全文HTML
--> --> --> 好氧颗粒污泥(aerobic granular sludge, AGS)是微生物细胞在一定选择压下自凝聚形成的一种规则而紧密的颗粒状污泥[1]。与普通活性污泥相比,AGS具有结构紧密、沉降性优良、微生物量高、同步脱氮除磷、耐有机负荷等优点[2-3],AGS形成机制日益成为污水处理领域的研究热点。污泥中的胞外聚合物(extracellular polymeric substances, EPS)是微生物在一定环境条件下分泌的高分子物质,其主要成分是蛋白质(protein, PN)和多糖(polysaccharides, PS),还有少量的腐殖酸、脂质、核酸以及富里酸类物质[4]。EPS作为细胞菌胶团的重要组分,其含量变化可改变微生物细胞表面特性,影响细胞间的相互凝聚能力[5],促进AGS形成和维持其颗粒状立体结构[6],对好氧污泥颗粒化具有重要作用。目前,有关AGS的EPS成分的研究结果具有较大差异。OLIVERIRA等[7]认为,在好氧污泥颗粒化过程中PN是EPS的主要成分,而LIN等[8]则发现EPS中PS含量最高。TAY等[9]认为,EPS中的PS可提高微生物细胞间的凝聚力,具有强化AGS结构稳定性的功能。WANG等[10]和ADAV等[11]也证明 PS作为AGS的核心并构成内部骨架以支撑整个颗粒状立体结构。但LIU等[12]和MCSWAIN等[13]认为PN是AGS的核心,CHEN等[14]亦发现PN是维持AGS结构稳定的关键物质。因此,在EPS对好氧污泥颗粒化的影响方面仍存在分歧。
本研究考察了污泥EPS中PN含量和污泥表面特性的相关性,采用三维荧光光谱(three-dimensional fluorescence spectrum, 3D-EEM)和傅里叶变换红外光谱(fourier transform infrared spectroscopy, FT-IR)等技术对接种污泥和AGS EPS组分和官能团进行了比较,明确了接种污泥和好氧颗粒污泥中EPS组成成分的差异,同时使用激光扫描共聚焦显微镜(confocal laser scanning microscope, CLSM)确定AGS EPS的分布情况,进一步了解EPS中PN对好氧污泥颗粒化的影响,以期为AGS形成的机理研究及其技术发展提供参考。
1.1. 接种污泥和实验用水
实验所用的接种污泥取自广州市猎德污水处理厂四期二沉池的回流污泥,接种污泥的理化特性为:接种污泥为絮状,污泥浓度为7 588 mg·L?1,污泥沉降比为84%,污泥容积指数为110.7 mL·g?1,污泥沉降速度为12.36 m·h?1。接种前淘洗掉污泥中的杂质,并对其进行曝气24 h。接种污泥的体积为3.7 L,约占序批式反应器(sequencing batch reactor, SBR)有效容积的1/2。实验用水采用人工配制的模拟废水,以C6H12O6和CH3COONa为混合碳源,NH4Cl为氮源,KH2PO4为磷源,用NaHCO3调节进水pH至7.5左右,并向进水中添加1 mL·L?1的微量元素溶液。人工配置的模拟废水水质组成如表1所示。
1.2. 实验装置及其运行方式
1)实验装置。在SBR中培养AGS,反应器主体为有机玻璃制成的圆柱体,其内径为9.8 cm,总高度为100 cm,有效高度为98 cm,有效高径比为10,反应器有效容积为 7.39 L。装置如图1所示。2)运行方式。在好氧污泥颗粒化过程中,SBR运行过程包括进水、曝气、沉淀、排水和闲置5个阶段,运行周期为6 h,包括进水4 min,曝气338~351 min,沉淀2~15 min,排水1 min,闲置2 min。反应器液体表面上升气速为0.86~4.64 cm·s?1,进水有机物浓度(以COD计)为800~1 800 mg·L?1,容积交换率为50%,通过水浴加热使SBR温度维持在25 ℃。采用调控COD、表面上升气速和污泥沉降时间以培养AGS。SBR运行的具体参数见表2。
