 , 俞辉1,2, 阳成强1,3, 林晓晓1, 盖艳波1
, 俞辉1,2, 阳成强1,3, 林晓晓1, 盖艳波1
 , 张为俊1,3
, 张为俊1,31. 中国科学院安徽光学精密机械研究所, 合肥 230031;
2. 中国科学技术大学, 合肥 230026;
3. 中国科学技术大学环境科学与光电技术学院, 合肥 230026
收稿日期: 2018-03-21; 修回日期: 2018-04-04; 录用日期: 2018-04-04
基金项目: 国家自然科学基金(No.41575125,91544228,41605102);国家重点研发计划项目(No.2016YFC0202205);中国科学院合肥物质科学研究院院长基金(No.YZJJ201508)
作者简介: 马乔(1990-), 男, E-mail:maqiao90@mail.ustc.edu.cn
通讯作者(责任作者): 盖艳波, E-mail:gaiyanbo@aiofm.ac.cn
摘要: 通过烟雾箱实验,研究了仲丁醇对苯乙烯臭氧化反应生成二次有机气溶胶(SOA)的影响.结果发现,在烟雾箱实验中,过量仲丁醇的加入导致生成SOA的产率明显下降.同时,结合MCM气相机理和气-粒分配理论,将Criegee中间体与醛类的双分子反应添加到箱式模型中模拟烟雾箱中SOA的生成过程.模拟结果表明,在没有仲丁醇存在的情况下,次级臭氧化物在SOA组分中占1/2左右的比例.仲丁醇的加入消耗了大量的·OH,同时使得[HO2]/[RO2]比值升高,影响自由基相关的反应机制,使得SOA产率下降.另外,研究发现,Criegee中间体与醛类反应的速率常数也是影响SOA生成模拟的一个重要参数,需要进一步开展相关的动力学实验和理论研究.
关键词:苯乙烯二次有机气溶胶烟雾箱箱式模型
Influence of 2-butanol on secondary organic aerosol formation from ozonolysis of styrene: Experimental and model studies
MA Qiao1,2
 , YU Hui1,2, YANG Chengqiang1,3, LIN Xiaoxiao1, GAI Yanbo1
, YU Hui1,2, YANG Chengqiang1,3, LIN Xiaoxiao1, GAI Yanbo1
 , ZHANG Weijun1,3
, ZHANG Weijun1,3 1. Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Hefei 230031;
2. University of Science and Technology of China, Hefei 230026;
3. School of Environmental Science and Optoelectronic Technology, University of Science and Technology of China, Hefei 230026
Received 21 March 2018; received in revised from 4 April 2018; accepted 4 April 2018
Supported by the National Natural Science Foundation of China(No.41575125, 91544228, 41605102), the National Key Research and Development Program of China(No.2016YFC0202205) and the Presidential Foundation of Hefei Institutes of Physical Science, Chinese Academy of Sciences, China(No.YZJJ201508)
Biography: MA Qiao(1990—), male, E-mail:maqiao90@mail.ustc.edu.cn
*Corresponding author: GAI Yanbo, E-mail:gaiyanbo@aiofm.ac.cn
Abstract: Influence of 2-butanol on secondary organic aerosol (SOA) formation from ozonolysis of styrene was studied using chamber experiments and model simulations in this work. The experimental results showed that the introduction of 2-butanol results in a decrease of SOA yield. Then, the reactions between Criegee intermediates with aldehydes were implemented into the master chemical mechanism (MCM) coupled with a gas-particle partitioning model to investigate their impact on SOA formation. And it is shown that the secondary ozonide accounts for about half of the SOA yield in the absence of 2-butanol. While the introducing of 2-butanol will result in the decrease of[OH] and the increase of[HO2]/[RO2] ratio, which is proposed to be the main factor leading to the decreases of SOA yield. In addition, it is found that the magnitude of SOA yield exhibits a strong dependence on the rate constants of the reactions between Criegee intermediates with aldehydes, which needs further experimental and theoretical studies in the future.
