 , 贾敏, 何若男, 余静, 吴兵
, 贾敏, 何若男, 余静, 吴兵

南京大学环境学院, 污染控制与资源化研究国家重点实验室, 南京 210023
收稿日期: 2021-02-22; 修回日期: 2021-03-28; 录用日期: 2021-03-28
基金项目: 国家重点研发计划(2017YFE0107200);国家自然科学基金(No.21976087,22022604)
作者简介: 陈玲(1994-), 女, E-mail: hhucl2013@163.com
通讯作者(责任作者): 吴兵(1983—), 男, 博士, 教授, 研究方向为复合污染识别与健康危害等.E-mail: bwu@nju.edu.cn
摘要:砷(As)是全球性毒害污染物,可与消毒副产物共存于水环境中,但目前缺乏两者对水生生物的系统联合毒性效应评估数据.本研究选用毒性较强的含氮消毒副产物—二氯乙酰胺(DCAcAm)为代表,研究其与无机三价As在不同性别成年斑马鱼肝脏、肠道、脑和鳃等器官中的联合毒性效应.结果表明,100 μg·L-1 As和300 μg·L-1 DCAcAm单一和联合暴露主要累积于肝脏,并在肝脏诱导最强的氧化损伤及炎症反应.As单一暴露对雌性斑马鱼表现出更强毒性,而DCAcAm单一暴露则在雄性斑马鱼中诱导更强毒性,两者的联合毒性效应在两种性别斑马鱼肝脏中均减弱.在雄性斑马鱼肠道、脑和鳃中,As和DCAcAm的毒性效应相互独立;而在雌性斑马鱼脑和鳃中联合毒性减弱,肠道中联合毒性增强.本研究结果可为As和DCAcAm联合毒性评估及健康风险评价提供数据支撑,对DCAcAm暴露的性别二态性响应的首次表征,可为DBPs类污染毒性效应评估提供另一维度的参考数据.
关键词:砷消毒副产物(DBPs)二氯乙酰胺(DCAcAm)斑马鱼联合毒性性别二态性
The sex dimorphism in accumulation and combined toxicity of arsenic and dichloroacetamide in adult zebrafish
CHEN Ling
 , JIA Min, HE Ruonan, YU Jing, WU Bing
, JIA Min, HE Ruonan, YU Jing, WU Bing

State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023
Received 22 February 2021; received in revised from 28 March 2021; accepted 28 March 2021
Abstract: Arsenic (As) is a global toxic pollutant and can coexist with disinfection by-products (DBPs) in the environment. However, there is a lack of data about their combined toxicity. In this study, the dichloroacetamide (DCAcAm), a highly toxic representative nitrogen DBPs, was selected to study the combined toxicity with inorganic trivalent As on liver, gut, brain, and gill in male and female adult zebrafish. The results showed that 100 μg·L-1 As or/and 300 μg·L-1 DCAcAm exposure mainly accumulated in the liver, and induced the strongest oxidative damage and inflammation in the liver. The combined toxicity of As and DCAcAm were weakened in the livers of male and female zebrafish. In the gut, brain and gills of male zebrafish, the toxicity of As and DCAcAm was independent of each other, while the combined toxicity of the two was weakened in the brain and gills of female zebrafish and enhanced in gut of female zebrafish. The results of this study can provide data support for the combined toxicity assessment and health risk assessment of As and DCAcAm, and the characterization of sex dimorphic response to DCAcAm exposure for the first time can provide another dimension of data for the evaluation of the DBPs toxicity.
Keywords: arsenicdisinfection by-products(DBPs)dichloroacetamide(DCAcAm)zebrafishcombined toxicitysexual dimorphism
1 引言(Introduction)砷(Arsenic, As)是水环境中广受关注的毒害污染物.据世界卫生组织(World Health Organization, WHO)统计, 全球共有遍布5个大洲的70多个国家和地区(Mondal et al., 2010), 超2亿人口通过饮用水等途径长期暴露于风险水平As浓度中(WHO安全标准10 μg · L-1)(Naujokas et al., 2013).在我国新疆、内蒙、山西的盆地地形区及江苏、广东等河流三角洲地形区局部地下水As浓度可达50 μg · L-1(Rodriguez-Lado et al., 2013; Guo et al., 2014).湖南渫水河流域地表水也被报道年平均As浓度高达155~9700 μg · L-1(Li et al., 2020).由于其高发性及高致癌性, As连续数十年名列美国毒性物质及疾病登记署(Agency for Toxic Substances and Disease Registry, ATSDR)物质优先级列表榜首(McDermott et al., 2020), 给水生生物及人体健康造成极大风险.As毒性可受其他环境污染物的影响, 例如, 1 mmol · L-1氟化钠可显著增加10 μmol · L-1 As对原代人脐静脉内皮细胞毒性(Ma et al., 2017).因此, 充分认识As与环境水体中其他污染物的联合毒性效应对评估环境水体健康风险有重要意义.
消毒副产物(Disinfection by-products, DBPs)是水环境中普遍存在的污染物.据报道, 在许多河流(Lin et al., 2020)、湖泊(Yu et al., 2015)、水库(Scott et al., 2005)、污水处理厂(Yu et al., 2019)和沿海海域(Yang et al., 2015)中均检测到DBPs的存在, 多类DBPs总量可高达120 μg · L-1.而美国疾控中心和环境公共卫生追踪网络于2000年起对各州社区供水系统中10类常见污染物长达10年的连续监测表明, As和DBPs已成为新墨西哥州饮用水污染的首要风险因素(DeFelice et al., 2017; Monti et al., 2019).在我国开展的研究也表明西安地区自来水中As和DBPs共存具有重大致癌风险(Zhang et al., 2018).可见As、DBPs复合污染问题日趋显著, 探明其联合毒性效应十分必要.
