 , 李晓东
, 李晓东
 , 陈彤
, 陈彤浙江大学热能工程研究所, 能源清洁利用国家重点试验室, 杭州 310027
收稿日期: 2018-12-11; 修回日期: 2019-01-24; 录用日期: 2019-01-24
基金项目: 国家重点研发计划战略性国际科技创新合作重点专项(No.2016YFE0202000)
作者简介: 张梦玫(1991-), 女, 博士生, E-mail:zhangmm@zju.edu.cn
通讯作者(责任作者): 李晓东, 教授、博士生导师, 中国履行斯德哥尔摩公约国家协调工作组专家、中国环境学会二
 英专家委员会委员, 主要从事煤和废弃物燃烧及污染控制、持久性有机污染物生成及控制的理论及技术等方面研究. E-mail:lixd@zju.edu.cn
英专家委员会委员, 主要从事煤和废弃物燃烧及污染控制、持久性有机污染物生成及控制的理论及技术等方面研究. E-mail:lixd@zju.edu.cn摘要: 氯化铜被认为是对二
 英(PCDD/F)生成促进作用最强的金属催化剂,温度和氧气含量是影响其催化二
英(PCDD/F)生成促进作用最强的金属催化剂,温度和氧气含量是影响其催化二 英生成的关键因素.本文选取250~550℃间8个不同温度点及0~20%间4种不同氧气含量,系统性地研究了温度和氧气含量对含氯化铜模拟飞灰(MFA)生成二
英生成的关键因素.本文选取250~550℃间8个不同温度点及0~20%间4种不同氧气含量,系统性地研究了温度和氧气含量对含氯化铜模拟飞灰(MFA)生成二 英的影响,并通过分析指纹特性探讨二
英的影响,并通过分析指纹特性探讨二 英的生成路径和机理.结果发现,290~350℃之间二
英的生成路径和机理.结果发现,290~350℃之间二 英生成量较高,且在290℃时二
英生成量较高,且在290℃时二 英总量达到最大值;氧气含量为10%时二
英总量达到最大值;氧气含量为10%时二 英生成量达到最高.研究二
英生成量达到最高.研究二 英同分异构体分布可从分子层面为研究其生成路径和机理提供重要信息,因此,本文分析了四至八氯代PCDD/F的全部异构体的分布.结果表明,在本文所选取的温度和氧含量范围内,温度对二
英同分异构体分布可从分子层面为研究其生成路径和机理提供重要信息,因此,本文分析了四至八氯代PCDD/F的全部异构体的分布.结果表明,在本文所选取的温度和氧含量范围内,温度对二 英生成路径的影响高于氧气含量.最后,重点讨论了2,3,7,8位氯取代的17种有毒异构体的分布随温度和氧气的变化情况及生成路径.利用主成分分析法(PCA)证实了氯化铜催化二
英生成路径的影响高于氧气含量.最后,重点讨论了2,3,7,8位氯取代的17种有毒异构体的分布随温度和氧气的变化情况及生成路径.利用主成分分析法(PCA)证实了氯化铜催化二 英从头合成过程中存在经由氯酚路径生成的异构体,并讨论了不同工况下氯酚路径对二
英从头合成过程中存在经由氯酚路径生成的异构体,并讨论了不同工况下氯酚路径对二 英生成的影响.
英生成的影响.关键词:氯化铜模拟飞灰二
 英催化指纹特性异构体分布
英催化指纹特性异构体分布Copper chloride catalyzed PCDD/F-formation: Experiments and PCDD/F-signatures
ZHANG Mengmei
 , LI Xiaodong
, LI Xiaodong
 , CHEN Tong
, CHEN Tong State Key Laboratory of Clean Energy Utilization, Institute for Thermal Power Engineering, Zhejiang University, Hangzhou 310027
Received 11 December 2018; received in revised from 24 January 2019; accepted 24 January 2019
Abstract: Copper chloride (CuCl2) has been identified as the strongest among various catalysts for formation of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/F). This study systematically explores the effect of temperature and oxygen on the formation of PCDD/F from CuCl2-catalyzed system, over a wide range of temperature (250~550℃) and oxygen content (0~20%). Both PCDD/F-output and its signature are extensively characterized, including homologue and isomer profiles. The temperature range between 290 and 350℃ largely promotes the formation of PCDD/F and a maximum output is reached at 290℃. An oxygen content of 10% in the gas phase is favorable for forming PCDD/F. Isomer-specific information is essential for researchers in their quest towards reaction mechanisms. In all cases a complete analysis on isomer distribution pattern is produced. Still, temperature exhibits a more significant influence on isomer profiles than oxygen content ranging from 5% to 20%. Special attention is paid to the seventeen 2, 3, 7, 8-substituted isomers, to their behaviour and relationship. The existence of chlorophenols (CP)-route congeners in CuCl2-catalyzed de novo formation is confirmed by principal component analysis (PCA); their contributions at different conditions are then discussed.