Keywords: styrenesecondary organic aerosolsmog chamberbox model
1 引言(Introduction)苯乙烯(C8H8)是一种高反应活性的烯烃, 其在大气中的浓度约为0.06~45 ppb (贾记红等, 2009; Cho et al., 2014; 区家敏等, 2014), 主要来源于人为源排放, 包括机动车尾气排放、石油加工、黏合剂挥发等(ATSDR, 2010; Uhde et al., 2007; 王伯光等, 2008).由于苯乙烯具有潜在的致癌和致突变特性, 若长时间或高浓度的暴露, 会对中枢神经系统和生殖系统造成损害.另外, 苯乙烯在大气中易被氧化剂(如臭氧O3、羟基自由基·OH、硝酸基自由基NO3·)氧化, 生成二次有机气溶胶(Secondary Organic Aerosol, SOA), 导致二次污染(王跃思等, 2010; 贾龙等, 2010; Wang et al., 2015).
与臭氧启动的其它烯烃的氧化机理类似, O3先通过1, 3-环加成至苯乙烯分子的C=C双键上, 生成不稳定的初级臭氧化物(Primary Ozonide, POZ), 随后分解成一种羰基氧化物(也称Criegee中间体)和一种醛(Criegee et al., 1949).Criegee中间体可与H2O、NO、NO2、SO2等发生双分子反应(Anglada et al., 2011; 2016;Lin et al., 2014; Long et al., 2011; 2016;Stone et al., 2014; Vereecken et al., 2012).大量研究表明, Criegee中间体会重新与醛类反应生成含五元环的次级臭氧化物(Secondary Ozonide, SOZ) (Fajgar et al., 1996; Griesbaum et al., 1998; Horie et al., 1999; Jalan et al., 2013; Neeb et al., 1996; Wei et al., 2015).图 1为Tuazon等(1993)提出的苯乙烯与O3反应生成SOZ的机理, 苯乙烯与臭氧反应生成的初级臭氧化物分解为两种Criegee中间体(C6H5HOO·与·CH2OO·)和两种醛(C6H5CHO与HCHO), 其中, C6H5HOO·可再与C6H5CHO反应生成次级臭氧化物3, 5-二苯基-1, 2, 4-三氧戊环, 该SOZ在室温下呈固态(NIST Chemistry WebBook, 2018), 极低的饱和蒸气压(5.9×10-8 Torr)使其极易通过气-粒转化进入气溶胶相(Na et al., 2006), 成为最终SOA产物的重要组分.
图 1(Fig. 1)
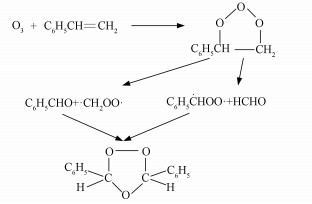 |
| 图 1 苯乙烯与臭氧反应生成SOZ的反应机理 Fig. 1Reaction mechanism of styrene reacting with ozone to generate SOZ |
在烯烃臭氧化反应中, 会伴随着大量·OH的产生, 这些·OH会与母体分子发生反应, 或与产物反应发生二次氧化, 使得反应变得更复杂(Iinuma et al., 2005; Jonsson et al., 2008).为了减少二次氧化的影响, 很多研究中都会加入·OH清除剂, 比如仲丁醇(Chew et al., 1996; 李时政等, 2015; Yuan et al., 2017)、环己烷(Gai et al., 2009; Li et al., 2016; Wu et al., 2017)或CO(Gutbrod et al., 1997).有无·OH清除剂的存在会对烯烃的臭氧化反应机理产生不同的影响(Jenkin, 2004).本文通过烟雾箱实验研究仲丁醇对苯乙烯臭氧化反应生成SOA的影响, 并结合箱式模型分析这一影响的背后机制.最后通过敏感性分析探索Criegee中间体与醛的反应速率对生成SOA产率的影响.
2 材料与方法(Materials and methods)2.1 烟雾箱实验苯乙烯(纯度为99%)、仲丁醇(纯度为99%)购自Sigma-Aldrich公司.实验中使用的背景气体为AADCO零空气发生系统(Model 737)产生的零空气.
自制室内6 m3烟雾箱系统的主反应器由Teflon材料制成, 位于绝热材料制成的壳体内部.实验前将苯乙烯和仲丁醇先后通过微量注射器注入到250 mL的加热玻璃瓶中(瓶内温度维持在约100 ℃), 随后通过零空气吹扫入烟雾箱反应器中.反应器内物质充分混匀后, 将臭氧发生器(COM-AD-01)产生的O3通入反应器并再次摇晃反应器使其充分混合.苯乙烯浓度使用美国Agilent公司的气相色谱仪(GC-FID, Agilent 7820A)测量, O3浓度使用美国Thermo公司的O3分析仪(Model 49i)测量, 生成的SOA质量浓度采用成套的SMPS仪器(Scanning Mobility Particle Sizer, SMPS 3936)进行测量.所有实验均在(25±1) ℃、相对湿度小于5 %的黑暗条件下进行.
