了耐盐的纤维素酶能够治理农业废弃物和纤维素类的生物材料的修复[19],因而,GluE1可作为良好的出发材料,通过进一步的突变等研究以提高热稳定性等,最终使其在环境治理过程中具一定的应用潜力。
参考文献
| [1] | Lin HL, Li WG, Guo CH, Qu SH, Ren NQ. Advances in the study of directed evolution for cellulases.Frontiers of Environmental Science & Engineering in China, 2011, 5(4): 519–525. |
| [2] | Panttil? ME, André L, Saloheimo M, Lehtovaara P, Knowles JKC. Expression of two Trichoderma reesei endoglucanases in the yeast Saccharomyces cerevisiae.Yeast, 1987, 3(3): 175–185DOI:10.1002/(ISSN)1097-0061. |
| [3] | King AJ, Cragg SM, Li J, Dymond J, Guille MJ, Bowles DJ, Bruce NC, Graham IA, McQueen-Mason SJ. Molecular insight into lignocellulose digestion by a marine isopod in the absence of gut microbes.Proceedings of the National Academy of Sciences of the United States America, 2010, 107(12): 5345–5350DOI:10.1073/pnas.0914228107. |
| [4] | Koga J, Baba Y, Shimonaka A, Nishimura T, Hanamura S, Kono T. Purification and characterization of a new family 45 endoglucanase, STCE1, from Staphylotrichum coccosporum and its overproduction in Humicola insolens.Applied and Environmental Microbiology, 2008, 74(13): 4210–4217DOI:10.1128/AEM.02747-07. |
| [5] | Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology.Microbiology and Molecular Biology Reviews, 2002, 66(3): 506–577DOI:10.1128/MMBR.66.3.506-577.2002. |
| [6] | Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics.Nucleic Acids Research, 2009, 37(S1): D233–D238. |
| [7] | Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities.Biochemical Journal, 1991, 280(2): 309–316DOI:10.1042/bj2800309. |
| [8] | Mernitz G, Koch A, Henrissat B, Schulz G. Endoglucanase Ⅱ (EGⅡ) of Penicillium janthinellum: cDNA sequence, heterologous expression and promotor analysis.Current Genetics, 1996, 29(5): 490–495DOI:10.1007/BF02221519. |
| [9] | Posta K, Béki E, Wilson DB, Kukolya J, Hornok L. Cloning, characterization and phylogenetic relationships of cel5B, a new endoglucanase encoding gene from Thermobifida fusca.Journal of Basic Microbiology, 2004, 44(5): 383–399DOI:10.1002/(ISSN)1521-4028. |
| [10] | Watson BJ, Zhang HT, Longmire AG, Moon YH, Hutcheson SW. Processive endoglucanases mediate degradation of cellulose by Saccharophagus degradans.Journal of Bacteriology, 2009, 191(18): 5697–5705DOI:10.1128/JB.00481-09. |
| [11] | Marques S, Pala H, Alves L, Amaral-Colla?o MT, Gama FM, Gírio FM. Characterisation and application of glycanases secreted by Aspergillus terreus CCMI 498 and Trichoderma viride CCMI 84 for enzymatic deinking of mixed office wastepaper.Journal of Biotechnology, 2003, 100(3): 209–219DOI:10.1016/S0168-1656(02)00247-X. |
| [12] | Oksanen T, Pere J, Paavilainen L, Buchert J, Viikari L. Treatment of recycled kraft pulps with Trichoderma reesei hemicellulases and cellulases.Journal of Biotechnology, 2000, 78(1): 39–48DOI:10.1016/S0168-1656(99)00232-1. |
| [13] | Wood TM. Properties of cellulolytic enzyme systems.Biochemical Society Transactions, 1985, 13(2): 407–410DOI:10.1042/bst0130407. |
| [14] | Bhat MK. Cellulases and related enzymes in biotechnology.Biotechnology Advances, 2000, 18(5): 355–383DOI:10.1016/S0734-9750(00)00041-0. |
