 ,1,2,3,*
,1,2,3,*New fossils of Late Pleistocene Sus scrofa from Yangjiawan Cave 2, Jiangxi, China
SUN Ji-Jia1,2,3, ZHANG Bei1,2,3, CHEN Xi4, DENG Li5, WEN Jun5, TONG Hao-Wen ,1,2,3,*
,1,2,3,*通讯作者: *tonghaowen@ivpp.ac.cn
收稿日期:2020-04-28网络出版日期:2021-01-20
| 基金资助: |
Received:2020-04-28Online:2021-01-20

摘要
江西萍乡杨家湾2号洞是发育在二叠系灰岩中的溶洞,其中充填了晚更新世的黏土和沙砾堆积;自2015年至今已经挖掘6次,出土了万余件哺乳动物化石标本,其中野猪牙齿化石约占49%, 代表目前我国更新世野猪牙齿化石最为丰富的地点。将杨家湾2号洞出土的猪科动物牙齿化石(尤其是犬齿和第三臼齿)与我国南方特有的化石种裴氏猪(Sus peii)和小猪(Sus xiaozhu)及盐井沟出土的野猪化石进行了牙齿形态学比较研究和一系列数据分析(包括散点图、回归分析、变异系数分析和线性判别分析等); 所有雄性下犬齿均属于野猪型,数据分析结果也表明,杨家湾2号洞出土的猪科化石可全部归入野猪种(Sus scrofa); 尽管雄性下犬齿和上第二及下第三臼齿测量数据的变异范围很大,但都在野猪的变异范围之内。在此基础上,还利用杨家湾2号洞出土的野猪第三臼齿化石进行了种内变异研究,上、下第三臼齿的散点图各自聚为两大聚集区,这一结果很可能是由性别差异所导致而非不同属种混合。中国南方地区早更新世之后的猪科动物基本只有野猪一种,这与毗邻的东南亚地区不同;东南亚的猪科动物十分多样,并且绝大多数具有爪哇疣猪型犬齿。
关键词:
Abstract
The YJW (Yangjiawan) Cave 2 of Pingxiang in Jiangxi Province is a karst cave that developed in the Permian limestone of the Changxing Formation, which is filled with clay and grit of Late Pleistocene age. Six excavations have been conducted at the site since 2015. More than ten thousand mammalian fossils have been unearthed, and the wild boar fossils account for approximately 49%, which represents the richest wild boar fossil tooth collection of Pleistocene age in southern China. This study focuses on the studies of the canine teeth and the third molars, and mainly compares fossils of Sus peii and S. xiaozhu in South China and the data of extant S. scrofa respectively in dental morphology and odontometric data analyses which includes scatter plot analysis, regression analysis, coefficient of variation analysis and linear discriminant analysis. The typical scrofic type of the male’s lower canine teeth confirmed the identification of the suid fossils from YJW Cave 2 as S. scrofa. Although the male’s lower canines, the M2s and m3s, are among the most variable teeth in sizes, they stay in the ranges of S. scrofa; furthermore, the scatterplots of both the upper and lower third molars form two distinct clusters respectively, which can probably be attributed to sexual dimorphism rather than resulting from a mixture of different suid species. The post-Early Pleistocene suid fauna in southern China is almost only composed of S. scrofa, which is quite different from the adjacent Southeast Asia where the suid fauna is quite taxonomically diversified and dominated by the verrucosic type.
Keywords:
PDF (3118KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
孙吉嘉, 张贝, 陈曦, 邓里, 文军, 同号文. 江西萍乡杨家湾2号洞晚更新世野猪化石研究. 古脊椎动物学报[J], 2021, 59(1): 64-80 DOI:10.19615/j.cnki.1000-3118.200819
SUN Ji-Jia, ZHANG Bei, CHEN Xi, DENG Li, WEN Jun, TONG Hao-Wen.
