 , 李筱琴1,2,3
, 李筱琴1,2,3

1. 华南理工大学环境与能源学院, 广州 510006;
2. 华南理工大学工业聚集区污染控制与生态修复教育部重点实验室, 广州 510006;
3. 固体废物处理与资源化广东省环境保护重点实验室, 广州 510006
收稿日期: 2018-09-26; 修回日期: 2018-11-13; 录用日期: 2018-11-13
基金项目: 广东省自然科学基金项目(No.2016A030313507)
作者简介: 梁莉(1993-), 女, E-mail:1245397315@qq.com
通讯作者(责任作者): 李筱琴(1974—), 女, 副教授, 主要研究方向为:重金属及有机污染土壤和水体的纳米修复技术;环境纳米材料的开发、表征、功能与调控机制研究;地下水和废水处理等.E-mail:xqli306@scut.edu.cn
摘要: 由于矿山开采、工业废水排放及农业施肥等人类活动,使得我国镉污染日益突出.本研究采用液相还原法制备硫化纳米零价铁(S-nZVI),研究其对Cd的去除行为.考察了不同硫化剂、合成方法、S/Fe比(物质的量比)及pH对Cd去除的影响,并采用扫描电子显微镜(SEM)、拉曼光谱(Raman)、X-射线衍射(XRD)等技术对反应前后的材料进行表征,结合批实验结果探讨Cd的去除机理.结果表明,采用一步法以硫化钠(Na2S)为硫化剂合成的S-nZVI对Cd的去除效率远高于其它方法,去除容量可达385.6 mg·g-1.反应120 min后,S-nZVI对Cd的去除率随S/Fe比的增加而升高,S-nZVI反应体系受pH影响较小.材料表征结果显示,S-nZVI颗粒由Fe0、Fe3O4、FeS组成,其中,FeS的含量随S/Fe比的升高而增加.S-nZVI对Cd的去除机理主要是Cd将FeS中的Fe置换,与S结合形成稳定的CdS.
关键词:镉硫化纳米零价铁硫化钠拉曼光谱
Removal of cadmium in aquatic environment by sulfidated nanoscale zero-valent iron(S-nZVI)
LIANG Li1,2,3
 , LI Xiaoqin1,2,3
, LI Xiaoqin1,2,3

1. School of Environment and Energy, South China University of Technology, Guangzhou 510006;
2. The Key Laboratory of Pollution Control and Ecosystem Restoration in Industry Clusters of the Ministry of Education, South China University of Technology, Guangzhou 510006;
3. Guangdong Environmental Protection Key Laboratory of Solid Waste Treatment and Recycling, Guangzhou 510006
Received 26 September 2018; received in revised from 13 November 2018; accepted 13 November 2018
Abstract: In China, cadmium pollution has become more and more severe due to human activities such as mining, industrial wastewater discharge, and agricultural fertilization. In this study, various sulfidated nanoscale zero-valent iron (S-nZVI) was synthesized and compared for the performance of Cd removal. The effects of sulfidation reagents, synthesis method, S/Fe molar ratio and pH on Cd removal efficiency were investigated. The removal mechanism of Cd was elucidated based on scanning electron microscopy (SEM), Raman spectroscopy (Raman), X-ray diffraction (XRD), and experimental results. The results showed that the removal efficiency of Cd by presynthesized S-nZVI with Na2S as the sulfidation reagent was much higher than other methods, and the removal capacity reached up to 385.6 mg·g-1. After reaction 120 min, the removal efficiency of Cd increased with the increase of S/Fe molar ratio. pH had little effect on the S-nZVI reaction with Cd. Characterization of S-nZVI showed that the particles were composed of Fe0, Fe3O4 and FeS. FeS increased with the increase of S/Fe molar ratio. The mechanism of the removal of Cd by S-nZVI was mainly via Cd displacement of Fe in FeS and form stable CdS.
Keywords: cadmiumS-nZVINa2SRaman spectroscopy
1 引言(Introduction)镉(Cd)是一种人体非必需元素, 主要来源有采矿、电镀、电池、冶炼、染料等工业废水的排放(Purkayastha et al., 2014).目前, 在环境介质(水体、土壤)中均能检测到Cd的存在(Purkayastha et al., 2014; Bonberg et al., 2017; He et al., 2017).与其它重金属相比, Cd的流动性高, 容易被植物吸收, 并可通过食物链富集于人体中, 从而对人体健康造成严重的危害(Mulligan et al., 2001; Purkayastha et al., 2014; He et al., 2017; Yu et al., 2018; Zhu et al., 2018).去除水中镉的修复技术主要有吸附法、沉淀法、离子交换及膜分离等(Hashim et al., 2011; Li et al., 2017; Kim et al., 2018).其中, 吸附法主要应用一些化学及生物吸附剂, 如活性炭、水凝胶、碳纳米管、甘蔗渣等(Hashim et al., 2011; He et al., 2017);沉淀法中常用的沉淀剂有硫化物、氢氧化物及铁氧化物(Purkayastha et al., 2014).这些处理技术由于成本或处理效率的问题, 限制了其大规模应用.因此, 迫切需要开发更多对Cd去除效率高、成本低、环境友好的治理方法.