| [15] | Dong JL, Hong YZ, Shao ZZ, Liu ZD. Molecular cloning, purification, and characterization of a novel, acidic, pH-stable endoglucanase from Martelella mediterranea.The Journal of Microbiology, 2010, 48(3): 393–398DOI:10.1007/s12275-010-9361-0. |
| [16] | Ito S. Alkaline cellulases from alkaliphilic Bacillus: enzymatic properties, genetics, and application to detergents.Extremophiles, 1997, 1(2): 61–66DOI:10.1007/s007920050015. |
| [17] | Hirasawa K, Uchimura K, Kashiwa M, Grant WD, Ito S, Kobayashi T, Horikoshi K. Salt-activated endoglucanase of a strain of alkaliphilic Bacillus agaradhaerens.Antonie van Leeuwenhoek, 2006, 89(2): 211–219DOI:10.1007/s10482-005-9023-0. |
| [18] | Voget S, Steele HL, Streit WR. Characterization of a metagenome-derived halotolerant cellulase.Journal of Biotechnology, 2006, 126(1): 26–36DOI:10.1016/j.jbiotec.2006.02.011. |
| [19] | Wang CY, Hsieh YR, Ng CC, Chan H, Lin HT, Tzeng WS, Shyu YT. Purification and characterization of a novel halostable cellulase from Salinivibrio spstrain NTU-05..Enzyme and Microbial Technology, 2009, 44(6/7): 373–379. |
| [20] | Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. Mega6: molecular evolutionary genetics analysis version 6.0..Molecular Biology and Evolution, 2013, 30(12): 2725–2729DOI:10.1093/molbev/mst197. |
| [21] | Zhou JP, Lu Q, Peng MZ, Zhang R, Mo MH, Tang XH, Li JJ, Xu B, Ding JM, Huang ZX. Cold-active and NaCl-tolerant exo-inulinase from a cold-adapted Arthrobacter sp. MN8 and its potential for use in the production of fructose at low temperatures..Journal of Bioscience and Bioengineering, 2015, 119(3): 267–274DOI:10.1016/j.jbiosc.2014.08.003. |
| [22] | Sakon J, Adney WS, Himmel ME, Thomas SR, Karplus PA. Crystal structure of thermostable family 5 endocellulase E1 from Acidothermus cellulolyticus in complex with cellotetraose.Biochemistry, 1996, 35(33): 10648–10660DOI:10.1021/bi9604439. |
| [23] | Hilge M, Gloor SM, Rypniewski W, Sauer O, Heightman TD, Zimmermann W, Winterhalter K, Piontek K. High-resolution native and complex structures of thermostable β-mannanase from Thermomonospora fusca-substrate specificity in glycosyl hydrolase family 5.Structure, 1998, 6(11): 1433–1444DOI:10.1016/S0969-2126(98)00142-7. |
| [24] | Hara Y, Hinoki Y, Shimoi H, Ito K. Cloning and sequence analysis of endoglucanase genes from an industrial fungus, Aspergillus kawachii.Bioscience, Biotechnology, and Biochemistry, 2003, 67(9): 2010–2013DOI:10.1271/bbb.67.2010. |
| [25] | Gupta V, Natarajan C, Kumar K, Prasanna R. Identification and characterization of endoglucanases for fungicidal activity in Anabaena laxa (Cyanobacteria).Journal of Applied Phycology, 2011, 23(1): 73–81DOI:10.1007/s10811-010-9539-1. |
| [26] | Krogh KBRM, Kastberg H, J?rgensen CI, Berlin A, Harris PV, Olsson L. Cloning of a GH5 endoglucanase from genus Penicillium and its binding to different lignins.Enzyme and Microbial Technology, 2009, 44(6/7): 359–367. |
| [27] | Yan X, Geng AL, Zhang J, Wei YJ, Zhang L, Qian CL, Wang QF, Wang SY, Zhou ZH. Discovery of (hemi-) cellulase genes in a metagenomic library from a biogas digester using 454 pyrosequencing.Applied Microbiology and Biotechnology, 2013, 97(18): 8173–8182DOI:10.1007/s00253-013-4927-5. |