1 Introduction
One of the IVPP’s (Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences) teams has been excavating YJW (Yangjiawan) Cave 1 since 2014 and YJW Cave 2 since 2015. These two caves are just 50 m apart (Zou et al., 2016).The Yangjiawan caves are located in Shangli County, Pingxiang, Jiangxi Province, and the exact position of Cave 2 is 27°46′25″N, 113°50′25″E. They are karst caves which are filled with Late Pleistocene clay and silt deposits. The mammalian fauna from YJW Cave 2 has been classified into 6 orders, 20 families and around 40 species; all of them are among the common elements of the Ailuropoda-Stegodon fauna (Zhang et al., 2017, 2018; Jiangzuo et al., 2018; Tong et al., 2018). The site bears the most abundant fossils and most species found in Jiangxi Province to date. More than 10000 mammalian tooth fossils have been unearthed, the majority of which belong to wild pigs.
Suiformes, including Suidae and Tayassuidae can be traced back to the Middle Eocene (Gentry and Hooker, 1988; Ducrocq, 1994; Ducrocq et al., 1998; Liu, 2001). The subfamily Suinae is among the most common elements in the Quaternary fauna of southern China. Three genera (Potamochoerus, Dicoryphochoerus and Sus) and about 13 species have been reported, whereas the validity of the Chinese Dicoryphochoerus as a genus will need further analysis when more material is recovered (Hou et al., 2018). Although the Quaternary Suinae fossils in southern China are very common, they are usually not well preserved and the crucial anatomical parts are missing. Therefore, many taxonomic issues remain unresolved. Comparative study of the skull features is one of the most effective vertebrate research methods. There are few skull specimens of fossil Suinae in the Quaternary deposits in China; meanwhile, there are a large number of isolated tooth remains, and the measurement and related analysis of the teeth are also important for species determination (Zeuner, 1963; B?k?nyi, 1974; Mayer et al., 1998). Researchers in the study of wild boars and domestic pigs usually measure the data of the second and the third molars; but the crown length of the second molar may have been reduced by abrasion with adjacent molars (Higham, 1968; Flannery, 1983; Stampfli, 1983; Payne and Bull, 1988; Mayer et al., 1998). There is clear sexual dimorphism in adult individuals with the most significant difference lying in the shape and size of the lower canine (Harrison and Bates, 1968; Payne and Bull, 1988; Mayer and Brisbin, 1991, 1993).
Like other cave sites in southern China, YJW Cave 2 lacks skull materials, but numerous tooth fossils have been unearthed. This study aims to identify the pig fossils in YJW Cave 2 by means of morphological characteristics observation and data measurements, and to compare the results with the data of S. xiaozhu and S. peii from LGC (Liucheng Gigantopithecus Cave), Guangxi and S. scrofa from Yanjinggou of Sichuan. In addition, the rich specimens are also helpful for other studies, especially the canine teeth and the third molars, and practical to conduct intraspecific variation studies, including the study of morphological characteristics and size variations between different individuals and genders.
2 Materials and methods
This study focused on the morphological identification and measurement data analysis of the canines, the fourth premolars, the second and third molars unearthed at YJW Cave 2 in 2018, and the observed specimens including 1 left I1 (IVPP V 26768.108), 1 left i1 (V 26768.11), 2 left i2s (V 26768.12-13), 10 male upper canines (V 26768.1-9, 115), 4 female upper canines (V 26768.10, 116-117, 362), 27 male lower canines (V 26768.14-22, 244-249, 363-374), 43 female lower canines (V 26768.23-37, 250-261, 375-390), 1 left P2 (V 26768.41), 1 left p2 (V 26768.38), 1 maxilla with P3 and P4 (V 26768.54), 1 left p3 (V 26768.39), 15 left P4s (V 26768.118-132), 12 right P4s (V 26768.42-53), 14 left p4s (V 26768.40, 262-274), 13 right p4s (V 26768.275-287), 1 left M1 (V 26768.56), 1 right m1 (V 26768.55), 46 left M2s (V 26768.57-71, 172-183, 391-409), 60 right M2s (V 26768.184-194, 410-458), 62 left m2s (V 26768.72-80, 311-319, 459-502), 55 right m2s (V 26768.320-329, 503-547), 41 left M3s (V 26768.81-89, 204-211, 548-571), 25 right M3s (V 26768.195-203, 572-587), 73 left m3s (V 26768.90-99, 330-361, 588-618) and 60 right m3s (V 26768.619-678). The main reference materials were the measurement data of the third molars of S xiaozhu and S. peii from LGC in Guangxi (Han, 1987).The classification system at high ranks is after McKenna and Bell (1997) and Frantz et al. (2016); the classification at the species level is after Groves (1981).