Keywords: copper chloridemodel fly ashpolychlorinated dibenzo-p-dioxins (PCDD) and dibenzofurans (PCDF)catalysissignature or fingerprintisomer distribution
1 引言(Introduction)二



自1977年首次报道在城市生活垃圾焚烧炉烟气中发现二







大量研究表明, 铜化合物尤其是氯化铜(CuCl2)对PCDD/F生成的促进作用最强(Olie et al., 1998; Takaoka et al., 2005a; Fujimori et al., 2009; Chin et al., 2012; Zhang et al., 2016).氯化铜既可为反应提供所必需的氯源, 氯化飞灰中的碳(Weber et al., 2001; Fujimori et al., 2009);又可催化碳—碳键断裂, 释放小分子碳结构, 以及催化碳环的闭合, 从而生成PCDD/F(Stieglitz, 1998; Takaoka et al., 2005b).温度和氧气含量是影响金属催化二


二









基于此, 本文在实验室条件下, 采用含有氯化铜的模拟飞灰, 系统地探究不同温度(250~550 ℃)及氧气含量(0~20%)对氯化铜催化生成二



2 试验材料和方法(Materials and methods)2.1 试验材料试验采用模拟飞灰(Model fly ash, MFA), 其配比为活性炭(3%(质量分数), 粒径0.037~0.075 mm)、氯化钠(NaCl, 10% Cl)、氯化铜(CuCl2·2H2O, 0.2% Cu), 其余为石英砂(SiO2, 粒径0.075~0.150 mm).活性炭及石英砂均用去离子水及丙酮分别淋洗3遍, 并在100 ℃下烘8 h至完全干燥.将氯化铜溶于少量蒸馏水, 所得溶液添加到其余固体组分中, 在研钵中共同混合研磨20 min至均匀.混合物在80 ℃下烘16 h至完全干燥, 遮光密封保存, 以备试验使用.另配有仅含活性炭(3%)、氯化钠(10% Cl)及石英砂的空白模拟飞灰(Blank MFA)作为对照.
2.2 试验装置和试验设计实验装置如图 1所示, 采用立式单段管式炉系统.管式炉总长0.4 m, 配有独立的加热和温控装置.石英管反应器内径20 mm, 中部设有石英砂芯样品床, 并配有探针式热电偶实时监测样品的反应温度.每次实验采用模拟飞灰2 g, 放置于样品床上, 并覆盖少量已去除有机物的玻璃棉.将石英管插入已预先加热至设定温度的管式炉中, 并通入混合均匀的反应气流(100 mL·min-1, O2/N2), 反应时间为60 min.具体实验工况见表 1, 其中, 工况S0为空白对照, S1~S8为不同反应温度的可比较工况, S4、S5、S9~S14为350 ℃及380 ℃时不同氧气含量的可比较工况.反应气氛为0%氧气工况时, 氮气瓶中输出的气流先通过质量分数为25%的焦性没食子酸水溶液以去除气流中可能夹杂的氧气, 再通入石英管反应器.每个样品加热结束后, 对XAD-Ⅱ树脂和甲苯吸收液收集的气相中二


图 1(Fig. 1)
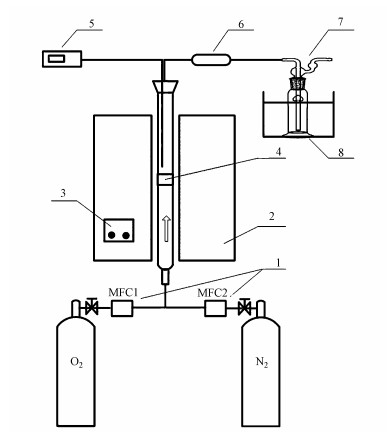 |
| 图 1 反应系统原理图(1.质量流量计, 2.立式管式炉和石英管, 3.管式炉温控装置, 4.样品床, 5.探针式热电偶, 6.XAD-Ⅱ树脂, 7.甲苯吸收液, 8.冰水浴) Fig. 1Schematic of the reactor system |
表 1(Table 1)
| 表 1 本研究试验工况 Table 1 Experimental program applied in this study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 1 本研究试验工况 Table 1 Experimental program applied in this study
