 , 李胜楠, 耿金菊
, 李胜楠, 耿金菊
 , 许柯, 任洪强
, 许柯, 任洪强南京大学环境学院, 污染控制与资源化研究国家重点实验室, 南京 210023
收稿日期: 2020-08-06; 修回日期: 2020-09-10; 录用日期: 2020-09-10
基金项目: 江苏省自然科学基金(No.BK20180010);国家自然科学基金(No.51978327, 21677071);水体污染控制与治理科技重大专项(No.2017ZX07202003)
作者简介: 石玉飞(1995-), 男, E-mail: 1214475212@qq.com
通讯作者(责任作者): 耿金菊, E-mail: jjgeng@nju.edu.cn
摘要:溶解性有机物(DOM)是发酵制药废水二级生化尾水深度处理工艺的主要去除对象,深入解析废水中DOM的特性是将其有效去除的前提和关键.本研究采集氨基酸类、头孢类、抗生素类、维生素类、阿维菌素类5种发酵制药废水二级出水,通过XAD-8树脂亲疏水性分离、凝胶色谱分子量分级、傅里叶变换红外光谱和三维荧光表征对二级出水中的DOM进行特性分析.结果表明,5种发酵制药废水二级出水的盐度和色度高,废水中有机物浓度高、波动大(48.60~245.40 mg·L-1).DOM大多数以疏水性组分为主,分子量分布广泛(210~10000 Da).二级出水DOM均含有大量的不饱和双键、苯环结构,同时含有—OH、—NH2和C=O等发色团和助色基团,造成废水色度较高.通过对发酵制药废水二级出水进行EEM-PARAFAC分析确定了4种荧光组分特征峰,包括3种腐殖质类(C1、C3、C4)和1种类蛋白类组分(C2).发酵制药废水二级出水中DOM主要是以类腐殖质有机物为主(C1、C3).全方面识别和解析发酵制药废水DOM的组成,可为废水深度处理工艺的优化提供指导.
关键词:制药废水溶解性有机物分子量亲疏水性特性解析
Characteristics of dissolved organic matter in the secondary effluent from fermentation pharmaceutical wastewater
SHI Yufei
 , LI Shengnan, GENG Jinju
, LI Shengnan, GENG Jinju
 , XU Ke, REN Hongqiang
, XU Ke, REN HongqiangState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing 210023
Received 6 August 2020; received in revised from 10 September 2020; accepted 10 September 2020
Abstract: The dissolved organic matter (DOM) in the secondary effluent from fermentation pharmaceutical wastewater is a key limiting factor for wastewater reclamation and reuse. An in-depth analysis of the characteristics of DOM will help to optimize the process operation to effectively remove DOM from wastewater. In this study, secondary effluents from five kinds of fermentation pharmaceutical wastewaters including amino acids, cephalosporins, antibiotics, vitamins and abamectin, were collected to investigate the characteristics of DOM. The characteristics of DOM in secondary effluent were analyzed using XAD-8 resin, high performance liquid chromatography-size exclusion chromatography, fourier transform infrared spectroscopy and fluorescence excitation-emission matrix spectroscopy. The results showed that, the secondary effluents of fermentation pharmaceutical wastewaters exhibited high salinity and color. The concentration of organic matters in the secondary effluent were about (48.6~232.2 mg·L-1). The hydrophobic fraction was the dominant composition of DOM with the molecular weight distributed widely (210~10000 Da) in secondary effluent. DOM in secondary effluent contained a large number of unsaturated double bonds and benzene ring structures, as well as chromophore and auxiliary groups such as —OH, —NH2, and C=O, resulting in strong color of wastewater. Three humic-like substances (C1, C3 and C4) and one protein-like substance (C2) were found by EEM-PARAFAC analysis. The humic-like substances (C1 and C3) were the largest group of DOM in the secondary effluent of fermentation pharmaceutical wastewater. The comprehensive identification of DOM composition would contribute to optimize the pharmaceutical wastewater treatment process.
Keywords: pharmaceutical wastewaterdissolved organic mattermolecular weighthydrophobic/hydrophilicperformance analysis
1 引言(Introduction)近年来我国水环境质量不断恶化, 对工业废水进行深度处理及有效回用是解决水资源危机的有效途径.发酵药物产品种类繁多, 生产工艺流程复杂及药品多为间歇式生产(Luo et al., 2018), 经二级生化处理后的废水, 仍具有成分复杂, 有机物浓度高, 难以被生物降解等特征, 且部分废水含有毒害成分(Zwienerm et al., 2000;Gadipelly et al., 2014;Ma et al., 2016), 不能达到直接排放和回用标准.二级出水中的溶解性有机物(dissolved organic matter, DOM)是发酵制药废水深度处理工艺去除的难点和重点, 其有机物浓度高, 污染物种类复杂, 甚至含有残留的活性药物成分, 不同组分理化性质高度异质, 具有多分散性, 且绝大多数组分在分子水平上仍未知, 具有一定的环境健康风险(Shi et al., 2017).常规的水质指标分析不能满足废水高标准排放要求, 难以揭示污染物的去除机理, 也制约了处理工艺优化设计和废水提标排放设计.深入解析废水中DOM的结构特征是将其有效去除的前提和关键.但已有的研究对发酵制药废水的DOM的组成和性质表征的研究和报道还较匮乏, 因此需要对制药废水二级出水中DOM进行全面综合系统表征.