The methods adopted are morphological observations and comparisons. Anatomical terms and methods of measuring of the tooth crown are from Hardjasasmita (1987), Van der Made (1996) and Fujita et al. (2000) (Fig. 1). The upper teeth and lower teeth were denoted by uppercase and lowercase letters respectively. The main analysis methods include scatter plot analysis, regression analysis, coefficient of variation analysis, and linear discriminant analysis.
Fig. 1
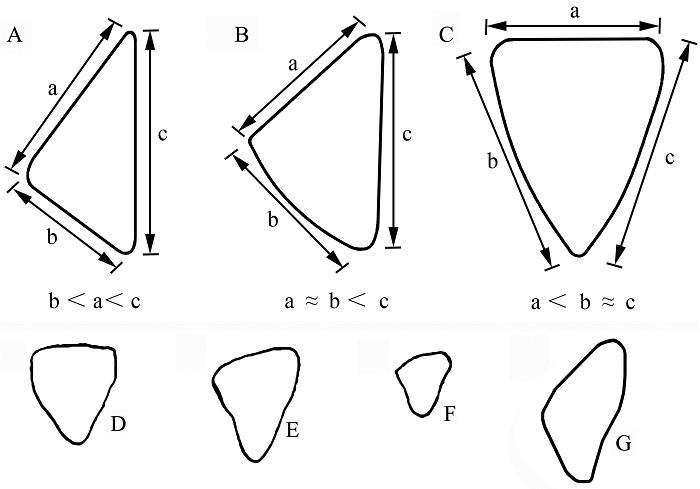 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 1Types of cross sections of the lower canines of different male suids
A. scrofic type; B. intermediate type; C. verrucosic type (modified from Fujita et al., 2000); D-E. Sus peii; F. S. xiaozhu (modified from Han, 1987); G. S. scrofa from Yangjiawan Cave 2 a. width of the posterior face; b. width of the labial (external) face; c. width of the lingual (internal) face
3 Systematic paleontology
Order Artiodactyla Owen, 1848Suborder Suiformes Jaeckel, 1911
Superfamily Suoidea Gray, 1821
Family Suidae Gray, 1821
Subfamily Suinae Zittel, 1893
Genus Sus Linnaeus, 1758
Sus scrofa Linnaeus, 1758
3.1 Descriptions and analysis of morphology
Out of all the bones and teeth, the anatomical features of the male lower canine are among the most crucial features for suid classifications (Wilkinson, 1976) and might even be considered to be the most important character in distinguishing different Sus species (Groves, 1981, 2007). There are three main types of lower canine cross-sections: the scrofic type, the verrucosic type and the intermediate type (Fujita, 2000) (Fig. 1A-C). In all the three types, the lingual (=anterior: Groves, 1981) face is always the broadest (Groves, 1981:11); in the scrofic type (Fig. 1A), the labial (inferior: Groves, 1981) face is the narrowest (Groves, 1981:11); in the verrucosic type, the posterior face is the narrowest (Hardjasamita, 1987) (Fig. 1C); in the intermediate type, the posterior face becomes as broad as the labial one (Fujita, 2000) (Fig. 1B). It’s worth mentioning that Groves (1981:11) said that the inferior surface of the verrucosic type is as broad as the posterior surface, which corresponds with