卤代乙酰胺是一类于2002年首次报道的新兴含氮DBPs.基于中国仓鼠卵巢癌细胞的研究表明, 卤代乙酰胺类物质的细胞毒性比卤乙酸和卤乙腈类物质高出2~142倍(Plewa et al., 2008; 2010).二氯乙酰胺(Dichloroacetamide, DCAcAm)是环境中最常检出的代表性卤代乙酰胺类物质(Ding et al., 2020).据报道, DCAcAm在污水处理厂出水浓度最高可达5.6 μg · L-1(Krasner et al., 2006).体外细胞毒性研究也表明DCAcAm的毒性是二氯乙酸的2.5倍.暴露于100 μg · L-1 DCAcAm可增加人胚胎肾细胞中(Reactive oxygen species, ROS)和乳酸脱氢酶水平, 降低ATP含量, 诱发细胞凋亡(Chen et al., 2019).而体内毒理学研究表明, 持续30 d暴露1 μg · L-1 DCAcAm可在斑马鱼腮部诱导活性氧ROS水平升高, 并诱发炎症(Zhang et al., 2019).进一步调查发现, As也被广泛报道能够在生物体内引起氧化应激和炎症反应(Pi et al., 2002; Yan et al., 2020).例如, 100 mg · L-1 As持续28 d暴露于不同性别大鼠, 可在雌性大鼠肾脏诱导谷胱甘肽含量降低, 雄大鼠肾脏谷胱甘肽(Glutathione, GSH)含量则不受影响, 而在雌性和雄性大鼠肝脏中均观察到GSH含量降低(Kucukkurt et al., 2015).因此, DCAcAm在致毒过程中可能在此与As发生性别特异的交互作用.
基于氧化应激和炎症反应为两者重要共同致毒途径的背景, 本研究选取斑马鱼为受试对象, 开展As和DCAcAm联合毒性系统性评估, 以探明两者联合毒性的性别差异性响应及毒性作用靶器官.以期为揭示DBPs对As毒性的影响及更有效地评估As和DBPs的健康风险提供数据支撑.
2 材料与方法(Materials and methods)2.1 实验材料2.1.1 化学试剂三价As标准溶液购自美国O2Si公司, DCAcAm标准品购自德国阿法埃莎;碳酸氢钠(NaHCO3)、氯化钠(NaCl)、浓硝酸(HNO3)及双氧水(H2O2)购自国药集团;乙腈及正己烷购自美国默克公司;过氧化氢酶(CAT)、GSH、总蛋白定量、细胞白介素IL-6及肿瘤坏死因子TNFα等生化试剂盒均购自南京建成;磷酸盐缓冲溶液购自南京凯基.
2.1.2 实验仪器生化分析采用Synergy H1酶标仪(美国BioTek公司);样品前处理设备包括SB-5200DTD超声仪、Ymnl-48组织均研磨器(南京以马内利仪器设备公司)、ETHOS UP微波辅助消解仪(意大利迈尔斯通公司)、UGC-45C氮吹仪(北京优晟联合公司)、低速离心机(美国艾本德公司).As和DCAcAm的化学检测仪器分别为美国铂金埃尔默公司电感耦合等离子体串联质谱(Inductively coupled plasma massspectrometry, ICP-MS, NexION 300X)及美国赛默飞世尔公司气相色谱连用仪(Gas chromatography/tandem mass spectrometry, GC-MS/MS, TSQ Quantum XLS).
2.2 实验方法2.2.1 斑马鱼饲养成年斑马鱼(4月龄)购自中国科学院水生生物研究所(武汉), 养殖在容积为15 L的玻璃鱼缸中.养殖水为经净化后的超纯水, 使用前紫外照射12 h, 并使用NaHCO3和NaCl调节pH为7.4±0.5, 电导率为(500±20) μS · cm-1, 温度维持在(28.0±0.5) ℃, 明/暗周期设定为14 h/10 h, 每天喂食2次活体丰年虾(滕晓强等, 2020).雄性和雌性斑马鱼分缸养殖.
2.2.2 暴露实验经适应2周后, 雄性及雌性斑马鱼均被随机分为4组, 且均设置单独的对照组, 雄性和雌性斑马鱼各剩余的3组分别暴露于100 μg · L-1 As、300 μg · L-1 DCAcAm和100 μg · L-1 As+300 μg · L-1 DCAcAm中23 d.环境中的无机As有三价及五价两种形态, 其中三价As的生物毒害更强, 本研究选用三价As进行实验, 暴露浓度参考Sun等(2019)报道的环境相关浓度.DCAcAm暴露浓度及暴露时长参考现有文献报道的效应浓度与时长进行选择(Yu et al., 2015).为确保结果具有可比性, 联合暴露组暴露浓度及时长与单一暴露组保持一致.每组各暴露50条斑马鱼, 暴露液体积为12 L.暴露期间, 每天更新1/3的暴露溶液.暴露结束后, 冰上麻醉, 解剖分离肝脏、脑、肠道及鳃等组织, 立即使用液氮进行速冻, 并转移至-80 ℃冰箱保存待用.
2.2.3 组织中As含量测定每组各称取3份重约50 mg(湿重)的肝脏、脑、肠道及鳃样本, 每份样本约由5~6条斑马鱼脏器混合而成.将样本转移至消解罐中, 加入2 mL浓HNO3(60%)和6 mL H2O2(30%).采用微波辅助消解仪在最大功率1800 W下进行消解, 消解程序设置为10 min内升温至160 ℃, 然后160 ℃保持10 min.待消解罐冷却后, 用超纯水将消解液稀释至总体积为10 mL, 每样品移取1.5 mL消解液过0.22 μm水系滤膜, 用于ICP-MS检测.ICP-MS工作射频功率为1550 W, 载气(氩气)流量为0.96 L · min-1;采用玻璃同心雾化器和石英雾化室, 镍采样锥和截取锥;碰撞气为氦气, 反应气为氨气.