纳米零价铁(nZVI)由于反应活性高而被广泛应用于地下水及土壤重金属的治理中(Mukherjee et al., 2015; Zou et al., 2016; Li et al., 2006a).nZVI具有独特的核壳结构, 其中核为Fe(0), 壳为以FeOOH为主的铁氧化物.在与重金属的反应过程中, 核作为有效的电子供体使nZVI具有还原作用, 壳为重金属的去除提供了吸附位点, 核与壳之间具有特殊的互补作用(Li et al., 2006b; Li et al., 2017;Ling et al., 2017).但nZVI对与其氧化还原电位接近的重金属Cd的去除效率低且不稳定(Calderon et al., 2015; Li et al., 2007).
近年来, 研究人员发现对nZVI进行硫化处理(S-nZVI)可进一步提高其反应活性和选择性, 具体与采用的硫化剂种类和用量、合成方法及目标污染物有关.制备S-nZVI常用的硫化剂主要有硫化钠(Na2S)、连二亚硫酸钠(Na2S2O4)及硫代硫酸钠(Na2S2O3), 其合成根据硫化剂是在nZVI形成前后加入可分为一步法和两步法(Kim et al., 2011; Su et al., 2015; Rajajayavel et al., 2015; Li et al., 2016; Han et al., 2016; Tang et al., 2016).Han等(2016)研究了不同硫化剂(Na2S、Na2S2O4、Na2S2O3)及合成方法对三氯乙烯(TCE)降解的影响, 发现TCE的降解与硫化剂及合成方法无关, S/Fe比(物质的量比)对TCE的降解速率影响较大, S-nZVI对TCE的去除率随着S/Fe比的增加而增加, 在S/Fe比为0.025时, TCE的去除率达到峰值.Fan等(2013)利用两步法以Na2S为硫化剂合成S-nZVI并去除高锝酸盐(99TcO4-), 结果表明, 99TcO4-被还原成TcO2并固定在表面, S/Fe比(物质的量比)低于0.056时, 99TcO4-去除速率随S/Fe比的升高而增大.
目前利用S-nZVI去除Cd的研究仅有少数几篇报道(Su et al., 2015; Su et al., 2016; Lv et al., 2018), 均利用一步法以Na2S2O4为硫化剂合成的S-nZVI去除Cd, 对Cd的去除容量分别为85、150 mg·g-1, 比nZVI提高2~4倍.Su等(2015)发现, S/Fe物质的量比为0.28时, Cd的去除率达到峰值.S-nZVI去除Cd的反应是表面介导过程, 硫化剂的种类、合成方法及S/Fe比对S-nZVI的微观结构及反应活性影响较大, 仍需要进一步优化.
因此, 本文通过研究不同硫化剂(Na2S2O4、Na2S2O3、Na2S)、合成方法(一步法、两步法)、S/Fe比(物质的量比)及pH对Cd去除的影响, 并用扫描电子显微镜、拉曼光谱、X-射线衍射等对S-nZVI进行表征, 结合批试验研究S-nZVI去除Cd的作用机理, 以期为Cd污染的治理提供理论指导和技术支撑.
2 材料与方法(Materials and methods)2.1 化学试剂六水合氯化铁(FeCl3·6H2O)、硼氢化钠(NaBH4)、九水合硫化钠(Na2S·9H2O)、连二亚硫酸钠(Na2S2O4)、五水合硫代硫酸钠(Na2S2O3·5H2O)、无水氯化镉(CdCl2)均为分析纯;硝酸(HNO3)、盐酸(HCl)、氢氧化钠(NaOH)为优级纯.所有试剂均直接使用无需进一步提纯.配置溶液及制备材料时均使用去离子水.
2.2 nZVI及S-nZVI的合成用两种方法制备S-nZVI, 即一步法和两步法.两步法是先采用液相还原法制备nZVI(Li et al., 2016), 即将1 L NaBH4(0.25 mol·L-1)逐滴加入到等体积的FeCl3(0.045 mol·L-1)溶液中, 同时用电动搅拌棒以600 r·min-1的转速搅拌溶液, 反应完成后继续搅拌15 min.将混合液用布氏漏斗过滤, 收集的固体颗粒用去离子水冲洗2次, 无水乙醇冲洗1次, 用含水率测定仪测定材料含水率后, 将其装在带盖瓶中置于冰箱中保存.然后称取10 g的nZVI, 向其中加入150 mL不同硫化剂溶液(Na2S(215.02 g·L-1)、Na2S2O4(77.93 g·L-1)、Na2S2O3(111.09 g·L-1)), 超声15 min, 获得S/Fe比(物质的量比)为0.75的S-nZVI.一步法S-nZVI制备和收集同nZVI, 除了将1 L NaBH4(0.25 mol·L-1)替换成NaBH4(0.25 mol·L-1)和硫化剂(Na2S/Na2S2O4/Na2S2O3)的混合溶液.