废水中DOM的组成、性质的表征是深度处理工艺设计和优化运行的前提(Pan et al., 2018).DOM的亲疏水性和分子量分布反映了污水处理中DOM的来源、转化(Mangal et al., 2020)、生物降解性和生物毒性(Zhang et al., 2010;Podgorski et al., 2018).DOM亲疏水性分布可用于评估废水的可处理性、膜污染, 以及潜在的有毒物质的形成(Tran et al., 2015;Xue et al., 2017).DOM分子量分布可用于评估化学, 物理和生物过程中去除废水中DOM的效率(Tran et al., 2015;Chon et al., 2017).三维荧光激发发射光谱法(Excitation-Emission Matrices, EEM)是通过廉价、无损的方法来获得含有荧光团的各种有机化合物的灵敏测量, 提供了大量关于DOM的信息(Shen et al., 2020).荧光光谱法已经被成功地应用于评估工农业污水的质量(Carstea et al., 2016), 污水处理的效率和选择性(Cohen et al., 2014).傅里叶变换红外光谱(Fourier transform infrared Spectrometer, FTIR)可用于识别DOM中有机官能团结构, 分析污水中的发色基团、共轭结构和助色团, 解析废水色度形成机制(Tang et al., 2014;Malik et al., 2019).全方面地识别和解析复杂混合物DOM的组成, 是进行废水处理工艺优化和实现废水达标排放和回用的先决条件.
本研究采集氨基酸类、头孢类、抗生素类、维生素类及阿维菌素类5种主要类型的发酵制药废水生化段二级出水, 对二级出水中DOM进行解析和表征.通过XAD-8树脂亲疏水性分离、凝胶色谱分子量分级、傅里叶变换红外光谱和三维荧光表征, 系统地解析二级出水中有机物的种类、分布及特征官能团等特性, 为深度处理工艺的优化提供指导.
2 材料和方法(Materials and methods)2.1 样品采集本研究所采集的5种发酵制药废水样品分别取自于某氨基酸类制药厂(W1)、某头孢类抗生素制药厂(W2)、某混合抗生素药业制药厂(W3)、某维生素类制药厂(W4)和某阿维菌素类制药厂(W5)的生化段二级出水.采集的样品保存于装有干冰冷藏的泡沫箱中运送回实验室进行分析.样品经0.45 μm的滤膜真空抽滤后避光保存于4 ℃冰箱中, 取部分水样测定基础水质指标, 剩余样品保存待用.
2.2 水质分析方法2.2.1 常规水质指标分析COD、总有机碳(TOC)和悬浮物(SS)均按照国家标准法测定(国家环保总局, 2002).色度采用铂-钴比色法测定(陈钊等, 2020);腐殖酸含量(mg·L-1)采用改性Lowry法测定(Vakondios et al., 2014);蛋白质含量(mg·L-1)采用BCA蛋白质定量试剂盒(南京生物工程研究所)测定;多糖含量(mg·L-1)采用硫酚法测定(Ma et al., 2019).
2.2.2 阴离子含量分析使用ICS-1000型号离子色谱测定样品中Cl-、NO3-、SO42-、PO43-等无机阴离子浓度, 具体分析条件为:使用AMMS-Ⅱ电导检测器和AS22型阴离子交换柱(4 mm × 250 mm), 流动相是缓冲盐溶液(4.5 mmol·L-1 Na2CO3+ 1.4 mmol·L-1 NaHCO3), 流速为1.2 mL·min-1, 柱温为30 ℃.
2.2.3 金属离子含量分析使用电感耦合等离子体直读光谱仪测定样品中Cu2+、Cr3+、Zn2+、Pb2+、As3+、Cd2+等金属离子浓度.
2.3 溶解性有机物分析2.3.1 紫外-可见吸收光谱测定使用紫外-可见分光光度计(Shimadzu, 日本)对样品进行200~700 nm全波段紫外光谱扫描.UV254是样品在254 nm处的紫外吸收值, 代表含苯环的芳香族化合物和含多个共轭双键有机物的相对含量, 一定程度上反映了样品DOM的不饱和度(Zhang et al., 2019).
2.3.2 三维荧光光谱表征使用荧光光谱仪(RF-6000, Shimadzu, 日本)测定水样中溶解性有机物的荧光组成, 设定仪器参数为:室温为25 ℃条件下, 检测器灵敏度设置为低灵敏度, PMT电压为700 V, 扫描速度为12000 min-1, 发射波长(Emission, Em)为280~550 nm, 增量为2 cm;激发波长(Excitation, Ex)为240~450 nm, 增量为5 nm, 发射、激发光谱带宽均为5 nm.以Mill-Q超纯水为空白, 使用1 cm石英比色皿进行测定, 将拉曼散射和瑞利散射荧光杂峰校正后, 得到样品三维荧光光谱数据, 由于制药废水二级出水中溶解性有机物浓度较高, 有机物荧光强度值较大, 为了减轻内部过滤效应, 测定前将待测样品进行稀释使其TOC < 10 mg·L-1(Li et al., 2020;Shi et al., 2020).