2.2.4 组织中DCAcAm含量测定每组各称取3份采集自5~6条斑马鱼的、重约50 mg(湿重)的肝脏、脑、肠道及鳃样本, 加入1 mL乙腈.在组织研磨器中以55.5 Hz的振荡频率间断研磨8 min, 转移至15 mL离心管, 在100%功率下超声30 min.超声浸提液在3000 r · min-1离心10 min, 将上清转移至50 mL离心管中, 向残渣中加入5 mL乙腈, 将超声浸提过程重复2次.向3次合并的浸提液中加入5 mL预先用乙腈饱和的正己烷进行脱脂处理.将下层清液移至15 mL玻璃离心管中, 于低于45 ℃的水浴中氮吹至干, 最后使用0.4 mL正己烷复溶, 过0.22 μm有机系滤膜后用于GC-MS检测.
GC-MS系统由Thermo TSQ Quantum XLS质谱配备Trace GC Ultra气相组成.色谱柱采用DB-5MS毛细管柱(30 m×0.25 mm, 0.25 μm), 色谱柱温度程序为初始温度40 ℃, 保持8 min, 再以40 ℃ · min-1升至150 ℃, 保持5 min;载气为氦气, 流速为1.2 mL · min-1;进样口温度为180 ℃, 进样量为1 μL, 进样方式为不分流进样;离子源为EI源, 源温度为250 ℃;碰撞气体为氩气, 溶剂延迟设置为8 min;数据采集模式为选择性离子检测模式(Selected ion monitor mode, SIM模式), 特征离子质量数为44、83、127.
2.2.5 氧化应激检测选用CAT、GSH评估不同性别斑马鱼各暴露结束后肝脏、脑、肠道及鳃的氧化损伤程度.每组称取3份重约10 mg(湿重)组织样本, 以1 g ∶ 9 mL的比例加入9倍体积的磷酸盐缓冲液, 放入组织研磨器中以55.5 Hz的振荡频率间断研磨8 min, 然后取出以2500 r · min-1离心10 min, 取上清.将CAT及GSH试剂盒中的工作试剂依次与前述上清液混合反应, 整个反应过程均按照试剂盒中说明书进行.最后采用酶标仪分别在405 nm及420 nm处测定吸光度(Optical density, OD).将上述上清液稀释10倍后, 用于总蛋白定量, 其中, 总蛋白量(TP)的测定过程依据试剂盒说明书进行.最后分别采用公式(1)和(2)计算单位蛋白中CAT活力(UCAT, U · mg-1, 以prot计)及GSH含量(CGSH, μg · mg-1, 以prot计).
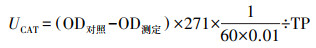 | (1) |
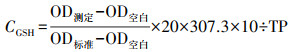 | (2) |
2.3 数据分析所有检测均设置3个样本平行, 数据统计方式为平均值±标准差, 图形绘制及统计学分析均采用GraphPad Prism8软件, 显著性差异分析方法采用非配对的双尾单因素方差分析(One way ANOWA), p≤0.05为显著性差异.热图的绘制采用R语言pheatmap包, 原始数据经归一化处理后读入, 并使用R语言scale函数进行中心化和标准化处理.
3 结果(Results)3.1 As在斑马鱼脏器中的富集对各组斑马鱼肝脏、脑、肠道及鳃组织中As含量分析结果表明, 雄性斑马鱼中平均As累积量低于雌性斑马鱼(图 1).从器官分布上来看, 雄性斑马鱼各器官中的累积量差异不明显(图 1a), As单一暴露组中雄性肝脏、肠道、脑及鳃平均累积量分别为0.17、0.24、0.20及0.14 mg · kg-1, 在联合暴露组中肝脏、肠道、脑及鳃平均累积量分别为0.39、0.34、0.27及0.22 mg · kg-1.
图 1(Fig. 1)
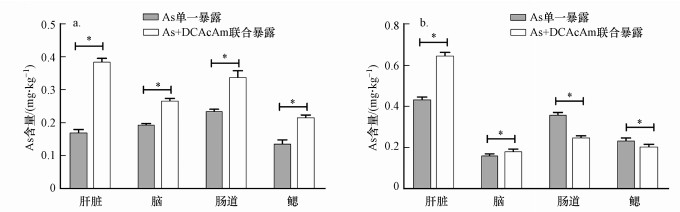 |
| 图 1 不同暴露方式下砷在斑马鱼器官中的累积 (a.雄性, b.雌性) Fig. 1The accumulation of As in organs of male and female zebrafish under different exposure mode (a.male, b.female) |
而雌性斑马鱼器官中As累积量存在显著器官差异性(图 1b), As单一暴露及联合暴露下, 肝脏累积量均显著高于其他组织, 分别为0.44 mg · kg-1和0.65 mg · kg-1;肠道累积量仅次于肝脏, 在As单一暴露及联合暴露组的平均累积量分别为0.36 mg · kg-1和0.25 mg · kg-1;鳃平均累积量在As单一暴露及联合暴露组均较小, 分别为0.24 mg · kg-1和0.21 mg · kg-1;脑则分别平均累积0.16 mg · kg-1和0.19 mg · kg-1.值得注意的是, 联合暴露下As在雌性斑马鱼肠道的累积量被显著降低.总体来看, 肝脏和肠道是As的主要累积器官.