1.3. 分析方法
1)常规指标分析。采用热提取法[15]提取EPS,EPS含量由PN和PS总含量表示;PS含量采用苯酚-硫酸法[16]测定;PN含量采用BCA(bicinchoninic acid)分光光度法[17]测定,其中EPS、PN和PS含量均以MLSS计;污泥Zeta 电位采用Zeta 电位分析仪(Zetasizer Nano ZS, Malvern)测定。颗粒污泥表面相对疏水性(relative hydrophobicity, Rh)。RH的具体测定过程如下:取泥水混合液10 mL,用 pH为7的三羟基甲基氨基甲烷缓冲液清洗2次;置于冰水浴中,用超声波细胞破碎仪(JY92-IIN, SCIENTZ)在 48 W 条件下超声 2 min;将超声后的悬浮液与10 mL正十六烷在分液漏斗中混合、摇匀 5 min;静置 30 min后测定相关污泥浓度,污泥浓度采用国家标准方法[18]进行测定。RH[19]根据式(1)进行计算。
式中:Q0为原污泥浓度,mg·L?1;Q1为分液后水相中的污泥浓度,mg·L?1。
2) 3D-EEM分析。采用荧光光谱仪(FluoroMax-4, HORIBA Jobin Yvon)对EPS进行分析。激发波长(excitation wavelength,Ex)和发射波长(emission wavelength, Em)分别为220~400 nm和290~500 nm,增量均为 5 nm,激发和发射狭缝宽度为3.6 nm,扫描速度为1 200 nm·min?1,响应时间为0.1 s。采用origin 8.0软件进行数据处理。
3) FTIR分析。采用傅里叶变换红外光谱仪(Nicolet IS50, Thermofisher)对EPS溶液进行官能团测定。操作过程如下:将提取的EPS溶液置于?80 ℃的冷冻干燥机(Virtis 4K, VIRTIS)内冷冻干燥至无水;将干燥后的EPS和溴化钾以质量比为1∶100的比例研磨并混合均匀,混合粉末压片成型后用红外光谱仪以分辨率为4 cm?1,扫描次数为32次,在4 000~500 cm?1内进行扫描,用origin 8.0软件进行数据处理。
4)荧光染色和CLSM分析。用荧光染料对AGS进行染色,以观察AGS样品中PN、PS、活细胞和死细胞的分布,选用的荧光染料[20-21]如表3所示。染色后的AGS样品使用激光共聚焦显微镜(LSM 800 with Airyscan, Carl Zeiss)进行观察。
2.1. AGS形成过程
污泥在SBR不同运行阶段的外观变化如图2所示。接种污泥为灰褐色、絮状,结构松散。当SBR运行到第30 天时出现少量细小的污泥颗粒,但絮状污泥占主体;在第50天,初期AGS形成,粒径较小。随着SBR持续运行,沉降性能较差的颗粒状污泥进一步被筛选出反应器,第70 天时AGS呈淡黄色,表面有一层绒毛,颗粒粒径主要集中在1.0~1.5 mm。第110 天时AGS培养成功,其外观为橙黄色,表面光滑,整体呈球状或椭球状的立体结构,颗粒粒径集中分布在1.43~2.26 mm。2.2. EPS、PN和PS含量以及PN/PS变化