2.3.3 亲疏水性测定采用XAD-8(Amberlite)大孔树脂对样品进行亲疏水性的分离, 将DOM分离为2种组分:亲水物质(HIS, hydrophilic substances)和疏水性物质(HOS, hydrophobic substances), 以TOC表示各组分的含量(Wei et al., 2008;Wang et al., 2013).
2.3.4 分子量测定采用高效液相尺寸排阻色谱(High performance liquid chromatography-size exclusion chromatography, HPSEC)对DOM分子量的分布进行测定, 高效液相色谱为Waters e2695型, 检测器为UV紫外检测器(Ultraviolet, UV), 凝胶渗透色谱柱为Protein Pak 125柱(7.8 mm× 300 mm, 10 μm, Waters, USA), 紫外检测波长设置为254 nm, 流动相为磷酸盐缓冲液(1.6 mmol·L-1 Na2HPO4 + 2.4 mmol·L-1 NaH2PO4)和0.025 mol·L-1 NaCl溶液的混合溶液, 流速为1.0 mL·min-1.样品进样体积为20 μL, 柱温度设置为30 ℃(Chon et al., 2017).分子量分别为210、4300、6800、17000、32000和77000 Da的聚苯乙烯磺酸钠(Polystyrene sulfonate standards, PSS)作为标准品进行测定, 得到标准物质的分子量与出峰时间的关系, 并以分子量的对数值和出峰时间绘制校准曲线.
2.3.5 傅里叶变换红外光谱测定取待测的样品放置于50 mL的离心管中, 先放置于-80 ℃冰箱冰冻24 h变成固体, 然后放置到真空冷冻干燥机上在-54 ℃条件下进行冻干, 收集获得的干燥粉末样品, 与KBr粉末以1∶100比例进行混合均匀后压片, 上机测定, 波长为500~4000 cm-1, 分辨率为4 cm-1, 测定样品中DOM的结构和化学特性(Tang et al., 2014;Wang et al., 2017).
2.4 数据统计与分析本实验中实验结果进行3次重复测定, 使用SPSS 18.0软件进行数据分析, 用Origin 8.5软件绘制图像.对EEM-PARAFAC分析是通过Matlab R2010a软件, 利用DOMFlour工具包, 通过残差分析和半数分析得到稳定的组分模型及其最大激发、发射波长和最大荧光强度(Fmax).
3 结果与讨论(Results and discussion)3.1 发酵制药废水二级出水常规水质指标分析对5种发酵制药废水二级生化出水进行常规水质指标分析, 各项水质指标测定结果分别如表 1和表 2所示.由表 1和表 2结果可以得到, 5种发酵制药废水二级出水的水质特点:①废水pH为7.4~8.5, 呈弱碱性, SS较大, 为22.50~660.80 mg·L-1, 废水较为浑浊;②5种二级出水有机物含量高, COD为156.90~736.30 mg·L-1, TOC为48.60~245.40 mg·L-1, 不同种类发酵制药废水二级出水中有机物浓度波动较大;③废水色度均较高, 表观色度呈棕黄色或红棕色, 含有大量带有发色官能团的有机物, 不能满足排放求;④废水中有机物主要以腐殖酸、蛋白为主, 浓度分别为54.70~481.30 mg·L-1和87.80~297.50 mg·L-1, 表明经过生物处理的二级出水仍含有较多的难生物降解的有机物.抗生素类、头孢类、维生素类废水中腐殖酸含量最大, 蛋白类含量次之;氨基酸类、阿维菌素类废水有机物以蛋白类为主, 腐殖酸含量次之;⑤废水中含盐量均较高, 主要以Cl-和SO42-为主, 不利于生物完全降解;⑥废水中有害金属离子如Pb2+、Zn2+、Cu2+、Cr3+含量较低, 未检出As3+、Cd2+等有毒重金属元素离子.制药工业废水采用生化方法进行处理, 对污染物去除效率低, 部分废水经二级生化处理不能达到排放标准要求, 需要进一步进行深度处理.刘峻峰等(2016)发现混装制剂制药工业废水经过生化处理后, 也难以达到国家排放标准.
表 1(Table 1)
| 表 1 发酵制药废水二级出水基本水质指标 Table 1 Water quality index of secondary effluent from fermentation pharmaceutical wastewater | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 1 发酵制药废水二级出水基本水质指标 Table 1 Water quality index of secondary effluent from fermentation pharmaceutical wastewater
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 2(Table 2)
| 表 2 发酵制药废水二级出水离子浓度 Table 2 Ion concentration of secondary effluent from fermentation pharmaceutical wastewater | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 2 发酵制药废水二级出水离子浓度 Table 2 Ion concentration of secondary effluent from fermentation pharmaceutical wastewater
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.2 发酵制药废水二级出水DOM亲疏水性分析5种发酵制药废水二级出水DOM亲疏水性占比如表 3所示, 发酵制药废水二级出水DOM大多数以疏水性组分为主, 占DOM的46.70%~85.30%, 亲水性组分占14.70%~53.30%.DOM中的疏水性物质主要是由腐殖酸和富里酸等组成, 亲水性物质主要由脂肪族碳氢化合物, 如糖类和氨基酸等(Du et al., 2014;Zheng et al., 2014;Xiao et al., 2016).因此, 发酵制药废水二级出水中腐殖酸和富里酸等难降解有机物的含量相对较多.腐殖质等疏水性物质是引起膜污染的主要物质(Shon et al., 2006;Zularisam et al., 2006), 因此发酵制药废水二级出水采用膜处理前需有效地去除腐殖质等疏水性物质, 以减轻膜污染.在氯化消毒过程中, 疏水酸性物质相比亲水物质也更易形成三卤甲烷(Lamsal et al., 2012).