2.3 材料表征2.3.1 扫描电子显微镜(SEM)采用Merlin场发射扫描电镜(德国ZEISS公司)对反应前后材料的形貌进行表征.取少量材料将其加入无水乙醇中, 超声分散30 min后, 取1滴滴于导电胶上, 待酒精风干后, 进行喷金.将制备好的样品置于SEM中在5.0/10.0 kV的加速电压下观测形貌.
2.3.2 X射线衍射(XRD)采用Empyrea锐影X射线衍射光谱仪(荷兰帕纳科公司)分析反应前后纳米颗粒的物相组成.测试条件:电流40 mA, 电压40 kV, 扫描速度7.14 s·步-1, 扫描范围10°~90°, 扫描步长0.02°, 铜靶, Kα射线(λ=0.15406 nm).
2.3.3 拉曼光谱(Raman)采用LabRAM Aramis显微拉曼光谱仪(法国H.J.Y公司)分析反应前后材料的结构组成.测试条件:激发波长为532 nm, 拉曼光谱的波数范围为100~800 cm-1.
2.3.4 Zeta电位(Zeta-potential)采用Zetasizer Nano ZS纳米粒度-Zeta电位测试仪(英国马尔文公司)测定材料在不同pH值时的Zeta电位, 绘制Zeta电位-pH的曲线, 得到材料的等电点.
2.4 批实验称取0.3262 g无水氯化镉用去离子水溶于1 L容量瓶中, 配置成200 mg·L-1 Cd储备溶液.以150 mL的蓝盖瓶作为反应器, 取100 mL目标污染物溶液, 通N2 5 min, 再加入一定量的nZVI或S-nZVI.将反应瓶置于150 r·min-1的常温摇床中振荡, 在预设的时间点从每个反应瓶中取1 mL反应液, 并用0.22 μm的针孔滤头将其过滤于5 mL离心管中, 测定反应试样中总镉及总铁的浓度.研究pH值的影响时, 用0.1 mol·L-1的NaOH或者0.1 mol·L-1的HCl调节溶液的pH.如无特别说明, 实验中采用以Na2S为硫化剂一步法合成的S-nZVI为反应材料, Cd的初始浓度为200 mg·L-1, S-nZVI的投加量为0.5 g·L-1, 溶液的初始pH值为6.2, 反应时间为2 h.
2.5 数据处理为了保证数据的可靠性, 批实验结果用3次实验数据平均值±标准偏差表示, 采用SPSS 19.0软件进行单因素方差分析, 选择Duncan法进行多重检验(α=0.05).
2.6 化学分析游离态总镉和总铁的浓度用原子吸收光谱仪(AAS)测定(AA-6300, Shimadzu, 日本), 测定总镉和总铁时所用标准溶液的浓度范围分别为0.1~0.8 mg·L-1、0.6~2 mg·L-1.若待测样品的浓度范围超过标准溶液的线性范围, 则用2%的硝酸稀释后测定.
3 结果与讨论(Results and discussion)3.1 不同硫化剂、合成方法对S-nZVI去除Cd的影响图 1为以Na2S2O4、Na2S2O3、Na2S为硫化剂, 分别用一步法(图 1a)和两步法(图 1b)合成的S-nZVI(S/Fe=0.75)对Cd去除的影响.由图可知, 两步法合成的3种材料对Cd的去除率均较低.用Na2S2O4、Na2S2O3及Na2S 3种硫化剂一步法制备的S-nZVI, 反应120 min后对Cd的去除率分别为22.5%、93.4%、96.4%(图 1a), 同时, 各去除率之间差异显著(p < 0.05).利用Na2S2O4合成的S-nZVI对Cd的去除效率最低, 可能是因为Na2S2O4在酸性条件下易水解生成硫代硫酸盐和亚硫酸盐, 使得合成的材料中能够与Cd键合的S2-较少(Han et al., 2016).鉴于以Na2S为硫化剂一步法合成的S-nZVI对Cd的去除率明显高于其它几种方法, 在后续实验中均采用此方法制备S-nZVI.