2.2 数值模拟零维箱式模型用来模拟苯乙烯与臭氧反应生成SOA的过程.气相机理使用英国Leeds大学开发的MCM v3.3.1 (http://mcm.leeds.ac.uk/MCM), 其苯乙烯和仲丁醇的子机理共包括245个化学物质和730个反应.由于在该体系中, Criegee中间体与醛的反应对SOA的生成有着重要贡献(Tuazon et al., 1993; Na et al., 2006), 因此, 反应(1)~(4)被添加到箱式模型中.
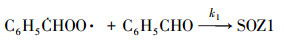 | (1) |
 | (2) |
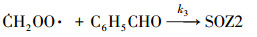 | (3) |
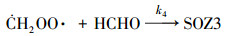 | (4) |
3 结果与讨论(Results and discussion)3.1 烟雾箱实验结果各实验的初始条件及SOA产率如表 1所示.实验共分两组进行, A组在无仲丁醇条件下进行, B组在过量仲丁醇条件下进行, 仲丁醇的初始浓度约为苯乙烯的50倍.为了使苯乙烯氧化完全, 所有实验在臭氧过量的条件下进行, [O3]0/[苯乙烯]0为2~4. SOA产率(Y)定义为生成的SOA质量浓度([SOA], μg·m-3)与消耗的碳氢化合物浓度(△[HC], μg·m-3)之比(式(5))(Odum et al., 1996; Pankow, 1994).
 | (5) |
图 2(Fig. 2)
 |
| 图 2 无仲丁醇和过量仲丁醇条件下苯乙烯臭氧化反应生成的SOA产率 Fig. 2The yield of SOA produced by ozonolysis of styrene in the absence and presence of 2-butanol |
表 1(Table 1)
| 表 1 实验的初始条件和最终的SOA产率 Table 1 Initial conditions and final SOA yields of chamber experiments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 1 实验的初始条件和最终的SOA产率 Table 1 Initial conditions and final SOA yields of chamber experiments
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.2 烟雾箱实验与数值模拟的对比Taatjes等(2012)通过实验中对·CH2OO·的直接测量, 得到·CH2OO·+CH3CHO反应在4 Torr条件下的绝对速率常数为(9.4±0.7)×10-13 cm3·molecule-1·s-1.另外在Stone等(2014)的实验中, 上述反应速率在低压极限条件下约为1.6×10-29 cm3·molecule-1·s-1, 而高压极限条件下约为1.7×10-12 cm3·molecule-1·s-1. MCM v3.3.1机理中尚不包括Criegee中间体与醛类的反应, 且反应(1)~(4)的速率常数缺乏直接的实验数据, 本文将反应(1)~(4)和k1=k2=k3=k4=1.0×10-13 cm3·molecule-1·s-1加入到箱式模型中, 以探索这类反应在SOA生成中所起的作用.以实验A3和B8为例, 烟雾箱实验和数值模拟结果对比如图 3所示.在本模拟条件下, 苯乙烯与实验值基本吻合, 臭氧浓度略高于实验值, 最终的SOA产率比实验值高出约50%.说明MCM v3.3.1机理基本上能重现苯乙烯与臭氧反应的气相过程.