表 3(Table 3)
| 表 3 5种发酵制药废水二级出水亲疏水性分析 Table 3 Hydrophobic/hydrophilic of DOM in secondary effluents from five fermentation pharmaceutical wastewaters | ||||||||||||||||||
表 3 5种发酵制药废水二级出水亲疏水性分析 Table 3 Hydrophobic/hydrophilic of DOM in secondary effluents from five fermentation pharmaceutical wastewaters
| ||||||||||||||||||
3.3 发酵制药废水二级出水分子量分析5种发酵制药废水二级出水DOM的分子量分布见图 1a.由图可见5种发酵制药废水二级出水DOM的分子量变化范围从几十到200 kDa, 出峰时间主要集中于10~20 min, 对应分子量为210~17000 Da, 二级出水仍然有大量的大分子DOM存在.相较于其他4种废水, 头孢类废水的UV响应值最高, 说明其所含有机物的紫外吸收能力大, 相对应的DOM中含有芳香族苯环及不饱和共轭双键等有机物占比最高.
图 1(Fig. 1)
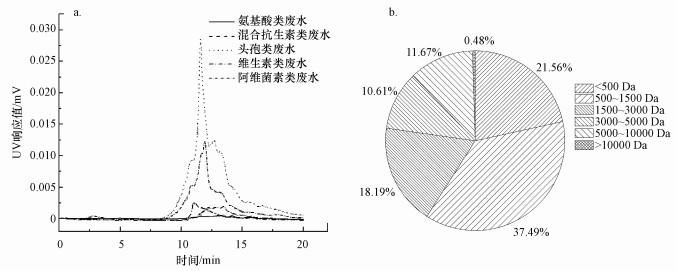 |
| 图 1 5种发酵制药废水二级出水DOM分子量分布(a)及抗生素废水二级出水DOM分子量占比(b) Fig. 1Distribution of the Molecular weight of DOM in secondary effluents from five fermentation pharmaceutical wastewater (a) and molecular weight ratio of DOM in antibiotic wastewater secondary effluent (b) |
抗生素类药品种类多且需求量大, 其废水水量较大且有机物浓度较高, 对二级出水分子量结果通过积分计算, 混合抗生素废水二级出水各分子量区间的有机物含量占比如图 1b所示, 分子量为500~1500 Da的有机物含量最高, 占比为37.49%;分子量小于500 Da的有机物占21.56%;分子量为1500~3000 Da的有机物占18.19%;分子量为3000~5000 Da的有机物占比10.61%;分子量为5000~10000 Da的有机物占11.67%;分子量大于10000 Da的有机物含量最少, 仅占0.48%.综上可知, 发酵制药废水二级出水中DOM分子量分布广泛主要在210~10000 Da范围内.Yan等(2007)发现混凝沉淀可显著去除分子量在3000~10000 Da的DOM.Gonzalez等(2013)发现臭氧或UV/H2O2可将大量高分子聚合物和腐殖质降解为小分子组分.因此, 发酵制药废水二级出水中DOM可以通过混凝预处理进一步去除, 或者通过高级氧化处理将大分子降解为小分子, 以提高其生物降解性.
3.4 发酵制药废水二级出水傅里叶变换红外光谱分析发酵制药废水二级出水含有大量发色溶解性有机物(CDOM), 发色基团和助色基团相互作用造成其色度较高, 通过FTIR图分析DOM的官能团特性, 将红外光谱按照波长范围分为4个峰区, 分别是第I(4000~2500 cm-1)、第Ⅱ(2500~1900 cm-1)、第Ⅲ(1900~1500 cm-1)及第Ⅳ(1500~600 cm-1)峰区(Qi et al., 2018;Malik et al., 2019).图 2a为5种发酵制药废水二级生化出水DOM的傅里叶变换红外光谱图, 不同DOM样品的透射峰的出峰位置具有相似性.第Ⅰ峰区主要的透射峰出现范围有3处, 在3300~ 3500 cm-1出峰推测是由于糖类和酚类等物质所含有的—OH或—NH2导致, 在2900~3000 cm-1有吸收峰推测为脂肪族等环形有机物甲基中所含的C—H键伸缩产生;在2500~2600 cm-1出峰推测为含有羧酸的有机物引起的;第Ⅱ峰区的主要透射峰出现的范围是1900~2000 cm-1, 主要是由于含有不饱和化合物中的共轭双键的不对称伸缩导致的;第Ⅲ峰区主要的透射峰出现范围是1600~1750 cm-1, 主要是由苯环、烯烃、芳香族等有机物所含有的C=C键及羧酸中的C=O键伸缩振动产生吸收峰, 在1500~1600 cm-1附近是酰胺C=O或仲酰胺N—H振动伸缩导致;第Ⅳ峰区主要透射峰较多, 其出现范围在1300~1400 cm-1, 反映出发酵制药废水二级出水DOM含有—CH3基团或含芳香烃类的吸收峰, 在1000~1200 cm-1多为醇类、脂类、醚类、多糖类及羧酸等有机物的C—O伸缩峰, 在600~870 cm-1是由于多肽类及蛋白类有机物所含的C—N、N—H及苯环上的C—H键面外弯曲形成的吸收峰(Kim et al., 2005;虞敏达等, 2017).综上, 5种二级出水DOM主要由芳香类、酚类、脂肪族类、羧酸类及蛋白类有机物组成, 这些物质含有大量的不饱和双键及发色基团和助色基团, 是导致发酵制药废水二级出水色度高的主要原因.