| [28] | Sun JP, Phillips CM, Anderson CT, Beeson WT, Marletta MA, Glass NL. Expression and characterization of the Neurospora crassa endoglucanase GH5-1.Protein Expression and Purification, 2011, 75(2): 147–154DOI:10.1016/j.pep.2010.08.016. |
| [29] | Halldórsdóttir S, Thórólfsdóttir ET, Spilliaert R, Johansson M, Thorbjarnardóttir SH, Palsdottir A, Hreggvidsson Gó, Kristjánsson JK, Holst O, Eggertsson G. Cloning, sequencing and overexpression of a Rhodothermus marinus gene encoding a thermostable cellulase of glycosyl hydrolase family 12.Applied Microbiology and Biotechnology, 1998, 49(3): 277–284DOI:10.1007/s002530051169. |
| [30] | Murashima K, Nishimura T, Nakamura Y, Koga J, Moriya T, Sumida N, Yaguchi T, Kono T. Purification and characterization of new endo-1,4-β-D-glucanases from Rhizopus oryzae.Enzyme and Microbial Technology, 2002, 30(3): 319–326DOI:10.1016/S0141-0229(01)00513-0. |
| [31] | Singh J, Batra N, Sobti RC. Purification and characterisation of alkaline cellulase produced by a novel isolate, Bacillus sphaericus JS1.Journal of Industrial Microbiology and Biotechnology, 2004, 31(2): 51–56DOI:10.1007/s10295-004-0114-0. |
| [32] | Berger E, Zhang D, Zverlov VV, Schwarz WH. Two noncellulosomal cellulases of Clostridium thermocellum, Cel9I and Cel48Y, hydrolyse crystalline cellulose synergistically.FEMS Microbiology Letters, 2007, 268(2): 194–201DOI:10.1111/fml.2007.268.issue-2. |
| [33] | Buckeridge MS, Goldman GH. Routes to cellulosic ethanol.New York: Springer, 2011. |
| [34] | Duan CJ, Xian L, Zhao GC, Feng Y, Pang H, Bai XL, Tang JL, Ma QS, Feng JX. Isolation and partial characterization of novel genes encoding acidic cellulases from metagenomes of buffalo rumens.Journal of Applied Microbiology, 2009, 107(1): 245–256DOI:10.1111/jam.2009.107.issue-1. |
| [35] | Liu N, Yan X, Zhang ML, Xie L, Wang Q, Huang YP, Zhou XG, Wang SY, Zhou ZH. Microbiome of fungus-growing termites: a new reservoir for lignocellulase genes.Applied and Environmental Microbiology, 2011, 77(1): 48–56DOI:10.1128/AEM.01521-10. |
| [36] | Yeh YF, Chang SCY, Kuo HW, Tong CG, Yu SM, Ho THD. A metagenomic approach for the identification and cloning of an endoglucanase from rice straw compost.Gene, 2013, 519(2): 360–366DOI:10.1016/j.gene.2012.07.076. |
| [37] | Akita M, Kayatama K, Hatada Y, Ito S, Horikoshi K. A novel β-glucanase gene from Bacillus halodurans C-125.FEMS Microbiology Letters, 2005, 248(1): 9–15DOI:10.1016/j.femsle.2005.05.009. |
| [38] | Benhmad I, Boudabbous M, Ya?ch A, Rebai M, Gargouri A. A novel neutral, halophile Stachybotrys microspora-based endoglucanase active impact on β-glucan.Bioprocess and Biosystems Engineering, 2016, 39(4): 685–693DOI:10.1007/s00449-016-1549-1. |
| [39] | Bergquist PL, Gibbs MD, Morris DD, Te’o VSJ, Saul DJ, Morgan HW. Molecular diversity of thermophilic cellulolytic and hemicellulolytic bacteria.FEMS Microbiology Ecology, 1999, 28(2): 99–110DOI:10.1111/fem.1999.28.issue-2. |