图 3(Fig. 3)
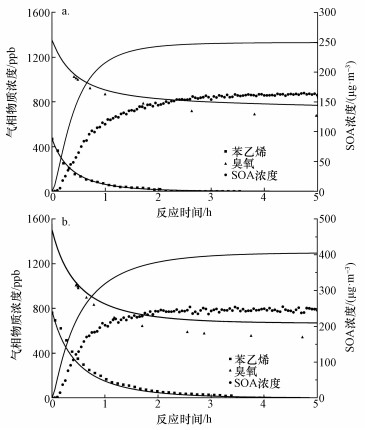 |
| 图 3 实验A3(a)和B8(b)反应过程中实验测量和数值模拟结果对比 (图中点代表实验测量值, 曲线代表对应物质的模拟值) Fig. 3Experimental and simulation results during the experiment A3(a) and B8(b) (The points represent the experimental results, and the lines represent the corresponding simulated values) |
对不同模拟条件下得到的气溶胶成分进行分析, 在没有加入仲丁醇作为·OH清除剂的情况下, 其主要SOA成分为3, 5-二苯基-1, 2, 4-三氧戊环(SOZ1)和2-过氧化氢基-1-苯基乙醇(STYRENOOH)(表 2), 均占到总SOA质量的1/2左右.STYRENOOH在MCM v3.3.1中生成路径如图 4所示, 在苯乙烯与臭氧反应过程中产生的·OH再与苯乙烯反应产生STYRENOOH, 并大量进入气溶胶相.当加入过量仲丁醇作为·OH清除剂后([仲丁醇]0/[苯乙烯]0约为70), 过量的仲丁醇消耗了大量的·OH, 使后者浓度下降了约50倍.这样更多的苯乙烯被O3所氧化, 而更少的苯乙烯被·OH氧化, 导致STYRENOOH产率的降低, 最终SOA产率也随之下降.另外, 仲丁醇的存在导致HO2浓度上升和RO2浓度下降.在对α-蒎烯和β-蒎烯等烯烃与O3反应的研究中也发现, ·OH清除剂的加入会引起[HO2]/[RO2]比的明显上升, 导致形成更多挥发性更强的产物, 进而影响了SOA的形成机制(Docherty et al., 2003; Jenkin, 2004; 兰辉等, 2011; Yuan et al., 2017).
表 2(Table 2)
| 表 2 不同模拟条件下得到的不同产物浓度 Table 2 Several product concentrations obtained under different simulation conditions | |||||||||||||||||||||||||||||||||||
表 2 不同模拟条件下得到的不同产物浓度 Table 2 Several product concentrations obtained under different simulation conditions
| |||||||||||||||||||||||||||||||||||
图 4(Fig. 4)
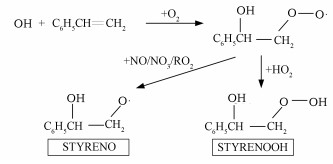 |
| 图 4 MCM v3.3.1中STYRENOOH的生成路径 Fig. 4STYRENOOH generation path in MCM v3.3.1 |
3.3 敏感性分析由于添加到箱式模型中的Criegee中间体与醛类反应的速率常数存在很大的不确定性, 因此, 针对这些速率常数对SOA生成的敏感性进行了分析.模拟条件如表 3所示, 其它初始条件同实验A3.模拟主要分两组展开, 第1组假设反应(1)~(4)的速率常数均相同, 第2组假设反应(1)~(4)的速率常数随着苯环官能团的增多而逐渐减小或增大.
表 3(Table 3)
| 表 3 箱式模型中使用的速率常数 Table 3 Rate constants used in box model | ||||||||||||||||||||||||||||||||||||||||||||
表 3 箱式模型中使用的速率常数 Table 3 Rate constants used in box model
| ||||||||||||||||||||||||||||||||||||||||||||
模拟结果如图 5所示, 图中黑色圆点为实验A3中所测得的SOA质量浓度.在假设k1=k2=k3=k4的情况下(图 5a), 随着Criegee中间体与醛类反应速率常数的增大, 该类反应的重要性逐渐增大, 得到SOZ产率增高, 同时生成的SOA质量浓度也越高; 而随着反应(2)~(4)反应速率相对于反应(1)的增大(图 5b), ·CH2OO·和HCHO与反应C6H5?HOO·+C6H5CHO→SOZ1产生更强的竞争反应, 得到的SOA质量浓度越低.模拟结果表明,反应(1)~(4)的反应速率对最终SOA形成有重要影响, 因此, 这一类反应的动力学研究有待进一步开展.