3.2 DCAcAm在斑马鱼脏器中的富集在DCAcAm单一暴露组中, 雄性斑马鱼脑累积量最大, 但与肝脏平均累积量差异不大, 分别为0.078 mg · kg-1和0.068 mg · kg-1;肠道和鳃的平均累积量极少, 分别为0.023 mg · kg-1和0.033 mg · kg-1(图 2a).而在联合暴露组中, 雄性斑马鱼肝脏累积量最高, 平均为0.143 mg · kg-1;脑次之, 平均累积量为0.099 mg · kg-1;肠道和鳃分别平均累积0.024 mg · kg-1和0.026 mg · kg-1.
图 2(Fig. 2)
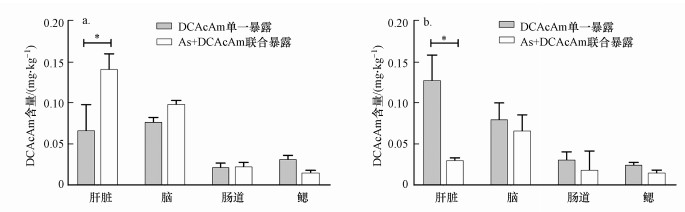 |
| 图 2 不同暴露方式下DCAcAm在雄性和雌性斑马鱼器官中的累积 (a.雄性, b.雌性) Fig. 2The accumulation of DCAcAm in organs of male and female zebrafish under different exposure mode (a.male, b.female) |
在DCAcAm单一暴露组, 雌性斑马鱼则在肝脏中平均累积量(0.128 mg · kg-1)最大, 且与位列第2的脑累积量(0.081 mg · kg-1)存在较大差异;肠道和鳃累积量较低, 平均分别累积0.032 mg · kg-1和0.033 mg · kg-1(图 2b).联合暴露组中, 雌性斑马鱼肝脏及脑平均累积量仍然高于肠道和鳃, 分别为0.031 mg · kg-1和0.067 mg · kg-1;肠道和鳃平均累积量则分别为0.019 mg · kg-1和0.016 mg · kg-1(图 2b).但与DCAcAm单一暴露相比, 雌性斑马鱼中肝脏及脑的绝对累积量被显著降低.
整体来看, 在不同组别及不同性别斑马鱼中肠道与鳃DCAcAm累积量均较低, 且差异较小.肝脏和脑在两种性别的斑马鱼器官中DCAcAm累积量均较其他器官高.雄性斑马鱼肝脏及脑的DCAcAm累积量在与As联合暴露后升高, 雌性斑马鱼肝脏及脑的DCAcAm累积量在与As联合暴露后大幅降低.
3.3 As和DCAcAm联合暴露对不同性别斑马鱼肝脏的损伤CAT和GSH是生物体内抗氧化系统的重要组成部分(Ighodaro et al., 2018), 测定两者的活力与含量能够表征As和DCAcAm暴露后斑马鱼肝脏的氧化损伤程度.本研究显示, 雄性和雌性斑马鱼肝脏中CAT活力在As及DCAcAm单一暴露组显著升高, 联合组无显著影响, 且与单一暴露组存在显著性差异(图 3a).而在雄性斑马鱼肝脏中DCAcAm单一暴露组和联合组显著诱导GSH含量降低, As单一暴露组无显著影响;与之相反, 仅As单一暴露组显著诱导雌性斑马鱼肝脏中GSH含量降低(图 3b).
图 3(Fig. 3)
 |
| 图 3 As和DCAcAm单一及联合暴露下斑马鱼肝脏氧化损伤及炎症响应 (a.CAT活力, b.GSH含量, c.IL-6含量, d.TNFα含量)(As代表As单一暴露组, DCAcAm代表DCAcAm单一暴露组, As+DCAcAm代表联合暴露组;*代表与对照组存在显著性差异, p≤0.05, 下同) Fig. 3Oxidative damage and inflammatory response of zebrafish liver after As or/and DCAcAm exposure (a.CAT activity, b.GSH content, c.IL-6 levels, d.TNFα levels)(As represent As alone exposure group, DCAcAm represents DCAcAm alone exposure group, As+DCAcAm represents the combined exposure group.Stars mark the significant differences, compared to control, p≤0.05, the same below) |
活性氧的产生及氧化应激的发生常被认为能够直接影响生物体免疫响应.TNF-α和IL-6是极重要的促炎细胞因子, 测定两者的浓度水平可直接反映机体炎症响应强弱(Liang et al., 2020).本研究测定结果表明, 仅DCAcAm单一暴露显著诱导雄性斑马鱼肝脏中IL-6含量升高, 联合组无显著性影响, 且与DCAcAm单一暴露组存在显著性差异;而在雌性斑马鱼肝脏中As和DCAcAm单一暴露组IL-6含量均显著升高, 联合组无显著性影响, 且与单一暴露组存在显著性差异(图 3c).雄性斑马鱼肝脏中, 所有组别TNFα含量均显著升高;雌性斑马鱼肝脏中除联合暴露组无显著影响外, As和DCAcAm单一暴露组TNFα含量均显著升高(图 3d).
上述结果表明, 300 μg · L-1 DCAcAm暴露在雄性斑马鱼肝脏中诱导更强的毒性, 而100 μg · L-1 As暴露则在雌性斑马鱼肝脏诱导更强的毒性.较之As或DCAcAm单一暴露, 联合暴露在雌雄斑马鱼肝脏中均诱导更低的毒性效应, 且同浓度下联合暴露在雄性斑马鱼肝脏的响应高于雌性斑马鱼肝脏.
3.4 As和DCAcAm联合暴露对不同性别斑马鱼肠道的损伤DCAcAm单一暴露及联合暴露组显著诱导雄性斑马鱼肠道中CAT活力升高, As单一暴露组无显著性影响, 而在雌性斑马鱼肠道中仅As单一暴露显著诱导CAT活力升高;与单一暴露组相比, 联合组的雄性及雌性斑马鱼肠道CAT活力均无显著性差异(图 4a).GSH含量分析结果表明(图 4b), 雄性斑马鱼肠道As单一暴露及联合暴露组GSH含量显著降低, 所有组别的雌性斑马鱼肠道均不受影响;联合组的雄性及雌性斑马鱼肠道GSH含量与单一暴露组均无显著性差异.