图 2(Fig. 2)
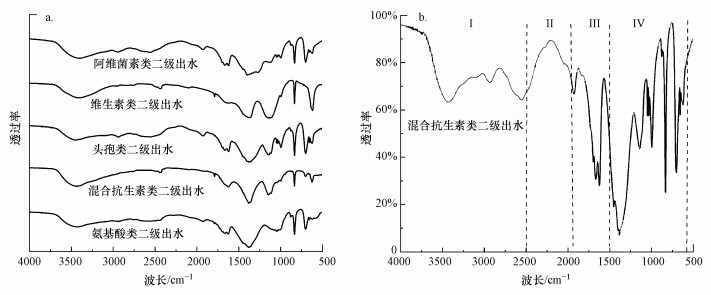 |
| 图 2 5种发酵制药废水二级出水(a)及抗生素废水二级出水傅里叶变换红外光谱图(b) Fig. 2Fourier transform infrared (FTIR) spectra in secondary effluent from fermentation pharmaceutical wastewater (a) and antibiotic wastewater secondary effluent (b) |
由图 2可见, 在头孢类废水、混合抗生素废水及维生素废水的DOM红外光谱图中1600~1690 cm-1处出现的透射峰的强度较大, 说明3种废水中不饱和有机物含量较高, 氨基酸类、头孢类及阿维菌素类废水的DOM红外光谱图中在950~1120 cm-1和600~870 cm-1处特征峰较多且峰强较高, 主要代表多肽、蛋白类和碳水化合物, 顺序为:头孢类>阿维菌素类>氨基酸类.5种二级出水DOM结构特性的不同点为:混合抗生素废水及维生素废水DOM以含芳香性和不饱和共轭键的有机物为主, 阿维菌素类和氨基酸类废水DOM以蛋白类为主, 头孢类废水中大分子不饱和含碳有机物等含量均较高.臭氧的强氧化性能够有效降解芳香族化合物或具有不饱和键结构的有机物(Van Geluwe et al., 2011), 因此发酵制药废水二级出水中的色度可以通过臭氧氧化进一步降低色度.
3.5 发酵制药废水二级出水荧光光谱分析平行因子分析法(PARAFAC)是基于对三维荧光光谱的降维处理, 从重叠的光谱中准确地分离出2~7个独立荧光组分, 方便快捷地进行有机物定性定量分析(Stedmon, 2008;Murphy et al., 2011).将5种发酵制药废水采集的样品进行荧光激发-发射光谱扫描, 5种发酵制药二级出水DOM经EEM-PARAFAC解析后, 得到4组分模型, 荧光特征峰位置及激发-发射载荷值如图 3所示.表 4中列出4组分的最大激发、发射波长和与文献中的相应组分的参比结果.C1组分含有两个荧光特征峰为Ex/Em=345(< 250)/430 nm, 属于类腐殖酸有机物(Yang et al., 2015);C2组分的荧光特征峰为Ex/Em=275/310 nm, 属于络氨酸类有机物(Zhang et al., 2020);C3组分的荧光特征峰为Ex/Em=380(270)/500 nm, 属于疏水性腐殖酸类有机物(Li et al., 2015), C4组分的荧光特征峰为Ex/Em=280(< 250)/370 nm, 属于类富里酸有机物(Qian et al., 2019).