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2.3 样品预处理及检测样品预处理按照EPA1613方法进行, 主要包括索氏提取、定容、过酸碱多级硅胶柱和氧化铝柱、氮吹(Zhang et al., 2018b).在索提、定容、氮吹过程中分别加入不同类型的同位素标样, 用于PCDD/F定量及计算标样回收率.使用日本JEOL公司的高分辨气相色谱/高分辨质谱(HRGC/HRMS, 型号: JMS-800D Ultra Focus)对样品中的PCDD/F进行检测.色谱条件:DB-5MS (60 m×0.25 mm×0.25 μm)毛细管柱;升温程序:初始温度为150 ℃, 然后以25 ℃·min-1的速率升至190 ℃, 再以3 ℃·min-1的速率升到280 ℃;进样口温度为270 ℃;载气采用氦气(99.999%), 流量为1.2 mL·min-1, 不分流自动进样, 每次的进样量为1 μL.质谱的电离方式为EI模式, 电离能为38 eV, 选择离子检测SIM, 分辨率大于10000.根据选择的离子及相对保留时间确定DB-5MS柱分离的色谱峰所对应的异构体.目标检测物是PCDD/F四至八氯代共136种异构体.由于部分异构体在DB-5MS色谱柱上的流出顺序极为接近, 实际上共94种异构体/异构体组能够被区分开来.
采用同位素稀释法对PCDD/F定量, 利用5种不同浓度的标样拟合每种化合物的标准曲线, 并计算出响应因子(陈彤, 2006).同位素标样从剑桥标准实验室购买, 包括除13C-OCDF以外的其余16种2, 3, 7, 8位氯取代的PCDD/F异构体的同位素.其中, 13C-1, 2, 3, 7, 8, 9-HxCDD被用作进机标, 因此, 1, 2, 3, 7, 8, 9-HxCDD采用13C-1, 2, 3, 4, 7, 8-HxCDD和13C-1, 2, 3, 6, 7, 8-HxCDD的平均响应因子来定量.13C-OCDF是潜在的干扰物, 因此用13C-OCDD来定量OCDF.对于其他无2, 3, 7, 8位氯取代的异构体, 则采用相同氯代水平的2, 3, 7, 8-PCDD/F的平均响应因子来定量.检测结束后, 计算各样品中同位素标样的回收率, 当且仅当回收率合格的样品数据才可算为有效.
3 结果和讨论(Results and discussion)3.1 温度对PCDD/F生成的影响温度是影响二



图 2(Fig. 2)
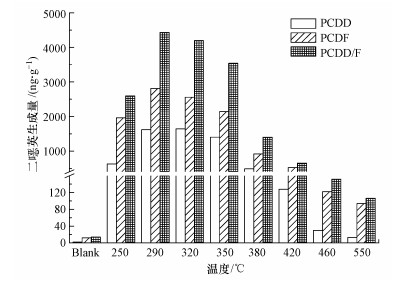 |
图 2 10% O2/N2气氛下温度对二 |
表 2(Table 2)
| 表 2 10% O2/N2气氛下毒性当量(TEQ)、PCDF/PCDD比值及氯代系数随温度的变化情况 Table 2 Toxic equivalence quantity (TEQ), the ratio of PCDF to PCDD and chlorination level at 10% O2/N2 as a function of temperature | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 2 10% O2/N2气氛下毒性当量(TEQ)、PCDF/PCDD比值及氯代系数随温度的变化情况 Table 2 Toxic equivalence quantity (TEQ), the ratio of PCDF to PCDD and chlorination level at 10% O2/N2 as a function of temperature
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PCDF/PCDD的比值如表 2所示.空白模拟飞灰的PCDF生成量远高于PCDD, CuCl2的加入减缓了PCDF与PCDD生成量的悬殊.所有温度下PCDF生成量均高于PCDD, PCDF/PCDD比值随温度升高呈先下降(250~350 ℃)后上升(≥380 ℃)的趋势.最适宜温度区间(290~350 ℃)内PCDD和PCDF均大量生成, 当温度高于380 ℃时, PCDD相对于PCDF的量急剧下降, 这可能与PCDF比PCDD性质更稳定有关(Luijk et al., 1994).高温时PCDD比PCDF更易降解, 因而相对含量降低.毒性当量(TEQ)变化趋势与PCDD/F总量变化趋势相同, 均在290 ℃达到峰值(表 2).