图 4(Fig. 4)
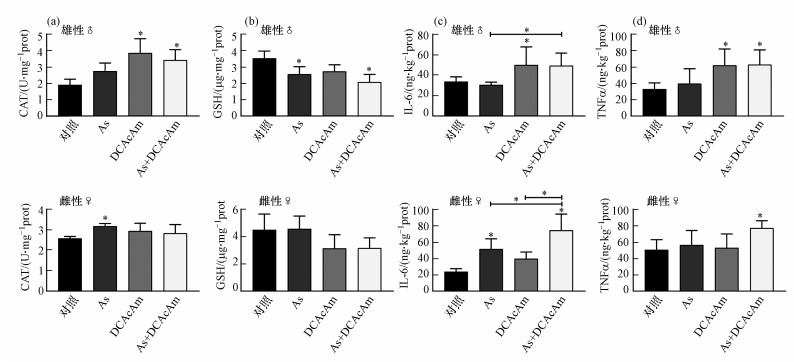 |
| 图 4 As和DCAcAm单一及联合暴露下斑马鱼肠道氧化损伤及炎症响应 (a.CAT活力, b.GSH含量, c.IL-6含量, d.TNFα含量) Fig. 4Oxidative damage and inflammatory response of zebrafish gut after As or/and DCAcAm exposure (a.CAT activity, b.GSH content, c.IL-6 levels, d.TNFα levels) |
仅DCAcAm单一暴露组在雄性斑马鱼肠道中显著诱导炎症因子IL-6水平升高, 而雌性斑马鱼肠道IL-6水平则受As单一暴露及联合暴露的显著影响而升高, 联合暴露组与As和DCAcAm单一暴露组存在显著性差异(图 4c).雄性斑马鱼肠道中TNFα水平在DCAcAm单一及联合暴露组中显著升高, 而雌性斑马鱼肠道中TNFα水平仅有联合组显著升高(图 4d).
以上结果表明, As及DCAcAm暴露对不同性别斑马鱼肠道毒性强弱差异性与肝脏研究结果一致;而两者的联合作用模式呈现性别差异, 雄性斑马鱼肠道中联合组毒性与单一暴露组差异不明显, 而在雌性斑马鱼肠道中联合组炎症响应程度强于单一暴露组(图 4d).
3.5 As和DCAcAm联合暴露对不同性别斑马鱼脑的损伤所有组别雄性和雌性斑马鱼脑中CAT活力均无显著性差异(图 5a).雄性斑马鱼脑中GSH含量仅受DCAcAm单一暴露诱导降低, 而雌性斑马鱼脑中所有组别均显著降低, 联合组表现出最强的影响, 但与单一暴露组不存在显著性差异(图 5b).在两种性别的斑马鱼脑中, DCAcAm单一暴露及联合暴露组均显著诱导炎症因子IL-6水平升高, As单一暴露组无显著影响(图 5c).所有组别的雄性斑马鱼脑中TNFα水平均未发生显著性变化, 而DCAcAm单一暴露及联合暴露组仍在雌性斑马鱼脑显著诱导炎症因子TNFα水平升高, As单一暴露组不受影响;值得注意的是, 联合组雌性斑马鱼脑中TNFα水平与As或DCAcAm单一暴露组均存在显著性差异(图 5d).可见, 与As单一暴露相比, DCAcAm单一暴露在两种性别的斑马鱼脑中均比As单一暴露具有更强的毒性效应.两者的联合暴露毒性在雄性斑马鱼脑与单一暴露相比差异不显著, 但在雌性斑马鱼脑联合作用表现出毒性减弱(图 5d).
图 5(Fig. 5)
 |
| 图 5 As和DCAcAm单一及联合暴露下斑马鱼脑氧化损伤及炎症响应 (a.CAT活力, b.GSH含量, c.IL-6含量, d.TNFα含量) Fig. 5Oxidative damage and inflammatory response of zebrafish brain after As or/and DCAcAm exposure (a.CAT activity, b.GSH content, c.IL-6 levels, d.TNFα levels) |
3.6 As和DCAcAm联合暴露对不同性别斑马鱼鳃的损伤所有组别雄性斑马鱼鳃中CAT活力均无显著差异, 而雌性斑马鱼鳃受As和DCAcAm单一暴露诱导CAT活力显著升高;与单一暴露组对照组相比, 联合组CAT活力并不存在显著性差异(图 6a).雄性斑马鱼鳃中GSH含量受As单一暴露及As、DCAcAm联合暴露影响而显著降低, 且联合组与单一暴露组不存在显著性差异;雌性斑马鱼鳃中GSH含量仅在As单一暴露组出现显著下降, 其他组别无显著差异, As单一暴露组雌性斑马鱼鳃中GSH含量与联合组存在显著性差异(图 6b).在两种性别的斑马鱼鳃中, As单一暴露及联合暴露组均显著诱导IL-6水平升高, DCAcAm单一暴露组无显著影响(图 6c).所有组别的雄性斑马鱼鳃中TNFα水平均未发生显著性变化, 而As单一暴露及联合暴露组仍在雌性斑马鱼脑显著诱导炎症因子TNFα水平升高(图 6d).与DCAcAm单一暴露相比, As单一暴露在两种性别的斑马鱼鳃诱导更强的毒性效应.两者的联合暴露毒性在雄性斑马鱼鳃与单一暴露相比差异不显著, 但在雌性斑马鱼鳃联合作用表现出毒性减弱(图 6b).