图 1(Fig. 1)
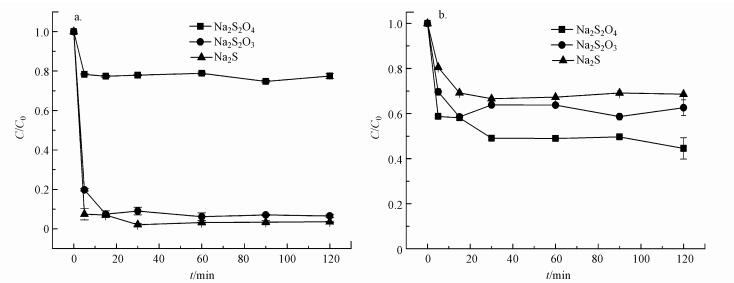 |
| 图 1 不同硫化剂及合成方法对Cd去除的影响 (a.一步法;b.两步法) Fig. 1The effect of different sulfidation reagents and synthesis methods on the removal of Cd |
3.2 材料的表征3.2.1 反应前SEM分析实验室新鲜制备的nZVI(图 2a)由于磁力作用团聚在一起, 呈链球状.进行硫化处理后, S-nZVI(图 2b)呈聚合片状结构, 其形貌与FeS的类似(Lyu et al., 2017).
图 2(Fig. 2)
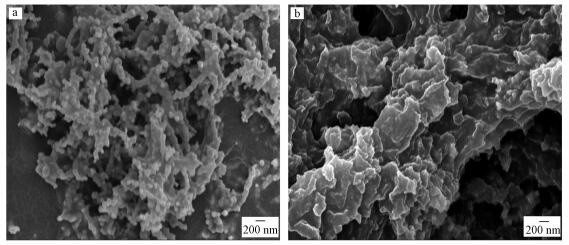 |
| 图 2 新鲜制备材料的扫描电镜图(a.nZVI;b. S-nZVI(S/Fe=1.0)) Fig. 2SEM images of fresh synthesized nanoparticles |
3.2.2 Zeta电位分析图 3为nZVI(S/Fe=0)及不同S/Fe比S-nZVI颗粒的Zeta电位随pH变化的关系.由图可知, S/Fe比为0、0.25、0.75、1.00的纳米颗粒的等电点分别为8.95、7.05、6.01、5.49, 可知随着S/Fe比的增加, S-nZVI颗粒的等电点降低.由于FeS的等电点在0.8~3.5(Su et al., 2015; Coles et al., 2000), 材料中FeS的含量势必会影响S-nZVI的表面负荷和等电点, 从而影响其与污染物的反应.
图 3(Fig. 3)
 |
| 图 3 不同S/Fe比S-nZVI的Zeta电位随pH的变化 Fig. 3Zeta potentials of the S-nZVI particles with various S/Fe molar ratios as a function of pH |
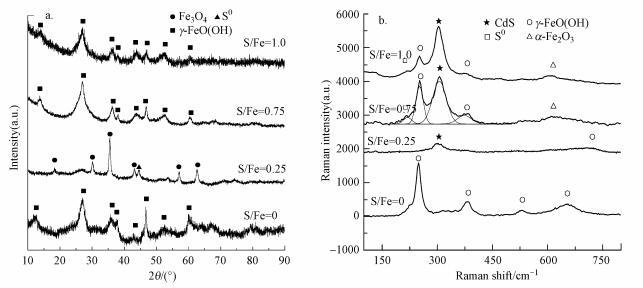
3.2.3 反应前XRD和Raman分析XRD结果显示(图 4a), nZVI在2θ=44.6°、64.9°和82.3°处出现的特征峰均归属于α-Fe0, 表明新鲜制备的nZVI主要以α-Fe0的形式存在.新鲜制备的nZVI中未发现铁氧化物的特征峰, 这可能是因为nZVI表面的铁氧化物含量低或结晶度较差, 以无定型状态存在(Zhang et al., 2013).不同S/Fe比的S-nZVI颗粒在2θ=44.6°处均检测到较宽的α-Fe0峰, 说明经过硫化处理后α-Fe0的结晶度变低, 同时检测到Fe3O4的特征峰.但未检测到FeS的特征峰, 可能是因为FeS以无定型状态存在, 这与其它研究结果一致(Han et al., 2016; Tang et al., 2016).