| [40] | Hreggvidsson GO, Kaiste E, Holst O, Eggertsson G, Palsdottir A, Kristjansson JK. An extremely thermostable cellulase from the thermophilic eubacterium Rhodothermus marinus.Applied and Environmental Microbiology, 1996, 62(8): 3047–3049. |
| [41] | Zhang S, Barr BK, Wilson DB. Effects of noncatalytic residue mutations on substrate specificity and ligand binding of Thermobifida fusca endocellulase cel6A.European Journal of Biochemistry, 2000, 267(1): 244–252DOI:10.1046/j.1432-1327.2000.00988.x. |
| [42] | Ko KC, Han Y, Choi JH, Kim GJ, Lee SG, Song JJ. A novel bifunctional endo-/exo-type cellulase from an anaerobic ruminal bacterium.Applied Microbiology and Biotechnology, 2011, 89(5): 1453–1462DOI:10.1007/s00253-010-2949-9. |
| [43] | Elvan H, Ertunga NS, Yildirim M, Colak A. Partial purification and characterisation of endoglucanase from an edible mushroom, Lepista flaccida.Food Chemistry, 2010, 123(2): 291–295DOI:10.1016/j.foodchem.2010.04.034. |
| [44] | Karnchanatat A, Petsom A, Sangvanich P, Piapukiew J, Whalley AJS, Reynolds CD, Gadd GM, Sihanonth P. A novel thermostable endoglucanase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm..Enzyme and Microbial Technology, 2008, 42(5): 404–413DOI:10.1016/j.enzmictec.2007.11.009. |
| [45] | Quay DHX, Bakar FDA, Rabu A, Said M, Illias RM, Mahadi NM, Hassan O, Murad AMA. Overexpression, purification and characterization of the Aspergillus niger endoglucanase, EglA, in Pichia pastoris.African Journal of Biotechnology, 2011, 10(11): 2101–2111. |
| [46] | Jabbar A, Rashid MH, Javed MR, Perveen R, Malana MA. Kinetics and thermodynamics of a novel endoglucanase (CMCase) from Gymnoascella citrina produced under solid-state condition.Journal of Industrial Microbiology & Biotechnology, 2008, 35(6): 515–524. |
| [47] | Lucas R, Robles A, García MT, Alvarez de Cienfuegos G, Gálvez A. Production, purification, and properties of an endoglucanase produced by the hyphomycete Chalara (Syn. Thielaviopsis) paradoxa CH32..Journal of Agricultural and Food Chemistry, 2001, 49(1): 79–85DOI:10.1021/jf000916p. |
| [48] | Kumble KD, Trivedi PN, Jaffar MB. Purification and characterisation of two endoglucanases from Arthrobotrys oligospora.Indian Journal of Biochemistry & Biophysics, 1990, 27(3): 146–150. |
| [49] | Lee KM, Jeya M, Joo AR, Singh R, Kim IW, Lee JK. Purification and characterization of a thermostable endo-β-1,4-glucanase from a novel strain of Penicillium purpurogenum.Enzyme and Microbial Technology, 2010, 46(3/4): 206–211. |
| [50] | Huchzermeyer B, Hausmann N, Paquet-Durant F, Koyro HW. Biochemical and physiological mechanisms leading to salt tolerance.Tropical Ecology, 2004, 45(1): 141–150. |
| [51] | Lanyi JK. Salt-dependent properties of proteins from extremely halophilic bacteria.Bacteriological Reviews, 1974, 38(3): 272–290. |
| [52] | Shi RR, Li ZM, Ye Q, Xu JH, Liu Y. Heterologous expression and characterization of a novel thermo-halotolerant endoglucanase Cel5H from Dictyoglomus thermophilum.Bioresource Technology, 2013, 142: 338–344DOI:10.1016/j.biortech.2013.05.037. |
| [53] | Rabinovich ML, Melnick MS, Bolobova AV. The structure and mechanism of action of cellulolytic enzymes.Biochemistry (Moscow), 2002, 67(8): 850–871DOI:10.1023/A:1019958419032. |