图 6(Fig. 6)
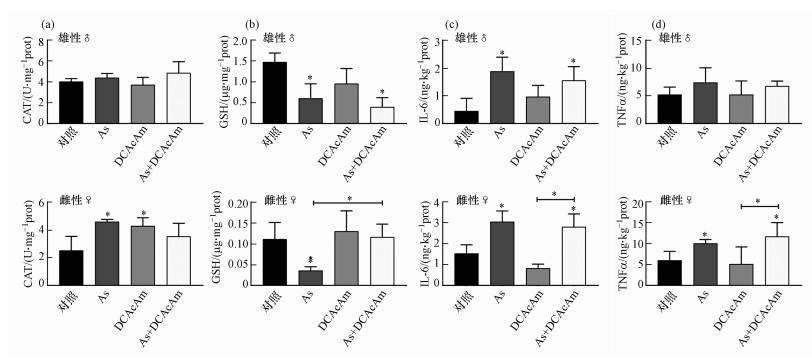 |
| 图 6 As和DCAcAm单一及联合暴露下斑马鱼鳃氧化损伤及炎症响应 (a.CAT活力, b.GSH含量, c.IL-6含量, d.TNFα含量) Fig. 6Oxidative damage and inflammatory response of zebrafish gill after As or/and DCAcAm exposure (a.CAT activity, b.GSH content, c.IL-6 levels, d.TNFα levels) |
4 讨论(Discussion)As和DBPs是水环境中普遍存在的高风险污染物, 越来越多的研究指出两者常共存于水环境中, 但其联合作用的毒理学数据十分缺乏, 亟需系统地开展两者的联合毒性效应评估.本研究分别将100 μg · L-1 As、300 μg · L-1 DCAcAm及100 μg · L-1 As+300 μg · L-1 DCAcAm暴露不同性别的成年斑马鱼.系统比较实验结果可见, As和DCAcAm的单一暴露在不同性别斑马鱼体内表现出毒性强弱的差异性(图 7).两者的联合作用在两种性别斑马鱼肝脏中均表现出联合毒性减弱, 而肠道、脑及鳃等器官在不同性别表现出极大差异.
图 7(Fig. 7)
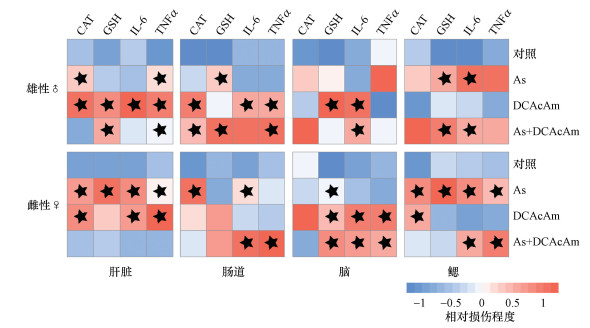 |
| 图 7 As和DCAcAm暴露在雄性和雌性斑马鱼肝脏、肠道、脑及鳃诱导的氧化损伤及炎症反应热图 (红色代表损伤, 蓝色代表正常, 黑色五角星代表与对照存在显著性差异) Fig. 7Heat map of oxidative damage and inflammation induced by As and DCAcAm exposure in male and female zebrafish liver, intestine, brain, and gills (Red represents damage and blue represents normal, the black star marks a significant difference compared to the control) |
肝脏是鱼类代谢最活跃的器官, 本研究发现肝脏是As和DCAcAm暴露毒性效应最为显著的器官.据报道, 50 μg · L-1三氧化二砷暴露于雌性斑马鱼30 d后, 可检测到斑马鱼肝脏CAT活力显著上升, GSH含量减少(Mondal et al., 2019).与Mondal等(2019)的研究结果相似, 本研究发现100 μg · L-1As暴露可在雌性斑马鱼肝脏诱导GSH含量减少, CAT活力、IL-6及TNFα水平升高, 而在雄性斑马鱼肝脏仅观察到CAT活力及TNFα水平升高, 表现出明显性别差异.化学检测结果显示, 雌性斑马鱼肝脏As累积量显著高于雄性斑马鱼肝脏, 这可能是As在不同性别斑马鱼肝脏毒性差异的原因.
氧化应激及炎症响应被广泛报道为DBPs的重要致毒途径(Jiang et al., 2017; Zhang et al., 2020).根据Lin、Yu等报道, 持续20 d暴露500 μg · L-1 DCAcAm即可在斑马鱼肝脏诱导氧化损伤, 表现为SOD活力升高, DNA损伤加剧(Yu et al., 2015; Lin et al., 2016).同样的结果也在本研究中出现, 300 μg · L-1DCAcAm暴露在雄性斑马鱼肝脏显著诱导CAT活力、IL-6及TNFα水平升高, 并降低了GSH含量, 而在雌性斑马鱼肝脏中仅诱导CAT活力、IL-6及TNFα水平升高;更有趣的是, 在DCAcAm单一暴露组雌性斑马鱼肝脏累积量高于雄性斑马鱼肝脏(图 2).尽管DCAcAm的这类性别差异性响应尚未见报道, 但氟化钠、硫丹等物质曾被报道可分别在斑马鱼脑和肝脏中诱导性别差异的氧化损伤, 且作者指出这可能是受性别特异的激素调节所致(Shao et al., 2012; Mukhopadhyay et al., 2015).
本研究发现, 与单一暴露相比, 雄性斑马鱼肝脏中累积更高量的As和DCAcAm, 雌性斑马鱼肝脏中则累积更多的As, 而DCAcAm的累积量小于单一暴露组.然而, As和DCAcAm的联合暴露在两种性别斑马鱼肝脏中诱导更弱的氧化损伤及炎症响应.可见As和DCAcAm的共暴露能在机体发生交互作用, 从而削弱两者的毒性效应.比对联合暴露在雄性及雌性斑马鱼肝脏的毒性效应, 仍然可发现性别差异性, 雄性斑马肝脏联合毒性效应强于雌性斑马鱼肝脏.以上有关As和DCAcAm的联合作用机制仍有待进一步探究.