图 3(Fig. 3)
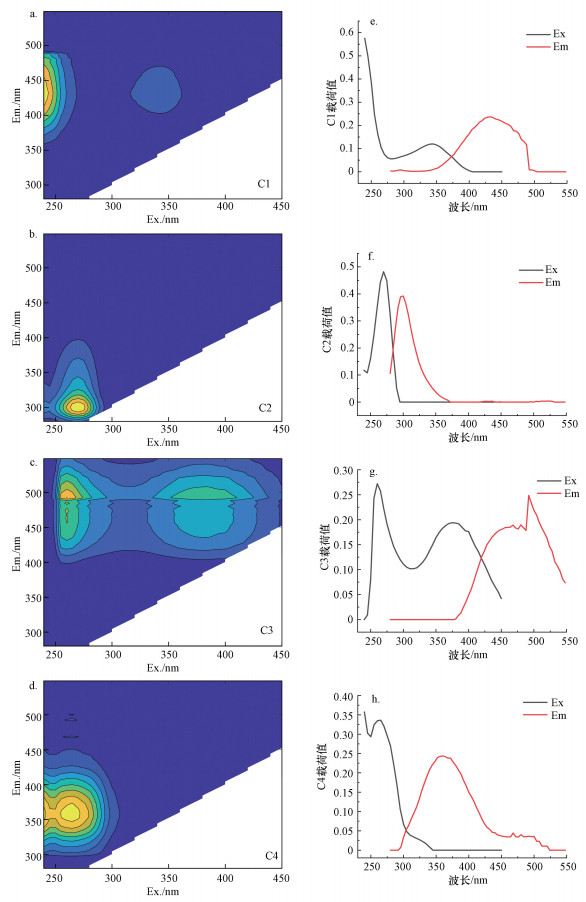 |
| 图 3 平行因子分析得出的4组分荧光光谱图及其Ex、Em对应的最大载荷值 Fig. 3PARAFAC components and wavelengths loadings (C1~C4) of the four components based on PARAFAC analysis |
表 4(Table 4)
| 表 4 发酵制药废水二级出水4荧光组分模型 Table 4 Fluorescence peak wavelength pairs for 4-component PARAFAC model | ||||||||||||||||||||
表 4 发酵制药废水二级出水4荧光组分模型 Table 4 Fluorescence peak wavelength pairs for 4-component PARAFAC model
| ||||||||||||||||||||
不同种类废水的4组分最大荧光强度具有差异性, 氨基酸类制药废水二级出水DOM中C1~C4组分的最大荧光强度排序均为:C1(289.08 R.U.)>C3(94.37 R.U.)>C2(9.38 R.U.)>C4(9.14 R.U.);头孢类抗生素废水二级出水DOM最大荧光强度值顺序为:C1(373.93 R.U.)>C3(71.25 R.U.)>C4(65.55 R.U.)>C2(14.01 R.U.);混合抗生素废水二级出水DOM最大荧光强度值顺序为:C1(120.45 R.U.)>C3(38.47 R.U.)>C4(20.04 R.U.)>C2(8.25 R.U.);维生素废水二级出水DOM最大荧光强度值顺序为:C1(126.21 R.U.)>C2(84.89 R.U.)>C3(24.22 R.U.)>C4(0);阿维菌素类废水二级出水DOM最大荧光强度值顺序为:C2(110.42 R.U.)>C1(107.68 R.U.)>C3(19.53 R.U.)>C4(4.21 R.U.).对5种废水的荧光光谱解析进行比较可知, 发酵制药废水二级出水中DOM主要是以类腐殖质有机物为主(C1、C3组分).
发酵制药废水二级出水DOM以疏水性组分为主、分子量分布广泛、含有大量的不饱和双键、苯环结构, 同时含有—OH、—NH2和C=O等发色团和助色基团, 以及以难生物降解类腐殖质有机物为主, 直接排放对水生生物和人类健康具有潜在的风险, 单一的深度处理工艺可能无法有效去除废水中DOM和满足发酵制药废水达标排放或回用的需求.针对发酵制药废水生化段二级出水中DOM的特性将不同深度处理工艺进行高效组合, 对研究发酵制药废水二级出水深度处理技术有潜在的应用意义和价值.
4 结论(Conclusions)1) 5种发酵制药废水二级出水呈高盐度和高色度.废水有机物浓度高, COD为156.90~736.30 mg·L-1, TOC为48.60~245.40 mg·L-1, 不同种类发酵制药废水二级出水中有机物浓度波动较大.废水中有机物主要以腐殖酸和蛋白类有机物为主.
2) 对5种发酵制药废水二级出水DOM表征, 通过亲疏水性分离可知, 发酵制药废水二级出水DOM大多数以疏水性组分为主, 占DOM的46.70%~85.30%.亲水性组分占14.70%~53.30%;通过分子量分析可知发酵制药废水二级出水中DOM分子量分布广泛, 主要在210~10000 Da范围内;通过红外光谱分析可知, 二级出水DOM均含有大量的不饱和双键、苯环结构, 同时含有—OH、—NH2和C=O等发色团和助色基团, 造成废水中色度较高;通过对发酵制药废水二级出水进行EEM-PARAFAC分析确定了4种荧光组分特征峰, 包括3种(C1、C3、C4)腐殖质类和1种(C2)类蛋白类组分.发酵制药废水二级出水中DOM主要是以类腐殖质有机物为主(C1、C3组分).