好氧污泥颗粒化过程中EPS、PN和PS含量以及PN/PS变化情况如图3所示,接种污泥的EPS、PN和PS含量分别为29.86、13.98、15.88 mg·g?1,PN/PS为0.88。在SBR运行初期,EPS、PN和PS含量均逐渐增加,PS含量略高于PN含量,这是因为污泥中的微生物在选择压的刺激下需分泌大量PS来维持正常的生命活动[22]。随后因选择压加强,干扰了微生物细胞间的信息交流和信号分子传递,打破了污泥中微生物生理活动的平衡,导致微生物新陈代谢活动受到抑制,因此,EPS、PN和PS含量从第45 天起均呈下降趋势,PN/PS出现明显波动。从第65 天起,可能是因为SBR中有大量细小颗粒污泥,微生物量较大且活性较高;另一方面,因为SBR长期运行,污泥中的部分微生物细胞自溶,所以EPS中的PN和PS含量增加。但微生物的代谢活动可消耗EPS中的PS,而PS含量的增加可能与其消耗近似处于平衡状态,所以PN/PS值随着PN含量的增加而增大[23]。第110 天时AGS培养成功,AGS的EPS、PN和PS的含量分别为68.60、41.86和26.74 mg·g?1,PN/PS为1.57,约是SBR启动时的2倍。在整个好氧污泥颗粒化过程中,EPS和PN含量及PN/PS变化整体均呈增大趋势,而PS维持在15.88~26.74 mg·g?1,说明AGS中EPS成分以PN占主导,这与CHEN等[20]研究结果一致。2.3. PN含量和污泥Zeta电位的相关性
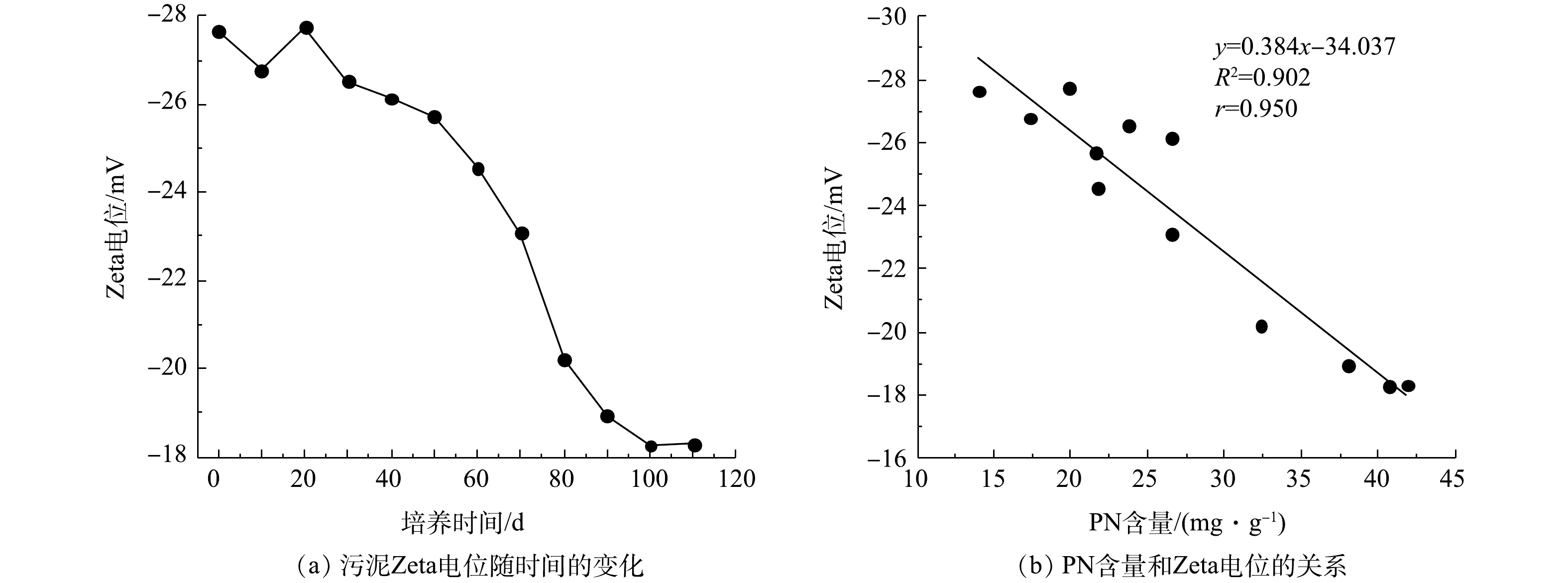
污泥Zeta电位变化及PN含量与Zeta电位之间的关系如图4所示。由图4(a)可知,接种污泥的Zeta电位为?27.64 mV,在SBR运行前30 d内,因接种污泥要适应新的环境,其生长状态不稳定,所以污泥Zeta电位有一定波动。随后污泥Zeta电位开始下降,在第100天,污泥Zeta电位下降到?18.27 mV。在第110天时AGS培养成功,其Zeta电位为?18.31 mV。如图4(b)所示,PN含量和污泥Zeta电位呈负相关,相关系数(r)为0.950。由于PN可与水中的金属离子发生离子键作用,压缩双电层,降低污泥Zeta电位[24]。此外,PN中带有正电荷的氨基类物质能够中和部分羟基和磷酸根基团中的负电荷,进一步降低污泥Zeta电位[25]。因此,污泥Zeta电位的降低可促进微生物间的相互凝聚,最终形成AGS。在本次实验过程中,PN含量基本处于增加趋势,而污泥Zeta电位趋于下降趋势,这说明EPS中PN含量增加,污泥Zeta电位降低。2.4. PN含量和RH的相关性
RH变化及PN含量与RH之间的关系如图5所示。在好氧污泥颗粒化过程中,RH整体呈增加趋势。接种污泥的RH为27.27%,在SBR运行初期,RH基本呈缓慢增加趋势,在第70天RH达到60.01%。随后因为初期AGS大量形成,反应器中絮状污泥被颗粒状污泥代替,所以RH开始显著增加,在第100天时RH为77.58%。随着AGS逐渐成熟稳定,第110 天时AGS的RH为76.80%,约是接种污泥的2.8倍(图5(a))。LIU等[26]发现RH和SBR运行期间的选择压密切相关,因此,其在好氧污泥颗粒化过程中具有重要作用。同样,DIGANCE等[27]的研究表明, 污泥EPS中的PN是污泥的主要疏水成分。在本实验中发现PN含量变化与RH变化存在密切关系,如图5(b)所示。PN含量和RH呈正相关关系,r为0.934。此外,张丽丽等[28]发现AGS的PN含量与RH密切相关,污泥RH随PN含量的增加而增大。结合2.3节的结果可知,在好氧污泥颗粒化过程中,PN含量增加,污泥Zeta电位降低且RH升高,有利于微生物细胞间的相互聚集,促进AGS形成。2.5. EPS的3D-EEM图谱分析