氯代系数(氯原子的质量平均取代个数)和同系物分布比例随温度的变化情况如表 2和图 3所示.无CuCl2时, 空白模拟飞灰生成PCDD/F主要以低氯代同系物(TCDD/F、PeCDD/F)为主, 除OCDD外其余同系物均呈现随氯代个数升高而占比逐渐降低的趋势(图 3), 因此, 整体氯代系数也较低(表 2).在模拟飞灰中氯总量不变的情况下, CuCl2的加入使得同系物分布趋势恰好相反:PCDD和PCDF均以高氯代同系物(OCDD/F、HpCDD/F)为主, 占比随氯代个数的升高而提高, 整体氯代系数也随之升高.这表明CuCl2可作为无机氯向有机氯转化的有效传递媒介(Luijk et al., 1994).PCDD/F同系物分布随温度升高而持续变化:250 ℃时, 由于温度较低, 氯化反应远高于脱氯化反应, PCDD和PCDF中均以OCDD/F为主;随着温度升高, OCDD和OCDF占比均先降低后升高, 在350 ℃时达到第2个峰值;而温度继续提高, 脱氯化反应渐强, OCDD/F占比呈递减趋势.相应的, PCDD/F整体氯代系数也在250 ℃时取得最大值, 经过先下降后上升并在350 ℃时取得第2个峰值后逐渐降低.不论是对于PCDD/F生成总量抑或是同系物分布(及氯代系数), 350~380 ℃均为其随温度变化的明显转折点.因此, 本文接下来选取350和380 ℃两个温度点分别探究氧气含量对PCDD/F生成的影响.
图 3(Fig. 3)
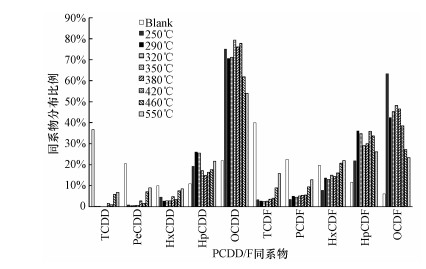 |
| 图 3 10% O2/N2气氛下温度对PCDD/F同系物分布的影响(PCDD 100%, PCDF 100%) Fig. 3Distribution of homologue groups (PCDD 100%, PCDF 100%) at 10% O2/N2 as a function of temperature |
3.2 氧气含量对PCDD/F生成的影响在二



图 4(Fig. 4)
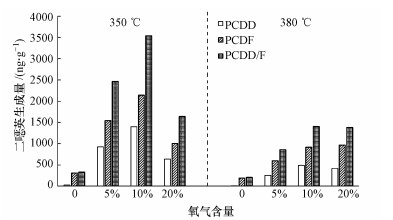 |
图 4 350 ℃和380 ℃时不同氧气含量对二 |
表 3(Table 3)
| 表 3 350 ℃和380 ℃时TEQ、PCDF/PCDD比值及氯代系数随氧气含量的变化情况 Table 3 TEQ, the ratio of PCDF to PCDD and chlorination level at 350 ℃ and 380 ℃ as a function of oxygen content | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 3 350 ℃和380 ℃时TEQ、PCDF/PCDD比值及氯代系数随氧气含量的变化情况 Table 3 TEQ, the ratio of PCDF to PCDD and chlorination level at 350 ℃ and 380 ℃ as a function of oxygen content
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
350和380 ℃时氯代系数和同系物分布比例随氧气含量的变化情况如表 3和图 5所示.无氧条件下高氯代同系物生成较少, 氯代系数也相应较低.由于模拟飞灰中氯含量充足, 有氧条件下所生成的PCDD和PCDF均倾向于高氯代同系物, 且同系物所占比例均随氯化度的升高而增加(图 5), 说明氧气能够有效促进无机氯向有机氯的转化(Pekárek et al., 2001).有氧条件下所生成的PCDD/F氯代系数均大于7, 10%氧含量下所生成的PCDD/F氯代系数略微低于5%和20%氧含量下的氯代系数, 原因是10%氧含量下PCDD/F各同系物均受到促进而大量生成, OCDD/F所占百分比略有降低.