图 5(Fig. 5)
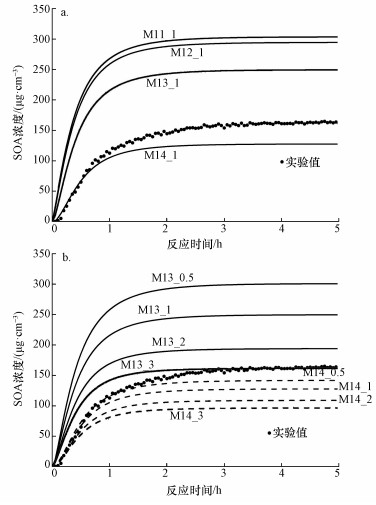 |
| 图 5 Criegee中间体与醛类反应的不同速率常数对模拟结果的影响 Fig. 5Effects of the rate constants of Criegee intermediates and aldehydes on simulation results |
4 结论(Conclusions)1) 过量仲丁醇的存在消耗了苯乙烯与臭氧反应产生的大量·OH, 影响了其中的自由基反应过程, 导致生成的SOA产率下降.
2) MCM v3.3.1气相化学机理能较好地重现烟雾箱中苯乙烯与臭氧的消耗速率,将速率系数为1.0×10-13 cm3·molecule-1·s-1的Criegee中间体+醛→SOZ反应添加到箱式模型后, 模拟得到的SOA产率较实验值高约50%, 且得到的产物主要为3, 5-二苯基-1, 2, 4-三氧戊环和2-过氧化氢基-1-苯基乙醇.
3) 针对Criegee中间体与醛反应在箱式模型中的影响进行了敏感性分析, 该反应对于苯乙烯与臭氧反应生成SOA的过程有着重要影响.但其反应速率系数缺乏直接的实验值和精确的理论值, 这些有待进一步的研究.
参考文献
| Anglada J M, Gonzalez J, Torrent-Sucarrat M. 2011. Effects of the substituents on the reactivity of carbonyl oxides.A theoretical study on the reaction of substituted carbonyl oxides with water[J]. Physical Chemistry Chemical Physics, 13(28): 13034–13045.DOI:10.1039/c1cp20872a |
| Anglada J M, Sole A. 2016. Impact of the water dimer on the atmospheric reactivity of carbonyl oxides[J]. Physical Chemistry Chemical Physics, 18(26): 17698–17712.DOI:10.1039/C6CP02531E |
| Agency for Toxic Substances and Disease Registry(ATSDR). 2010. Toxicological profile for styrene[OL]. 2018-03-21. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=421&tid=74. |
| 陈文泰, 邵敏, 袁斌, 等. 2013. 大气中挥发性有机物(VOCs)对二次有机气溶胶(SOA)生成贡献的参数化估算[J]. 环境科学学报, 2013, 33(1): 163–172. |
| Chew A A, Atkinson R. 1996. OH radical formation yields from the gas-phase reactions of O3 with alkenes and monoterpenes[J]. Journal of Geophysical Research:Atmospheres, 101(D22): 28649–28653.DOI:10.1029/96JD02722 |
| Cho J, Roueintan M, Li Z. 2014. Kinetic and dynamic investigations of OH reaction with styrene[J]. The Journal of Physical Chemistry A, 118(40): 9460–9470.DOI:10.1021/jp501380j |
| Criegee R, Wenner G. 1949. Die ozonisierung des 9, 10-oktalins[J]. Justus Liebigs Annalen der Chemie, 564(1): 9–15.DOI:10.1002/(ISSN)1099-0690 |
| Docherty K S, Ziemann P J. 2003. Effects of stabilized criegee intermediate and OH radical scavengers on aerosol formation from reactions of β-Pinene with O3[J]. Aerosol Science and Technology, 37(11): 877–891.DOI:10.1080/02786820300930 |
| Fajgar R, Vítek J, Haas Y, et al. 1996. Observation of secondary 2-butene ozonide in the ozonation of trans-2-butene in the gas phase[J]. Tetrahedron Letters, 37(19): 3391–3394.DOI:10.1016/0040-4039(96)00554-0 |
| Gai Y B, Ge M F, Wang W G. 2009. Rate constants for the gas phase reaction of ozone with n-butyl acrylate and ethyl methacrylate[J]. Chemical Physics Letters, 473: 57–60.DOI:10.1016/j.cplett.2009.03.070 |
| Griesbaum K, Miclaus V, Jung I C. 1998. Isolation of ozonides from gas-phase ozonolyses of terpenes[J]. Environmental Science & Technology, 32(5): 647–649. |
| Gutbrod R, Meyer S, Rahman M M, et al. 1997. On the use of CO as scavenger for OH radicals in the ozonolysis of simple alkenes and isoprene[J]. International Journal of Chemical Kinetics, 29(9): 717–723.DOI:10.1002/(ISSN)1097-4601 |
| Horie O, Sch?fer C, Moortgat G K. 1999. High reactivity of hexafluoro acetone toward criegee intermediates in the gas-phase ozonolysis of simple alkenes[J]. International Journal of Chemical Kinetics, 31(4): 261–269.DOI:10.1002/(ISSN)1097-4601 |