图 4(Fig. 4)
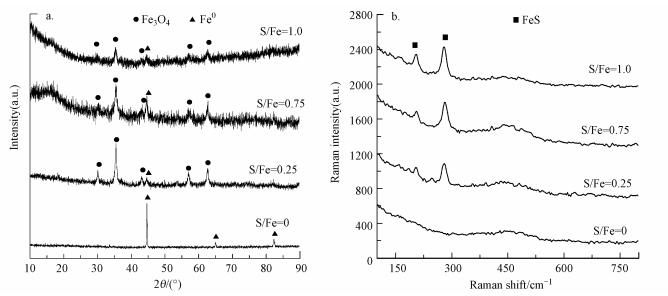 |
| 图 4 反应前不同S/Fe比的S-nZVI的X-射线衍射图(a)和拉曼光谱图(b) Fig. 4XRD(a) and Raman patterns(b) of S-nZVI with various S/Fe molar ratios before reaction |
新鲜制备S-nZVI的拉曼光谱(图 4b)在203.1 cm-1和280.3 cm-1处均出现明显的谱峰, 对应为无定型的Fe—S振动(Genchev et al., 2016; Bourdoiseau et al., 2008), 表明在硫化过程中形成了FeS, 并且FeS含量随着S/Fe比的升高而增加.在拉曼光谱中未检测到Fe3O4的特征峰, 这可能是因为含铁物质共存时, Fe3O4的拉曼信号较弱(Das et al., 2011).结合图 4a可知, 新鲜制备的不同S/Fe比的S-nZVI均由Fe0、Fe3O4及无定型态的FeS组成.
3.3 S-nZVI对Cd的去除3.3.1 S/Fe比对Cd去除的影响图 5a显示了不同S/Fe比的S-nZVI对Cd的去除情况.nZVI(S/Fe=0)去除Cd时, 在前30 min内Cd的去除率不断增加, 在30 min时, Cd的去除率达到最大, 随着反应的进行, 部分Cd释放回溶液中, 导致去除率降低.反应120 min后, Cd的去除率随着S/Fe比的升高而增加, 但当S/Fe比大于0.75时, 材料(S/Fe=0.75、1.00)对Cd的去除率之间无显著差异(p>0.05).S-nZVI(S/Fe=0.75)对Cd的去除容量为385.6 mg·g-1, 远高于文献报道值(85、150 mg·g-1)(Su et al., 2015; Lv et al., 2018).总的来说, 经过硫化处理后S-nZVI对Cd去除效率高且稳定.虽然S/Fe比为0.75和1.00的S-nZVI对Cd的去除量之间无显著差异(p>0.05), 但后者释放到溶液中的Fe含量明显增多(图 5b).因此, 在实际应用时, 在保证去除率的前提下, 要选择合适S/Fe比的S-nZVI, 以防过多的Fe释放到水体中.
图 5(Fig. 5)
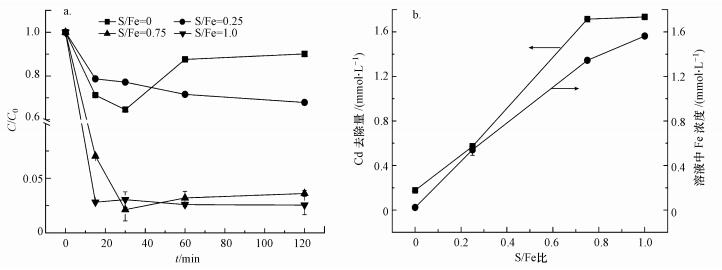 |
| 图 5 S/Fe比对Cd去除的影响(a)及反应120 min后Cd的去除量与溶液中Fe含量的关系(b) Fig. 5Effect of S/Fe molar ratios on the removel of Cd(a) and the relation between Cd removal amount and total Fe in final solution after 120 min(b) |
3.3.2 初始pH对不同S/Fe比的S-nZVI去除Cd的影响图 6a显示了pH对不同S/Fe比的S-nZVI去除Cd的影响.nZVI及不同S/Fe比的S-nZVI对Cd的去除率分别在pH=7、pH=3时达到最大.当pH从3增加到5时, S/Fe比为0.25、0.75、1.00的S-nZVI对Cd的去除率分别下降了6.30%、0.64%、0.60%.S/Fe比为0.25和0.75的S-nZVI在酸性条件下对Cd的去除率之间均差异显著(p < 0.05).而3种材料(S/Fe=0.25、0.75、1.00)在中性和弱碱性条件下对Cd的去除率之间均无显著差异(p>0.05).总的来说, pH对S-nZVI去除Cd影响较小.由图 6b可知, nZVI及S-nZVI反应体系中Fe的溶解量均随着pH从3增加到5而降低, 继续增加pH, Fe的溶解量变化不大.