图 5(Fig. 5)
 |
| 图 5 350 ℃和380 ℃时氧气含量对PCDD/F同系物分布的影响(PCDD 100%, PCDF 100%) Fig. 5Distribution of homologue groups(PCDD 100%, PCDF 100%) at 350 ℃ and 380 ℃ as a function of oxygen content |
3.3 PCDD/F同分异构体分布分析同分异构体分布是指该异构体在其对应的同系物中所占的比例.以TCDD为例, 该同系物的同分异构体分布是指TCDD所有同分异构体各自的量占TCDD总量的百分比.研究同分异构体分布能够为分析PCDD/F的生成路径及生成机理提供重要信息(Weber et al., 1999; Zhang et al., 2008).不同金属催化剂催化生成PCDD/F的同分异构体分布具有各自不同的特点(Zhang et al., 2016; Zhang et al., 2017).本文中PCDD/F同分异构体分布随温度和氧含量的变化情况分别如图 6、图 7所示.结果表明, 尽管不同温度、氧含量下氯化铜模拟飞灰的二

图 6(Fig. 6)
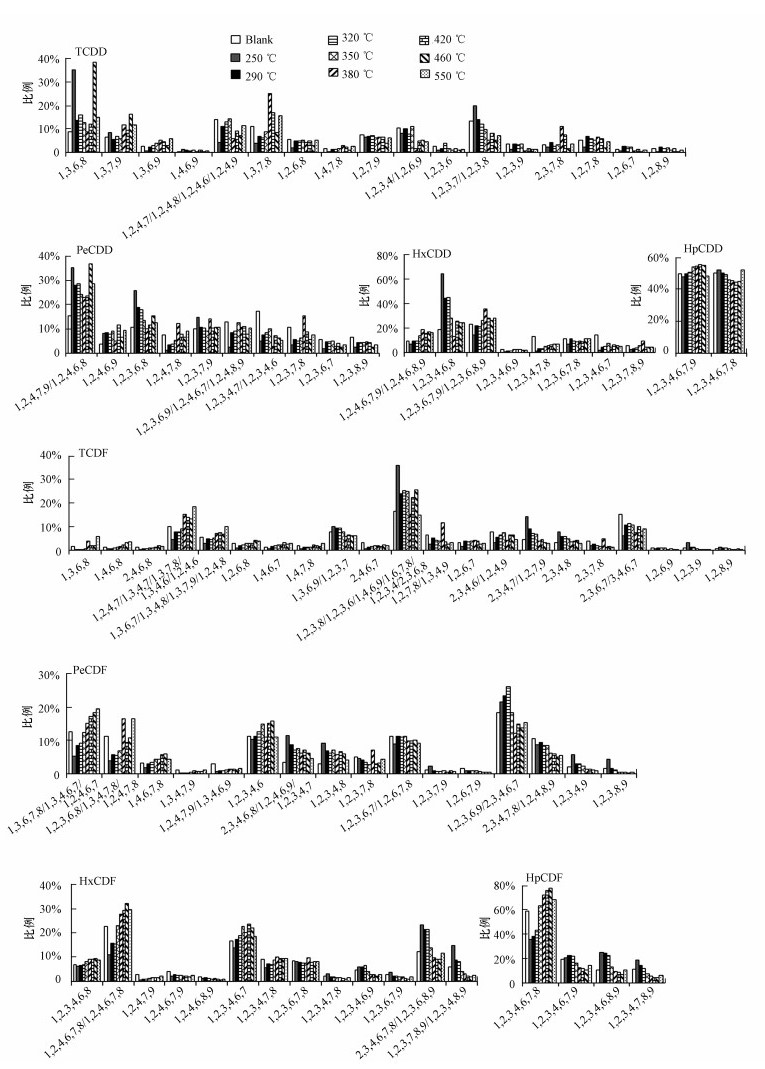 |
| 图 6 10% O2/N2气氛下温度对PCDD/F同分异构体分布的影响 Fig. 6Isomer distribution of PCDD/F at 10% O2/N2 as a function of temperature |
图 7(Fig. 7)
 |
| 图 7 350 ℃和380 ℃时氧气含量对PCDD/F同分异构体分布的影响 Fig. 7Isomer distribution pattern of PCDD/F at 350 ℃ and 380 ℃ as a function of oxygen content |
从二






表 4(Table 4)
| 表 4 10% O2/N2气氛下温度对17种有毒PCDD/F同分异构体的Hagenmaier分布值的影响 Table 4 Hagenmaier profiles of the 17 toxic PCDD/F isomers at 10% O2/N2 as a function of temperature | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 4 10% O2/N2气氛下温度对17种有毒PCDD/F同分异构体的Hagenmaier分布值的影响 Table 4 Hagenmaier profiles of the 17 toxic PCDD/F isomers at 10% O2/N2 as a function of temperature
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 5(Table 5)