| Hu C, Ma Q, Liu Z, et al. 2017. Influence of NO2 on secondary organic aerosol formation from ozonolysis of limonene[J]. Atmospheric Chemistry & Physics, 2017: 1–58. |
| Iinuma Y, Boge O, Miao Y, et al. 2005. Laboratory studies on secondary organic aerosol formation from terpenes[J]. Faraday Discussions, 130: 279–294.DOI:10.1039/b502160j |
| Jalan A, Allen J W, Green W H. 2013. Chemically activated formation of organic acids in reactions of the Criegee intermediate with aldehydes and ketones[J]. Physical Chemistry Chemical Physics, 15(39): 16841–16852.DOI:10.1039/c3cp52598h |
| Jenkin M E. 2004. Modelling the formation and composition of secondary organic aerosol from α-and β-pinene ozonolysis using MCM v3[J]. Atmospheric Chemistry & Physics, 4(7): 1741–1757. |
| 贾记红, 黄成, 陈长虹, 等. 2009. 炼焦过程挥发性有机物排放特征及其大气化学反应活性[J]. 环境科学学报, 2009, 29(5): 905–912.DOI:10.3321/j.issn:0253-2468.2009.05.002 |
| 贾龙, 徐永福. 2010. 苯乙烯-NOx光照的二次有机气溶胶生成[J]. 化学学报, 2010, 68(23): 2429–2435. |
| Jonsson ? M, Hallquist M, Ljungstr m E. 2008. Influence of OH scavenger on the water effect on secondary organic aerosol formation from ozonolysis of limonene, △3-carene, and α-pinene[J]. Environmental Science & Technology, 42(16): 5938–5944. |
| 兰辉, 陈敏东, 马嫣. 2011. 1-甲基环己烯气相臭氧氧化生成二次有机气溶胶产物的研究[J]. 环境化学, 2011, 30(6): 1144–1151. |
| Li H, Chen Z M, Huang L B, et al. 2016. Organic peroxides' gas-particle partitioning and rapid heterogeneous decomposition on secondary organic aerosol[J]. Atmospheric Chemistry and Physics, 16: 1837–1848.DOI:10.5194/acp-16-1837-2016 |
| 李时政, 马嫣, 郑军, 等. 2015. α-蒎烯臭氧氧化反应中二次有机气溶胶理化特性与云凝结核活性[J]. 环境化学, 2015, 34(9): 1633–1641. |
| Lin X X, Liu Y R, Huang T, et al. 2014. Theoretical studies of the hydration reactions of stabilized Criegee intermediates from the ozonolysis of β-pinene[J]. RSC Advances, 4(54): 28490–28498.DOI:10.1039/c4ra04172k |
| Long B, Bao J L, Truhlar D G. 2016. Atmospheric chemistry of criegee intermediates:Unimolecular reactions and reactions with water[J]. Journal of the American Chemical Society, 138(43): 14409–14422.DOI:10.1021/jacs.6b08655 |
| Long B, Tan X F, Long Z W, et al. 2011. Theoretical studies on reactions of the stabilized H2COO with HO2 and the HO2···H2O complex[J]. The Journal of Physical Chemistry A, 115(24): 6559–6567.DOI:10.1021/jp200729q |
| Na K, Song C, Cocker D R. 2006. Formation of secondary organic aerosol from the reaction of styrene with ozone in the presence and absence of ammonia and water[J]. Atmospheric Environment, 40(10): 1889–1900.DOI:10.1016/j.atmosenv.2005.10.063 |
| Nannoolal Y, Rarey J, Ramjugernath D. 2008. Estimation of pure component properties:Part 3.Estimation of the vapor pressure of non-electrolyte organic compounds via group contributions and group interactions[J]. Fluid Phase Equilibria, 269(1): 117–133. |
| Nannoolal Y, Rarey J, Ramjugernath D, et al. 2004. Estimation of pure component properties:Part 1.Estimation of the normal boiling point of non-electrolyte organic compounds via group contributions and group interactions[J]. Fluid Phase Equilibria, 226: 45–63.DOI:10.1016/j.fluid.2004.09.001 |