参考文献
| Carstea E M, Bridgeman J, Baker A, et al. 2016. Fluorescence spectroscopy for wastewater monitoring: A review[J]. Water Research, 95: 205-219. DOI:10.1016/j.watres.2016.03.021 |
| Chon K, Chon K, Cho J. 2017. Characterization of size fractionated dissolved organic matter from river water and wastewater effluent using preparative high performance size exclusion chromatography[J]. Organic Geochemistry, 103: 105-112. DOI:10.1016/j.orggeochem.2016.11.003 |
| 陈钊, 李生彬, 封云杉. 2020. 铂钴比色法测定水中色度不确定的分析[J]. 内蒙古科技与经济, 3: 52-56. |
| Cohen E, Levy G J, Borisover M. 2014. Fluorescent components of organic matter in wastewater: Efficacy and selectivity of the water treatment[J]. Water Research, 55: 323-334. DOI:10.1016/j.watres.2014.02.040 |
| Du X, Xu Z, Li J, et al. 2014. Characterization and removal of dissolved organic matter in a vertical flow constructed wetland[J]. Ecological Engineering, 73: 610-615. DOI:10.1016/j.ecoleng.2014.09.098 |
| Gadipelly C, Perezgonzalez A, Yadav G D, et al. 2014. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse[J]. Industrial & Engineering Chemistry Research, 53(29): 11571-11592. |
| Gonzalez O C, Justo A, Bacardit J, et al. 2013. Characterization and fate of effluent organic matter treated with UV/H2O2 and ozonation[J]. Chemical Engineering Journal, 226: 402-408. DOI:10.1016/j.cej.2013.04.066 |
| 国家环保总局. 2002. 水和废水监测分析方法[M]. 北京: 中国环境科学出版社. |
| Kim H, Yu M. 2005. Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control[J]. Water Research, 39(19): 4779-4789. DOI:10.1016/j.watres.2005.09.021 |
| Lamsal R, Montreuil K R, Kent F C, et al. 2012. Characterization and removal of natural organic matter by an integrated membrane system[J]. Desalination, 303: 12-16. DOI:10.1016/j.desal.2012.06.025 |
| Li J, Wang L, Geng J, et al. 2020. Distribution and removal of fluorescent dissolved organic matter in 15 municipal wastewater treatment plants in China[J]. Chemosphere, 251: 126375. DOI:10.1016/j.chemosphere.2020.126375 |
| Li W, Xu Z, Wu Q, et al. 2015. Characterization of fluorescent-dissolved organic matter and identification of specific fluorophores in textile effluents[J]. Environmental Science and Pollution Research, 22(6): 4183-4189. DOI:10.1007/s11356-014-3201-4 |
| 刘峻峰, 黄琳琳, 李姗姗, 等. 2016. 电催化氧化对制药废水二级出水的深度处理效能[J]. 环境工程学报, 10(11): 6269-6274. DOI:10.12030/j.cjee.201507033 |
| Luo H, Yang R, Zhao Y, et al. 2018. Recent advances and strategies in process and strain engineering for the production of butyric acid by microbial fermentation[J]. Bioresource Technology, 253: 343-354. DOI:10.1016/j.biortech.2018.01.007 |
| Ma K, Qin Z, Zhao Z, et al. 2016. Toxicity evaluation of wastewater collected at different treatment stages from a pharmaceutical industrial park wastewater treatment plant[J]. Chemosphere, 158: 163-170. DOI:10.1016/j.chemosphere.2016.05.052 |
| Ma S J, Ma H J, Hu H D, et al. 2019. Effect of mixing intensity on hydrolysis and acidification of sewage sludge in two-stage anaerobic digestion: Characteristics of dissolved organic matter and the key microorganisms[J]. Water Research, 148: 359-367. DOI:10.1016/j.watres.2018.10.058 |
| Malik S N, Khan S M, Ghosh P C, et al. 2019. Treatment of pharmaceutical industrial wastewater by nano-catalyzed ozonation in a semi-batch reactor for improved biodegradability[J]. Science of the Total Environment, 678: 114-122. DOI:10.1016/j.scitotenv.2019.04.097 |
| Mangal V, Degasparro S, Beresford D V, et al. 2020. Linking molecular and optical properties of dissolved organic matter across a soil-water interface on Akimiski Island(Nunavut, Canada)[J]. Science of the Total Environment, 704: 135415. DOI:10.1016/j.scitotenv.2019.135415 |
| Murphy K R, Hambly A, Singh S, et al. 2011. Organic matter fluorescence in municipal water recycling schemes: toward a unified PARAFAC model[J]. Environmental Science & Technology, 45(7): 2909-2916. |
| Pan H, Yu H, Wang Y, et al. 2018. Investigating variations of fluorescent dissolved organic matter in wastewater treatment using synchronous fluorescence spectroscopy combined with principal component analysis and two-dimensional correlation[J]. Environmental Technology, 39(19): 2495-2502. DOI:10.1080/09593330.2017.1357759 |
| Podgorski D C, Zito P, Mcguire J T, et al. 2018. Rebuttal to comment on "examining natural attenuation and acute toxicity of petroleum-derived dissolved organic matter with optical spectroscopy"[J]. Environmental Science & Technology, 52: 11962-11963. |
| Qi W, Zhang H, Hu C, et al. 2018. Effect of ozonation on the characteristics of effluent organic matter fractions and subsequent associations with disinfection by-products formation[J]. Science of the Total Environment, 610-611: 1057-1064. DOI:10.1016/j.scitotenv.2017.08.194 |
| Qian F, He M, Wu J, et al. 2019. Insight into removal of dissolved organic matter in post pharmaceutical wastewater by coagulation-UV/H2O2[J]. Journal Environmental Science (China), 76: 329-338. DOI:10.1016/j.jes.2018.05.025 |