| 表 5 350 ℃和380 ℃时不同氧气含量对17种有毒PCDD/F同分异构体的Hagenmaier分布值的影响 Table 5 Hagenmaier profiles of the 17 toxic PCDD/F isomers at 350 ℃ and 380 ℃ as a function of oxygen content | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 5 350 ℃和380 ℃时不同氧气含量对17种有毒PCDD/F同分异构体的Hagenmaier分布值的影响 Table 5 Hagenmaier profiles of the 17 toxic PCDD/F isomers at 350 ℃ and 380 ℃ as a function of oxygen content
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
本文利用主成分分析法(Principal component analysis, PCA)研究各异构体分布变化的整体情况.图 8是利用SPSS 20.0软件基于S1~S14共14个工况所得出的数据对TCDD、PeCDD、HxCDD和TCDF内同分异构体分布的主成分分析, 提取因子数为2.两个因子的累积方差贡献率分别为80.8%(TCDD)、84.7% (PeCDD)、84.0% (HxCDD)和75.2% (TCDF), 说明2个因子可代表14个工况变量中的大部分信息.观察TCDD各同分异构体在图中的分布发现, 1, 3, 6, 8-和1, 3, 7, 9-TCDD远离其余所有同分异构体且分布在其对立面.同样地, 1, 2, 4, 7, 9/1, 2, 4, 6, 8-、1, 2, 3, 6, 8-和1, 2, 3, 7, 9-PeCDD在PeCDD中的分布及1, 2, 3, 4, 6, 8-HxCDD在HxCDD中的分布都是类似情况, 表明这7种异构体的生成路径与其余PCDD异构体有所不同.尽管大部分PCDD异构体的具体生成途径尚不明确, 但这7种PCDD异构体已确定为经由氯酚(Chlorophenols, CP)作为中间产物缩聚而生成(Tuppurainen et al., 2003; Ryu et al., 2005a), 被称为氯酚路径异构体(CP-route isomers).本文采用活性炭作为碳源进行从头合成试验, 未添加任何二

图 8(Fig. 8)
 |
| 图 8 基于14组CuCl2模拟飞灰PCDD/F生成数据对TCDD、PeCDD、HxCDD和TCDF内同分异构体分布的主成分分析(PCA) Fig. 8Principal component analysis (PCA) of isomer profiles within TCDD, PeCDD, HxCDD and TCDF (based on the 14 sets of CuCl2-MFA data) |
由图 6可知, 相比于空白模拟飞灰,CuCl2的加入明显提高了氯酚路径PCDD/F异构体的占比, 即CuCl2能够选择性地促进PCDD/F从头合成过程中经由氯酚路径生成.不同于2, 3, 7, 8-PCDD/F异构体, 氯酚路径PCDD/F异构体比例的变化情况具有一定共性, 即随温度升高先降低(多在380 ℃取得最小值)后升高(多在460 ℃达到峰值)再降低.250和460 ℃最有利于PCDD/F经由氯酚路径生成, 350和380 ℃下氧气对于氯酚路径的影响较为复杂(图 7), 尚不能得出明确结论.