肠道、脑及鳃等器官也不同程度地受到As和DCAcAm单一或联合暴露的影响.整体上, As单一暴露在两种性别斑马鱼腮诱导更强毒性, DCAcAm单一暴露则在斑马鱼脑部诱导更强毒性, 肠道对两者单一暴露则呈现与肝脏类似的性别差异性响应.DCAcAm单一暴露时, 在雌性斑马鱼脑的累积量分别为0.081 mg · kg-1和0.078 mg · kg-1, 这可能是雌性斑马鱼脑部损伤强于雄性的原因.而在联合暴露后, 雌性斑马鱼脑DCAcAm累积量下降为0.067 mg · kg-1, 在雌性斑马鱼脑联合毒性效应也表现出减弱(图 2).此外, 雌性斑马鱼鳃毒性效应强于雄性的可能原因为As在雌性斑马鱼鳃的累积量高于雄性斑马鱼鳃, 分别为0.24 mg · kg-1和0.014 mg · kg-1, 联合暴露也使得雌性斑马鱼鳃As累积量下降, 可能因此导致联合毒性效应减弱.与对照相比, 雄性斑马鱼肠道DCAcAm暴露诱导多毒性终点表现显著差异, 比雌性斑马鱼肠道表现出更强的毒性(图 7).但本研究尚未有充分的证据说明雌性斑马鱼肠道中As和DCAcAm联合作用增强的原因.
5 结论(Conclusions)1) 不同性别斑马鱼对As及DCAcAm具有敏感性差异, 肝脏及肠道毒性结果表明, DCAcAm暴露对雄性斑马鱼具有更强毒性, 而As暴露对雌性斑马鱼具有更强毒性.
2) As及DCAcAm暴露对斑马鱼脑和鳃等器官的毒性效应差异较大, As在两种性别的斑马鱼鳃中表现出的毒性强于DCAcAm, 而在脑中则DCAcAm表现出更高的毒性.雌性斑马鱼脑和鳃炎症响应强度强于雄性斑马鱼脑和鳃.
3) 在不同器官及不同性别中As和DCAcAm联合作用模式均表现差异性.在两种性别的斑马鱼肝脏中, 两者联合毒性减弱, 表现出拮抗性.在雄性斑马鱼肠道、脑及鳃中As和DCAcAm毒性效应相互独立, 而在雌性斑马鱼肠道联合作用增强, 脑和鳃联合作用减弱.
参考文献
| Chen Y W, Xu T, Yang X Y, et al. 2019. The toxic potentials and focus of disinfection byproducts based on the human embryonic kidney (HEK293) cell model[J]. Science of the Total Environment, 664: 948-957. DOI:10.1016/j.scitotenv.2019.01.361 |
| DeFelice N B, Leker H G, Gibson J M. 2017. Annual cancer risks from chemicals in North Carolina community water systems[J]. Human and Ecological Risk Assessment, 23: 974-991. DOI:10.1080/10807039.2017.1292842 |
| Ding X L, Zhu J Y, Zhang J, et al. 2020. Developmental toxicity of disinfection by-product monohaloacetamides in embryo-larval stage of zebrafish[J]. Ecotoxicology and Environmental Safety, 189. DOI:10.1016/j.ecoenv.2019.110037 |
| Guo H M, Wen D G, Liu Z Y, et al. 2014. A review of high arsenic groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes[J]. Applied Geochemistry, 41: 196-217. DOI:10.1016/j.apgeochem.2013.12.016 |
| Ighodaro O M, Akinloye O A. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid[J]. Alexandria Journal of Medicine, 54: 287-293. DOI:10.1016/j.ajme.2017.09.001 |
| Jiang W B, Li B, Chen Y Y, et al. 2017. The toxic influence of dibromoacetic acid on the hippocampus and pre-frontal cortex of rat: involvement of neuroinflammation response and oxidative stress[J]. Metabolic Brain Disease, 32: 2009-2019. DOI:10.1007/s11011-017-0095-0 |
| Krasner S W, Weinberg H S, Richardson S D, et al. 2006. Occurrence of a new generation of disinfection byproducts[J]. Environmental Science & Technology, 40: 7175-7185. |
| Kucukkurt I, Ince S, Demirel H H, et al. 2015. The effects of boron on arsenic-induced lipid peroxidation and antioxidant status in male and female rats[J]. Journal of Biochemical and Molecular Toxicology, 29: 564-571. DOI:10.1002/jbt.21729 |
| Lin X H, Xu J C, Keller A A, et al. 2020. Occurrence and risk assessment of emerging contaminants in a water reclamation and ecological reuse project[J]. Science of the Total Environment, 744. |
| Li W X, Liu J, Hudson-Edwards K A. 2020. Seasonal variations in arsenic mobility and bacterial diversity: The case study of Huangshui Creek, Shimen Realgar Mine, Hunan Province, China[J]. Science of the Total Environment, 749. DOI:10.1016/j.scitotenv.2020.142353 |
| Liang X M, Wang F, Li K B, et al. 2020. Effects of norfloxacin nicotinate on the early life stage of zebrafish (Danio rerio): Developmental toxicity, oxidative stress and immunotoxicity[J]. Fish & Shellfish Immunology, 96: 262-269. |
| Lin T, Zhou D J, Yu S L, et al. 2016. The removal process of 2, 2-dichloroacetamide (DCAcAm), a new disinfection by-product, in drinking water treatment process and its toxicity on zebrafish[J]. Chemosphere, 159: 403-411. DOI:10.1016/j.chemosphere.2016.06.029 |
| Ma Y Q, Ma Z H, Yin S Q, et al. 2020. Arsenic and fluoride induce apoptosis, inflammation and oxidative stress in cultured human umbilical vein endothelial cells[J]. Chemosphere, 167: 454-461. |
| McDermott T R, Stolz J F, OremLand RS. 2020. Arsenic and the gastrointestinal tract microbiome[J]. Environmental Microbiology Reports, 12: 136-159. DOI:10.1111/1758-2229.12814 |
| Mondal D, Banerjee M, Kundu M, et al. 2010. Comparison of drinking water, raw rice and cooking of rice as arsenic exposure routes in three contrasting areas of West Bengal, India[J]. Environmental Geochemistry and Health, 32: 463-477. DOI:10.1007/s10653-010-9319-5 |