图 6(Fig. 6)
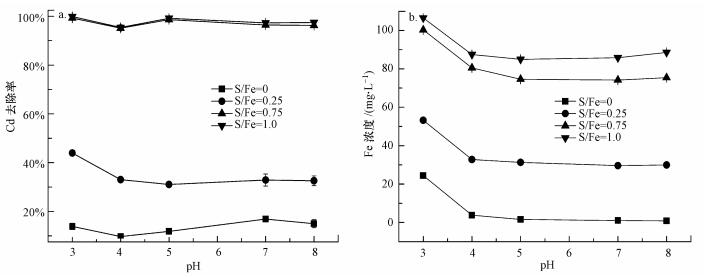 |
| 图 6 pH对不同S/Fe比的S-nZVI去除Cd的影响(a)及反应过程中Fe的释放情况(b) Fig. 6Effect of pH on the removel of Cd by S-nZVI with various S/Fe molar ratios(a) and the release of Fe during the reaction(b) |
3.4 S-nZVI去除Cd的反应机理图 7为反应后材料的扫描电镜图.由图可知, 与Cd反应后, nZVI及S-nZVI颗粒均被氧化为无规则片状体.
图 7(Fig. 7)
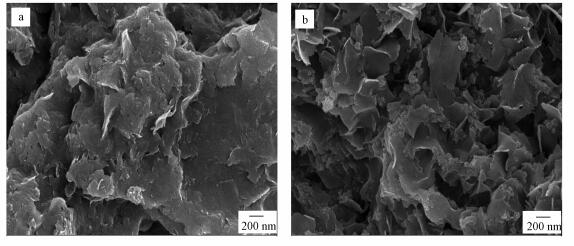 |
| 图 7 反应后材料的扫描电镜图 (a.nZVI;b.S-nZVI(S/Fe=1.0)) Fig. 7SEM images of nanoparticles after reaction |
不同S/Fe比的S-nZVI与Cd反应后的XRD见图 8a.由图 8a可知, nZVI及S/Fe比为0.75、1.00的S-nZVI反应后颗粒中均检测到较强的γ-FeO(OH)的特征峰, 而S/Fe=0.25的S-nZVI与Cd反应后材料中的铁氧化物仍以Fe3O4的形式存在.
图 8(Fig. 8)
 |
| 图 8 反应后不同S/Fe比的S-nZVI的X-射线衍射图(a)和拉曼光谱图(b) Fig. 8XRD(a) and Raman patterns(b) of S-nZVI with various S/Fe molar ratios after reaction |
不同S/Fe比的S-nZVI与Cd反应2 h后FeS的特征峰消失, 均检测到CdS(~305 cm-1)的拉曼特征峰(Reddy et al., 2017), 并且其强度随S/Fe比的升高而增强(图 8b).表明随着S/Fe比增加, 更多的Cd会与FeS中的Fe发生置换生成CdS.当S/Fe比分别为0.75和1.00时, 检测到微弱的S0的拉曼振动峰, Li等(2018)的研究也显示, 利用S-nZVI去除污染物的过程中会有少量S0生成, 这可能是由于反应过程中FeS与溶液中残留的O2作用所致(Bourdoiseau et al., 2008; Holmes et al., 2013; Xia et al., 2010).结合图 8a可知, nZVI与Cd反应后, 材料中的铁氧化产物主要为γ-FeO(OH), S/Fe比为0.75、1.00的S-nZVI的铁氧化产物主要有γ-FeO(OH)和α-Fe2O3, 表明S对Fe的氧化过程产生了影响.而S/Fe比为0.25的S-nZVI中的铁氧化产物主要为Fe3O4, 这可能是由于溶液的初始pH值为6.2, 而S-nZVI(S/Fe=0.25)的Zeta电位为7.05(图 3), 在此pH条件下材料带正电, 反应过程中能够吸附于材料表面的H+较少, 从而使得Fe3O4不易转化为γ-FeO(OH)(Coles et al., 2000).
4 结论(Conclusions)1) 以Na2S为硫化剂采用一步法合成的S-nZVI对Cd的去除效率远高于其它方法.经过硫化处理后, S-nZVI呈聚合片状.S-nZVI颗粒由Fe0、Fe3O4、FeS组成, 其中, FeS的含量随S/Fe比的升高而增加.S-nZVI的等电点随着S/Fe比的增大而降低.S-nZVI对Cd的去除机理主要是Cd将FeS中的Fe置换, 与S结合形成稳定的CdS.同时, S对Fe的氧化过程也产生影响.
2) 反应120 min后, S-nZVI对Cd的去除率随S/Fe比的增加而升高.与nZVI相比, S-nZVI对Cd的去除效率高且更稳定.S-nZVI反应体系受pH影响较小.0.5 g·L-1的S-nZVI(S/Fe=0.75、1.00)对200 mg·L-1 Cd的去除率在30 min内均可达96%以上.根据反应过程中Fe的释放量可知, S/Fe=0.75的材料更有利于Cd的去除, 去除容量可达385.6 mg·g-1.