| Shen J, Liu B, Wu J, et al. 2020. Characterization of fluorescent dissolved organic matters in metalworking fluid by fluorescence excitation-emission matrix and high-performance liquid chromatography[J]. Chemosphere, 239: 124703. DOI:10.1016/j.chemosphere.2019.124703 |
| Shi Y, Li S, Wang L, et al. 2020. Characteristics of DOM in 14 AAO processes of municipal wastewater treatment plants[J]. Science of the Total Environment, 742: 140654. DOI:10.1016/j.scitotenv.2020.140654 |
| Shi X, Leong K Y, Ng H Y. 2017. Anaerobic treatment of pharmaceutical wastewater: A critical review[J]. Bioresource Technology, 245(Pt A): 1238-1244. |
| Shon H K, Vigneswaran S, Kim I S, et al. 2006. Fouling of ultrafiltration membrane by effluent organic matter: A detailed characterization using different organic fractions in wastewater[J]. Journal of Membrane Science, 278(1): 232-238. |
| Stedmon C A. 2008. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial[J]. Limnology and Oceanography-methods, 6(11): 572-579. DOI:10.4319/lom.2008.6.572 |
| Tang X, Wu Q Y, Zhao X, et al. 2014. Transformation of anti-estrogenic-activity related dissolved organic matter in secondary effluents during ozonation[J]. Water Research, 48: 605-612. DOI:10.1016/j.watres.2013.10.016 |
| Tran N H, Ngo H H, Urase T, et al. 2015. A critical review on characterization strategies of organic matter for wastewater and water treatment processes[J]. Bioresource Technology, 193: 523-533. DOI:10.1016/j.biortech.2015.06.091 |
| Vakondios N, Koukouraki E E, Diamadopoulos E. 2014. Effluent organic matter (EfOM) characterization by simultaneous measurement of proteins and humic matter[J]. Water Research, 63: 62-70. DOI:10.1016/j.watres.2014.06.011 |
| Van Geluwe S, Braeken L, Der Bruggen B V, et al. 2011. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: A review[J]. Water Research, 45(12): 3551-3570. DOI:10.1016/j.watres.2011.04.016 |
| Wang D, Zhao Y, Xie J, et al. 2013. Characterizing DOM and removal by enhanced coagulation: A survey with typical Chinese source waters[J]. Separation and Purification Technology, 110: 188-195. DOI:10.1016/j.seppur.2013.03.020 |
| Wang H W, Li X Y, Hao Z P, et al. 2017. Transformation of dissolved organic matter in concentrated leachate from nanofiltration during ozone-based oxidation processes (O3, O3/H2O2 and O3/UV)[J]. Journal of Environmental Management, 191: 244-251. DOI:10.1016/j.jenvman.2017.01.021 |
| Wei Q, Wang D, Wei Q, et al. 2008. Size and resin fractionations of dissolved organic matter and trihalomethane precursors from four typical source waters in China[J]. Environmental Monitoring and Assessment, 141(1/3): 347-357. |
| Xiao K, Sun J, Shen Y, et al. 2016. Fluorescence properties of dissolved organic matter as a function of hydrophobicity and molecular weight: case studies from two membrane bioreactors and an oxidation ditch[J]. RSC Advances, 6(29): 24050-24059. DOI:10.1039/C5RA23167A |
| Xue S, Jin W, Zhang Z, et al. 2017. Reductions of dissolved organic matter and disinfection by-product precursors in full-scale wastewater treatment plants in winter[J]. Chemosphere, 179: 395-404. DOI:10.1016/j.chemosphere.2017.02.106 |
| Yan M, Wang D, Shi B, et al. 2007. Effect of pre-ozonation on optimized coagulation of a typical North-China source water[J]. Chemosphere, 69(11): 1695-1702. DOI:10.1016/j.chemosphere.2007.06.014 |
| Yang L, Hur J, Zhuang W. 2015. Occurrence and behaviors of fluorescence EEM-PARAFAC components in drinking water and wastewater treatment systems and their applications: a review[J]. Environmental Science and Pollution Research, 22(9): 6500-6510. DOI:10.1007/s11356-015-4214-3 |
| 虞敏达, 何小松, 檀文炳, 等. 2017. 污水厂出水颗粒态与溶解态有机物的红外和荧光光谱特征[J]. 光谱学与光谱分析, 37(8): 2467-2473. |
| Zhang B, Shan C, Hao Z, et al. 2019. Transformation of dissolved organic matter during full-scale treatment of integrated chemical wastewater: Molecular composition correlated with spectral indexes and acute toxicity[J]. Water Research, 157: 472-482. DOI:10.1016/j.watres.2019.04.002 |
| Zhang H, Qu J, Liu H. 2010. Effect of chlorination and ozone pre-oxidation on the photobacteria acute toxicity for dissolved organic matter from sewage treatment plants[J]. Science China Chemistry, 53: 2394-2398. DOI:10.1007/s11426-010-4040-x |
| Zhang P, Huang P, Xu X, et al. 2020. Spectroscopic and molecular characterization of biochar-derived dissolved organic matter and the associations with soil microbial responses[J]. Science of the Total Environment, 708: 134619. DOI:10.1016/j.scitotenv.2019.134619 |
| Zheng X, Khan M T, Croue J, et al. 2014. Contribution of effluent organic matter (EfOM) to ultrafiltration (UF) membrane fouling: Isolation, characterization, and fouling effect of EfOM fractions[J]. Water Research, 65: 414-424. DOI:10.1016/j.watres.2014.07.039 |
| Zularisam A W, Ismail A F, Salim R, et al. 2006. Behaviours of natural organic matter in membrane filtration for surface water treatment-a review[J]. Desalination, 194(1/3): 211-231. |
| Zwienerm C, Frimmel F H. 2000. Oxdative treatment of phramaceuticals in water[J]. Water Research, 34(6): 5. |