4 结论(Conclusions)1) 通过在温度250~550 ℃范围内选取8个不同温度点, 明确了氯化铜模拟飞灰生成二



2) 同时研究了350 ℃及380 ℃下氧气含量对二




3) 在温度250~550 ℃、氧气含量0~20%的范围内, 温度对二




参考文献
| Addink R, Olie K. 1995. Mechanisms of formation and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans in heterogeneous systems[J]. Environmental Science & Technology, 29(6): 1425–1435. |
陈彤.2006.城市生活垃圾焚烧过程中二二 英英的形成机理及控制技术研究[D].杭州: 浙江大学http://www.cnki.com.cn/Article/CJFDTotal-DTSJ201801008.htm 英英的形成机理及控制技术研究[D].杭州: 浙江大学http://www.cnki.com.cn/Article/CJFDTotal-DTSJ201801008.htm |
| Chin Y, Lin C, Chang-Chien G, et al. 2012. PCDD/F formation catalyzed by the metal chlorides and chlorinated aromatic compounds in fly ash[J]. Aerosol and Air Quality Research, 12: 228–236.DOI:10.4209/aaqr.2011.09.0139 |
| Fiedler H. 1998. Thermal formation of PCDD/PCDF:A survey[J]. Environmental Engineering Science, 15(1): 49–58. |
付建英, 李晓东, 詹明秀, 等. 2015. 生活垃圾循环流化床焚烧锅炉飞灰中二 英热解析特性研究[J]. 环境科学学报, 2015, 35(6): 1833–1841. 英热解析特性研究[J]. 环境科学学报, 2015, 35(6): 1833–1841. |
| Fujimori T, Takaoka M. 2009. Direct chlorination of carbon by copper chloride in a thermal process[J]. Environmental Science & Technology, 43(7): 2241–2246. |
| Fujimori T, Takaoka M, Takeda N. 2009. Influence of Cu, Fe, Pb, and Zn chlorides and oxides on formation of chlorinated aromatic compounds in MSWI fly ash[J]. Environmental Science & Technology, 43(21): 8053–8059. |
| Hatanaka T, Imagawa T, Takeuchi M. 2002. Effects of copper chloride on formation of polychlorinated dibenzofurans in model waste incineration in a laboratory-scale fluidized-bed reactor[J]. Chemosphere, 46(3): 393–399.DOI:10.1016/S0045-6535(01)00059-5 |
| Hatanaka T, Kitajima A, Takeuchi M. 2004. Role of copper chloride in the formation of polychlorinated dibenzo-p-dioxins and dibenzofurans during incineration[J]. Chemosphere, 57(1): 73–79.DOI:10.1016/j.chemosphere.2004.04.058 |
| Hell K, Stieglitz L, Altwicker E R, et al. 2001. Reactions of 2, 4, 6-trichlorophenol on model fly ash:oxidation to CO and CO2, condensation to PCDD/F and conversion into related compounds[J]. Chemosphere, 42(5): 697–702. |
| Huang H, Buekens A. 1995. On the mechanisms of dioxin formation in combustion processes[J]. Chemosphere, 31(9): 4099–4117.DOI:10.1016/0045-6535(95)80011-9 |
| Lin W, Wu Y, Tu L, et al. 2010. The emission and distribution of PCDD/Fs in municipal solid waste incinerators and coal-fired power plant[J]. Aerosol and Air Quality Research, 10: 519–532.DOI:10.4209/aaqr.2010.03.0017 |
| Liu G, Jiang X, Wang M, et al. 2015. Comparison of PCDD/F levels and profiles in fly ash samples from multiple industrial thermal sources[J]. Chemosphere, 133: 68–74.DOI:10.1016/j.chemosphere.2015.03.073 |
| López N, Gómez-Segura J, Marín R P, et al. 2008. Mechanism of HCl oxidation (Deacon process) over RuO2[J]. Journal of Catalysis, 255(1): 29–39.DOI:10.1016/j.jcat.2008.01.020 |
| Luijk R, Akkerman D M, Slot P, et al. 1994. Mechanism of formation of polychlorinated dibenzo-p-dioxins and dibenzofurans in the catalyzed combustion of carbon[J]. Environmental Science & Technology, 28(2): 312–321. |
| Olie K, Addink R, Schoonenboom M. 1998. Metals as catalysts during the formation and decomposition of chlorinated dioxins and furans in incineration processes[J]. Journal of the Air & Waste Management Association, 48(2): 101–105. |
| Olie K, Vermeulen P L, Hutzinger O. 1977. Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some municipal incinerators in the Netherlands[J]. Chemosphere, 6(8): 455–459.DOI:10.1016/0045-6535(77)90035-2 |
| Pekárek V, Grabic R, Marklund S, et al. 2001. Effects of oxygen on formation of PCB and PCDD/F on extracted fly ash in the presence of carbon and cupric salt[J]. Chemosphere, 43(4): 777–782. |