| Mondal P, Shaw P, Bandyopadhyay A, et al. 2019. Mixture effect of arsenic and fluoride at environmentally relevant concentrations in zebrafish (Danio rerio) T liver: Expression pattern of Nrf2 and related xenobiotic metabolizing enzymes[J]. Aquatic Toxicology, 213. DOI:10.1016/j.chemosphere.2020.128678 |
| Monti M M, David F, Shin M, et al. 2019. Community drinking water data on the National Environmental Public Health Tracking Network: A surveillance summary of data from 2000 to 2010[J]. Environmental Monitoring and Assessment, 191. DOI:10.1007/s10661-019-7710-y |
| Mukhopadhyay D, Priya P, Chattopadhyay A. 2015. Sodium fluoride affects zebrafish behaviour and alters mRNA expressions of biomarker genes in the brain: Role of Nrf2/Keap1[J]. Environmental Toxicology and Pharmacology, 40: 352-359. DOI:10.1016/j.etap.2015.07.003 |
| Naujokas M F, Anderson B, Ahsan H, et al. 2013. The Broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem[J]. Environmental Health Perspectives, 121: 295-302. DOI:10.1289/ehp.1205875 |
| Plewa M J, Muellner M G, Richardson S D, et al. 2008. Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: An emerging class of nitrogenous drinking water disinfection byproducts[J]. Environmental Science & Technology, 42: 955-961. |
| Plewa M J, Simmons J E, Richardson S D, et al. 2010. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products[J]. Environmental and Molecular Mutagenesis, 51: 871-878. DOI:10.1002/em.20585 |
| Pi J B, Yamauchi H, Kumagai Y, et al. 2002. Evidence for induction of oxidative stress caused by chronic exposure of chinese residents to arsenic contained in drinking water[J]. Environmental Health Perspectives, 110: 331-336. DOI:10.1289/ehp.02110331 |
| Rodriguez-Lado L, Sun G F, Berg M, et al. 2013. Groundwater arsenic contamination throughout China[J]. Science, 341: 866-868. DOI:10.1126/science.1237484 |
| Scott B F, Spencer C, Martin J W, et al. 2005. Comparison of haloacetic acids in the environment of the northern and southern hemispheres[J]. Environmental Science & Technology, 39: 8664-8670. |
| Sun H J, Zhang J Y, Wang Q, et al. 2019. Environmentally relevant concentrations of arsenite induces developmental toxicity and oxidative responses in the early life stage of zebrafish[J]. Environmental Pollution, 254. DOI:10.1016/j.envpol.2019.113022 |
| Shao B, Zhu L S, Dong M, et al. 2012. DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio)[J]. Ecotoxicology, 21: 1533-1540. DOI:10.1007/s10646-012-0907-2 |
| 滕晓强, 陆宏峰, 郭心月, 等. 2020. 二氯苯醌对斑马鱼胚胎发育及超氧化物歧化酶的影响[J]. 环境科学学报, 40(7): 2659-2664. |
| Yan N, Xu G W, Zhang C C, et al. 2020. Chronic arsenic exposure induces the time-dependent modulation of inflammation and immunosuppression in spleen[J]. Cell and Bioscience, 10. DOI:10.1186/s13578-020-00448-6 |
| Yang G P, Li L, Lu X L, et al. 2015. Distributions and sea-to-air fluxes of volatile halocarbons in the southern Yellow Sea and the East China Sea[J]. Acta Oceanologica Sinica, 34: 9-20. |
| Yu S L, Lin T, Chen W, et al. 2015. The toxicity of a new disinfection by-product, 2, 2-dichloroacetamide (DCAcAm), on adult zebrafish (Danio rerio) and its occurrence in the chlorinated drinking water[J]. Chemosphere, 139: 40-46. DOI:10.1016/j.chemosphere.2015.05.079 |
| Yu Y, Ma X, Chen R Y, et al. 2019. The occurrence and transformation behaviors of disinfection byproducts in drinking water distribution systems in rural areas of eastern China[J]. Chemosphere, 228: 101-109. |
| Zhang H, Chang S, Wang L B, et al. 2018. Estimating and comparing the cancer risks from THMs and low-level arsenic in drinking water based on disability-adjusted life years[J]. Water Research, 145: 83-93. DOI:10.1016/j.watres.2018.08.012 |
| Zhang Y, Sun H J, Zhang J Y, et al. 2019. Chronic exposure to dichloroacetamide induces biochemical and histopathological changes in the gills of zebrafish[J]. Environmental Toxicology, 34: 781-787. |
| Zhang Z X, Zhu Q Y, Huang C, et al. 2020. Comparative cytotoxicity of halogenated aromatic DBPs and implications of the corresponding developed QSAR model to toxicity mechanisms of those DBPs: Binding interactions between aromatic DBPs and catalase play an important role[J]. Water Research, 170. DOI:10.1016/j.watres.2019.115283 |