参考文献
| Bonberg N, Pesch B, Ulrich N, et al. 2017. The distribution of blood concentrations of lead (Pb), cadmium (Cd), chromium (Cr) and manganese (Mn) in residents of the German Ruhr area and its potential association with occupational exposure in metal industry and/or other risk factors[J]. International Journal of Hygiene and Environmental Health, 220: 998–1005.DOI:10.1016/j.ijheh.2017.05.009 |
| Bourdoiseau J A, Jeannin M, Sabot R, et al. 2008. Characterisation of mackinawite by Raman spectroscopy:Effects of crystallisation, drying and oxidation[J]. Corrosion Science, 50(11): 3247–3255.DOI:10.1016/j.corsci.2008.08.041 |
| Calderon B, Fullana A. 2015. Heavy metal release due to aging effect during zero valent iron nanoparticles remediation[J]. Water Research, 83: 1–9.DOI:10.1016/j.watres.2015.06.004 |
| Coles C A, Rao S R, Yong R N. 2000. Lead and cadmium interactions with mackinawite:retention mechanisms and the role of pH[J]. Environmental Science & Technology, 34(6): 996–1000. |
| Das S, Hendry M J. 2011. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes[J]. Chemical Geology, 290(3/4): 101–108. |
| Fan D M, Anitori R P, Tebo B M, et al. 2013. Reductive sequestration of pertechnetate (99TcO4-) by nano zerovalent iron (nZVI) transformed by abiotic sulfide[J]. Environmental Science & Technology, 47(10): 5302–5310. |
| Genchev G, Erbe A. 2016. Raman spectroscopy of mackinawite FeS in anodic iron sulfide corrosion products[J]. Journal of the Electrochemical Society, 163(6): C333–C338.DOI:10.1149/2.1151606jes |
| Han Y L, Yan W L. 2016. Reductive Dechlorination of trichloroethene by zero-valent iron nanoparticles:reactivity enhancement through sulfidation treatment[J]. Environmental Science & Technology, 50: 12992–13001. |
| Hashim M A, Mukhopadhyay S, Sahu J N, et al. 2011. Remediation technologies for heavy metal contaminated groundwater[J]. Journal of Environmental Management, 92(10): 2355–2388.DOI:10.1016/j.jenvman.2011.06.009 |
| He H, Tam N F Y, Yao A, et al. 2017. Growth and Cd uptake by rice(Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag[J]. Chemosphere, 189: 247–254.DOI:10.1016/j.chemosphere.2017.09.069 |
| He J Y, Li Y L, Wang C M, et al. 2017. Rapid adsorption of Pb, Cu and Cd from aqueous solutions by β-cyclodextrin polymers[J]. Applied Surface Science, 426: 29–39.DOI:10.1016/j.apsusc.2017.07.103 |
| Holmes P R, Crundwell F K. 2013. Polysulfides do not cause passivation:Results from the dissolution of pyrite and implications for other sulfide minerals[J]. Hydrometallurgy, 139(3): 101–110. |
| Kim E J, Kim J H, Azad A M, et al. 2011. Facile synthesis and characterization of Fe/FeS nanoparticles for environmental applications[J]. ACS Applied Materials & Interfaces, 3(5): 1457–1462. |
| Kim S, Chu K H, Al-Hamadani Y A J, et al. 2018. Removal of contaminants of emerging concern by membranes in water and wastewater:A review[J]. Chemical Engineering Journal, 335: 896–914.DOI:10.1016/j.cej.2017.11.044 |
| Li B, Yang L, Wang C Q, et al. 2017. Adsorption of Cd(Ⅱ) from aqueous solutions by rape straw biochar derived from different modification processes[J]. Chemosphere, 175: 332–340.DOI:10.1016/j.chemosphere.2017.02.061 |
| Li D, Mao Z, Zhong Y, et al. 2016. Reductive transformation of tetrabromobisphenol A by sulfidated nano zerovalent iron[J]. Water Research, 103: 1–9.DOI:10.1016/j.watres.2016.07.003 |
| Li J X, Zhang X Y, Liu M C, et al. 2018. Enhanced reactivity and electron selectivity of sulfidated zerovalent iron toward chromate under aerobic conditions[J]. Environmental Science & Technology, 52: 2988–2997. |
| Li S, Wang W, Liang F, et al. 2017. Heavy metal removal using nanoscale zero-valent iron(nZVI):Theory and application[J]. Journal of Hazardous Materials, 322: 163–171.DOI:10.1016/j.jhazmat.2016.01.032 |
| Li X Q, Elliott D W, Zhang W X. 2006a. Zero-valent iron nanoparticles for abatement of environmental pollutants:materials and engineering aspects[J]. Critical Reviews in Solid State and Materials Sciences, 31: 111–122.DOI:10.1080/10408430601057611 |