| Ryu J, Choi K, Mulholland J A. 2006. Polychlorinated dibenzo-p-dioxin (PCDD) and dibenzofurna (PCDF) isomer patterns from municipal waste combustion:Formation mechanism fingerprints[J]. Chemosphere, 65(9): 1526–1536.DOI:10.1016/j.chemosphere.2006.04.002 |
| Ryu J, Mulholland J A, Kim D H, et al. 2005a. Homologue and isomer patterns of polychlorinated dibenzo-p-dioxins and dibenzofurans from phenol precursors:Comparison with municipal waste incinerator data[J]. Environmental Science & Technology, 39(12): 4398–4406. |
| Ryu J, Mulholland J A, Takeuchi M, et al. 2005b. CuCl2-catalyzed PCDD/F formation and congener patterns from phenols[J]. Chemosphere, 61(9): 1312–1326.DOI:10.1016/j.chemosphere.2005.03.062 |
| Ryu J, Mulholland J A. 2005c. Metal-mediated chlorinated dibenzo-p-dioxin (CDD) and dibenzofuran (CDF) formation from phenols[J]. Chemosphere, 58(7): 977–988.DOI:10.1016/j.chemosphere.2004.08.084 |
| Schecter A, Birnbaum L, Ryan J J, et al. 2006. Dioxins:An overview[J]. Environmental Research, 101(3): 419–428.DOI:10.1016/j.envres.2005.12.003 |
| Stanmore B R. 2004. The formation of dioxins in combustion systems[J]. Combustion and Flame, 136(3): 398–427.DOI:10.1016/j.combustflame.2003.11.004 |
| Stieglitz L. 1998. Selected topics on the de novo synthesis of PCDD/PCDF on fly ash[J]. Environmental Engineering Science, 15(1): 5–18.DOI:10.1089/ees.1998.15.5 |
| Takaoka M, Yamamoto T, Shiono A, et al. 2005a. The effect of copper speciation on the formation of chlorinated aromatics on real municipal solid waste incinerator fly ash[J]. Chemosphere, 59(10): 1497–1505.DOI:10.1016/j.chemosphere.2004.12.049 |
| Takaoka M, Shiono A, Nishimura K, et al. 2005b. Dynamic change of copper in fly ash during de novo synthesis of dioxins[J]. Environmental Science & Technology, 39(15): 5878–5884. |
| Tuppurainen K, Asikainen A, Ruokoj?rvi P, et al. 2003. Perspectives on the formation of polychlorinated dibenzo-p-dioxins and dibenzofurans during municipal solid waste (MSW) incineration and other combustion processes[J]. Accounts of Chemical Research, 36(9): 652–658.DOI:10.1021/ar020104+ |
| Wang M, Liu G, Jiang X, et al. 2015. Formation and potential mechanisms of polychlorinated dibenzo-p-dioxins and dibenzofurans on fly ash from a secondary copper smelting process[J]. Environmental Science and Pollution Research, 22(11): 8747–8755.DOI:10.1007/s11356-014-4046-6 |
| Weber P, Dinjus E, Stieglitz L. 2001. The role of copper (Ⅱ) chloride in the formation of organic chlorine in fly ash[J]. Chemosphere, 42(5-7): 579–582.DOI:10.1016/S0045-6535(00)00230-7 |
| Weber R, Hagenmaier H. 1999. PCDD/PCDF formation in fluidized bed incineration[J]. Chemosphere, 38(11): 2643–2654.DOI:10.1016/S0045-6535(98)00472-X |
| Wikstr?m E, Ryan S, Touati A, et al. 2003. Key parameters for de novo formation of polychlorinated dibenzo-p-dioxins and dibenzofurans[J]. Environmental Science & Technology, 37(9): 1962–1970. |
| Zhang H, Ni Y, Chen J, et al. 2008. Influence of variation in the operating conditions on PCDD/F distribution in a full-scale MSW incinerator[J]. Chemosphere, 70(4): 721–730.DOI:10.1016/j.chemosphere.2007.06.054 |
| Zhang M, Buekens A, Li X. 2018a. Statistical analysis as a tool for discriminating dioxin formation pathways[J]. Journal of Material Cycles and Waste Management, 20(3): 1516–1529.DOI:10.1007/s10163-018-0715-8 |
| Zhang M, Buekens A, Ma S, et al. 2018b. Iron chloride catalysed PCDD/F-formation:Experiments and PCDD/F-signatures[J]. Chemosphere, 191: 72–80.DOI:10.1016/j.chemosphere.2017.09.130 |
| Zhang M, Buekens A, Olie K, et al. 2017. PCDD/F-isomers signature - effect of metal chlorides and oxides[J]. Chemosphere, 184: 559–568.DOI:10.1016/j.chemosphere.2017.05.176 |
| Zhang M, Yang J, Buekens A, et al. 2016. PCDD/F catalysis by metal chlorides and oxides[J]. Chemosphere, 159: 536–544.DOI:10.1016/j.chemosphere.2016.06.049 |