采用3D-EEM技术对接种污泥和AGS的EPS组分进行比较。接种污泥和AGS的EPS的3D-EEM如图6所示。接种污泥和AGS的EPS的3D-EEM中均出现荧光峰A(Ex: 270~285 nm,Em: 295~320 nm)、荧光峰B(Ex: 270~295 nm,Em: 325~390 nm)和荧光峰 C(Ex: 310~380 nm,Em: 400~470 nm),其中荧光峰A和荧光峰B分别代表酪氨酸和色氨酸类蛋白质,均属于溶解性微生物代谢产物,酪氨酸类蛋白质是AGS形成的重要成分,可促进絮状污泥颗粒化[29-30];而色氨酸类蛋白质为疏水性物质,其与EPS中芳环氨基酸结构共同作用,可提高AGS结构稳定性[31]。AGS中EPS的荧光峰A和荧光峰B的荧光强度明显高于接种污泥,这表明在好氧污泥颗粒化过程中,污泥EPS中酪氨酸和色氨酸类蛋白质含量有所增加。AGS的EPS的3D-EEM出现2个新荧光峰:即代表芳香族蛋白类物质的荧光峰D(Ex: 220~230 nm,Em: 290~310 nm)和代表富里酸类物质的荧光峰E(Ex: 220~240 nm,Em: 400~470 nm),而芳香族蛋白类物质的存在有利于AGS的形成[28]。2.6. EPS的FT-IR分析
为了确定接种污泥和AGS的EPS官能团的差异,在4000~500 cm?1内进行了EPS的FT-IR分析,结果如图7所示。1 650~1 600 cm?1吸收峰是由蛋白质二级结构(酰胺Ⅰ)中的C=O拉伸振动引起的β-折叠和α-螺旋,1 550 cm?1吸收峰是由酰胺Ⅱ中的N—H弯曲产生[32-33]。AGS EPS在1 650~1600 cm?1和1 550 cm?1处的吸收峰面积分别为133.87、52.51(见表4)。有研究[34-35]发现,酰胺Ⅰ中的蛋白质二级结构具有促进微生物絮凝,加快推进好氧污泥颗粒化进程的作用,而从接种污泥提取的 EPS中未出现相似的吸收峰。接种污泥和AGS的EPS分别在1 400 cm?1和1 410 cm?1处出现与C=O对称拉伸有关的吸收峰[36]。接种污泥的EPS中存在与PN(酰胺Ⅲ)有关的C—N拉伸所产生的1 260 cm?1吸收峰,吸收峰面积为36.43(见表3),而从AGS中提取的EPS中并未出现类似吸收峰。因PS中C—O伸缩震动和C—OH变形震动造成在1 140 cm?1和1 100 cm?1处出现吸收峰,2处吸收峰面积分别为85.89和108.65(见表4),表明好氧污泥颗粒化过程中PS含量没有明显变化,这与PS含量测定结果(图3)相一致。
2.7. EPS的CLSM分析
为确定AGS EPS分布情况,采用CLSM对AGS EPS分布进行了研究。如图8所示,AGS表面附着PN(绿色区域)和PS(蓝色区域)。EPS分布在AGS外表层,从而将微生物细胞包裹在EPS中。一方面,EPS在AGS表面形成保护层,在一定程度上可用来抵御环境恶化以及有毒物质对微生物细胞的危害;另一方面,在营养物质匮乏时,微生物将EPS作为新的能源物质来维持自身的生命活动。AGS表面的PS为高分子粘性物质,可以作为细胞间的连接基质,与丝状菌嵌合形成交叉的网状骨架结构(图8(b)),为微生物细胞与其他微小颗粒间形成架桥。在外界选择压的改变下,附着在AGS表面的PN改变细胞表面的特性,进一步促进微生物细胞间以及微生物与污泥微粒间的相互凝聚并形成微生物聚集体,最终形成好氧颗粒污泥。由图8(b)和图8(d)发现,绿色区域面积大于蓝色区域面积,表明AGS EPS中PN含量高于PS含量,与图3中的结果相符合。2) PN含量和污泥Zeta电位、RH分别呈负相关和正相关,r分别为0.950、0.934。PN含量增加,污泥Zeta电位降低,污泥RH升高,有利于微生物细胞间的相互聚集,可促进好氧污泥颗粒化。
3) EPS中代表酪氨酸和色氨酸类蛋白质的荧光强度增强,AGS EPS中出现芳香族蛋白和富里酸类物质以及含有N—H官能团的蛋白质,为好氧污泥颗粒化奠定了物质基础。
4) EPS分布在AGS表层并包裹微生物细胞。
参考文献


 下载:
下载: 





 点击查看大图
点击查看大图