| Li X Q, Zhang W X. 2006b. Iron nanoparticles:the coreshell structure and unique properties for Ni(Ⅱ) sequestration[J]. Langmuir, 22: 4638–4642.DOI:10.1021/la060057k |
| Li X Q, Zhang W X. 2007. Sequestration of metal cations with zerovalent iron nanoparticles-a study with high resolution X-ray photoelectron spectroscopy(HR-XPS)[J]. Journal of Physical Chemistry C, 111: 6939–6946.DOI:10.1021/jp0702189 |
| Li Y, Li X Q, Xiao Y, et al. 2016. Catalytic debromination of tetrabromobisphenol A by Ni/nZVI bimetallic particles[J]. Chemical Engineering Journal, 284: 1242–1250.DOI:10.1016/j.cej.2015.09.079 |
| Ling L, Huang X Y, Li M R, et al. 2017. Mapping the reactions in a single zero-valent iron nanoparticle[J]. Environmental Science & Technology, 51: 14293–14300. |
| Lv D, Zhou X X, Zhou J S, et al. 2018. Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium(Ⅱ) removal from aqueous solutions[J]. Applied Surface Science, 442: 114–123.DOI:10.1016/j.apsusc.2018.02.085 |
| Lyu H H, Tang J C, Huang Y, et al. 2017. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite[J]. Chemical Engineering Journal, 322: 516–524.DOI:10.1016/j.cej.2017.04.058 |
| Mukherjee R, Kumar R, Sinha A, et al. 2015. A review on synthesis, characterization, and applications of nano zero valent iron(nZVI) for environmental remediation[J]. Critical Reviews in Environmental Science and Technology, 46: 443–466. |
| Mulligan C N, Yong R N, Gibbs B F. 2001. Remediation technologies for metal-contaminated soils and groundwater:an evaluation[J]. Engineering Geology, 60: 193–207.DOI:10.1016/S0013-7952(00)00101-0 |
| Purkayastha D, Mishra U, Biswas S. 2014. A comprehensive review on Cd(Ⅱ) removal from aqueous solution[J]. Journal of Water Process Engineering, 2: 105–128.DOI:10.1016/j.jwpe.2014.05.009 |
| Rajajayavel S R, Ghoshal S. 2015. Enhanced reductive dechlorination of trichloroethylene by sulfidated nanoscale zerovalent iron[J]. Water Research, 78: 144–153.DOI:10.1016/j.watres.2015.04.009 |
| Reddy C V, Shim J, Cho M. 2017. Synthesis, structural, optical and photocatalytic properties of CdS/ZnS core/shell nanoparticles[J]. Journal of Physics and Chemistry of Solids, 103: 209–217.DOI:10.1016/j.jpcs.2016.12.011 |
| Su Y M, Adeleye A S, Keller A A, et al. 2015. Magnetic sulfide-modified nanoscale zerovalent iron(S-nZVI) for dissolved metal ion removal[J]. Water Research, 74: 47–57.DOI:10.1016/j.watres.2015.02.004 |
| Su Y M, Adeleye A S, Huang Y X, et al. 2016. Direct synthesis of novel and reactive sulfide-modified nano iron through nanoparticle seeding for improved cadmium-contaminated water treatment[J]. Scientific Reports, 6: 24358.DOI:10.1038/srep24358 |
| Tang J, Tang L, Feng H P, et al. 2016. pH-dependent degradation of p-nitrophenol by sulfidated nanoscale zerovalent iron under aerobic or anoxic conditions[J]. Journal of Hazardous Materials, 320: 581–590.DOI:10.1016/j.jhazmat.2016.07.042 |
| Xia J L, Yang Y, He H, et al. 2010. Surface analysis of sulfur speciation on pyrite bioleached by extreme thermophile acidianus manzaensis using Raman and XANES spectroscopy[J]. Hydrometallurgy, 100: 129–135.DOI:10.1016/j.hydromet.2009.11.001 |
| Yu Y, Wan Y N, Camara A Y, et al. 2018. Effects of the addition and aging of humic acid-based amendments on the solubility of Cd in soil solution and its accumulation in rice[J]. Chemosphere, 196: 303–310.DOI:10.1016/j.chemosphere.2018.01.002 |
| Zhang Y L, Su Y M, Zhou X F, et al. 2013. A new insight on the core-shell structure of zerovalent iron nanoparticles and its application for Pb(Ⅱ) sequestration[J]. Journal of Hazardous Materials, 263: 685–693.DOI:10.1016/j.jhazmat.2013.10.031 |
| Zhu C, Wen H, Zhang Y, et al. 2018. Cd isotope fractionation during sulfide mineral weathering in the Fule Zn-Pb-Cd deposit, Yunnan Province, Southwest China[J]. Science of the Total Environment, 616-617: 64–72.DOI:10.1016/j.scitotenv.2017.10.293 |
| Zou Y D, Wang X X, Khan A, et al. 2016. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions:a review[J]. Environmental Science & Technology, 50: 7290–7304. |