| Neeb P, Horie O, Moortgat G K. 1996. Formation of secondary ozonides in the gas-phase ozonolysis of simple alkenes[J]. Tetrahedron Letters, 37(52): 9297–9300.DOI:10.1016/S0040-4039(97)82946-2 |
| Odum J R, Hoffmann T, Bowman F, et al. 1996. Gas/particle partitioning and secondary organic aerosol yields[J]. Environmental Science & Technology, 30(8): 2580–2585. |
| Pankow J F. 1994. An absorption model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol[J]. Atmospheric Environment, 28(2): 189–193.DOI:10.1016/1352-2310(94)90094-9 |
| 区家敏, 冯小琼, 刘郁葱, 等. 2014. 珠江三角洲机动车挥发性有机物排放化学成分谱研究[J]. 环境科学学报, 2014, 34(4): 826–834. |
| Stone D, Blitz M, Daubney L, et al. 2014. Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure[J]. Physical Chemistry Chemical Physics, 16(3): 1139–1149.DOI:10.1039/C3CP54391A |
| Taatjes C A, Welz O, Eskola A J, et al. 2012. Direct measurement of Criegee intermediate (CH2OO) reactions with acetone, acetaldehyde, and hexafluoroacetone[J]. Physical Chemistry Chemical Physics, 14(30): 10391–10400.DOI:10.1039/c2cp40294g |
| Topping D, Barley M, Bane M K, et al. 2016. UManSysProp v1.0:an online and open-source facility for molecular property prediction and atmospheric aerosol calculations[J]. Geoscientific Model Development, 9(2): 899–914.DOI:10.5194/gmd-9-899-2016 |
| Tuazon E C, Arey J, Atkinson R, et al. 1993. Gas-phase reactions of 2-vinylpyridine and styrene with hydroxyl and NO3 radicals and ozone[J]. Environmental Science & Technology, 27(9): 1832–1841. |
| Uhde E, Salthammer T. 2007. Impact of reaction products from building materials and furnishings on indoor air quality-A review of recent advances in indoor chemistry[J]. Atmospheric Environment, 41(15): 3111–3128.DOI:10.1016/j.atmosenv.2006.05.082 |
| Vereecken L, Harder H, Novelli A. 2012. The reaction of Criegee intermediates with NO, RO2, and SO2, and their fate in the atmosphere[J]. Physical Chemistry Chemical Physics, 14(42): 14682–14695.DOI:10.1039/c2cp42300f |
| 王伯光, 张远航, 邵敏, 等. 2008. 广州地区大气中C2~C9非甲烷碳氢化合物的人为来源[J]. 环境科学学报, 2008, 28(7): 1430–1440.DOI:10.3321/j.issn:0253-2468.2008.07.028 |
| Wang H H, Ji Y M, Gao Y P, et al. 2015. Theoretical model on the formation possibility of secondary organic aerosol from OH initialed oxidation reaction of styrene in the presence of O2/NO[J]. Atmospheric Environment, 101: 1–9.DOI:10.1016/j.atmosenv.2014.10.042 |
| 王跃思, 周立, 王明星, 等. 2010. 北京大气中可形成气溶胶的有机物[J]. 气候与环境研究, 2010, 5(1): 13–19. |
| Wei W W, Yang X, Zheng R H, et al. 2015. Theoretical studies on the reactions of the simplest Criegee intermediate CH2OO with CH3CHO[J]. Computational and Theoretical Chemistry, 1074: 142–149.DOI:10.1016/j.comptc.2015.10.013 |
| Wu H H, Wang Y, Li H, et al. 2017. The OH-initiated oxidation of atmospheric peroxyacetic acid:experimental and model studies[J]. Atmospheric Environment, 164: 61–70.DOI:10.1016/j.atmosenv.2017.05.038 |
| Yuan C, Ma Y, Diao Y W, et al. 2017. CCN activity of secondary aerosols from terpene ozonolysis under atmospheric relevant conditions[J]. Journal of Geophysical Research:Atmospheres, 122: 4654–4669.DOI:10.1002/2016JD026039 |