the “intermediate type” by Fujita et al. (2000). The feature of suid canine fossils unearthed in this deposit is clearly the scrofic type with the lingual surface being the broadest and the labial face being the narrowest; the posterior face is enamel-less. The lower canines of S. xiaozhu and S. peii from LGC are the intermediate type (Chen, 2004), but closer to the verrucosic type according to the figures by Han (1987:fig. 3). In terms of lower canine teeth, fossils from YJW Cave 2 are closer to S. scrofa (Figs. 1G; 2: 8c, 9c). The size of the male lower canine from YJW Cave 2 varies quite a bit, which should represent different age classes according to the study by Endo et al. (1994). Although the females of S. scrofa have the largest canine teeth among the Sus species, which even overlaps with the male’s range (Groves, 1981), it’s still not difficult to distinguish the male’s lower canines from those of the females, because the female’s lower canine is not only obviously smaller in size, but also has a developed enamel-less tooth-root (Mayer and Brisbin, 1988). Furthermore, the present authors also noticed that the female’s lower canine has a relatively narrower posterior surface (Fig. 2: 17-24).Fig. 2
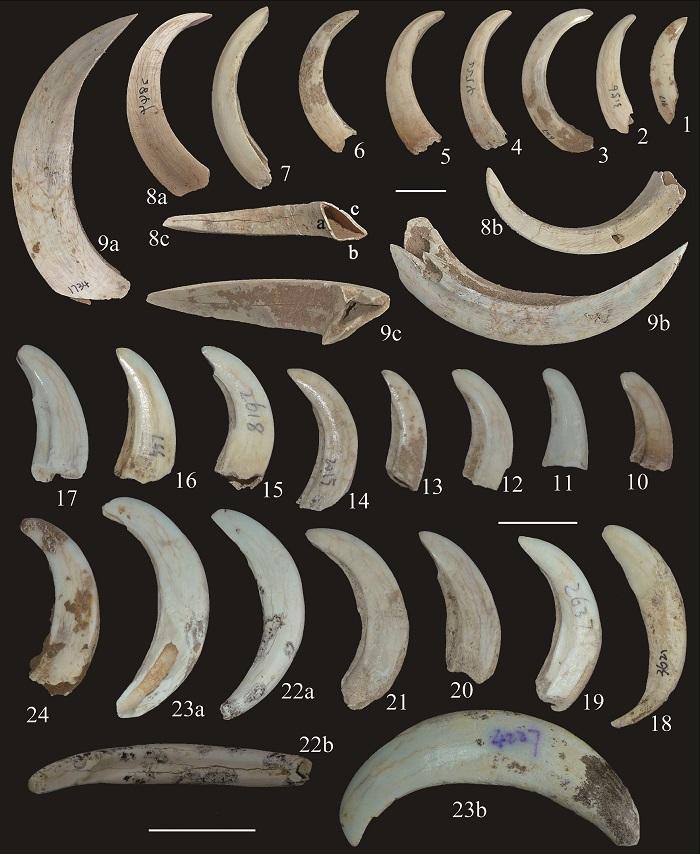 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 2Lower canines of both sexes of Sus scrofa from Yangjiawan Cave 2
1-3, 6, 9. male right lower canines (IVPP V 26768.14-18); 4-5, 7-8. male left lower canines (horizontally flipped) (V 26768.19-22); 10, 17, 20-24. female left lower canine (horizontally flipped) (V 26768.23-29); 11-16, 18-19. female right lower canine (V 26768.30-37) 1-7, 8a, 9a, 23b. anterior (or lingual) views; 8b, 9b, 10-24. inferior (or labial) views; 8c, 9c, 22b. posterior views. Scale bars=2 cm
The male lower canines of S. scrofa from YJW Cave 2 are fairly variable in size, which is closely related to their age stages (Fig. 2: 1-9); none of them has a root, which means the male lower canine can continue growing throughout their life span. In the contrast, the female lower canines are much smaller in size and with developed roots, but the size is far less variable relative to that of the males (Fig. 2: 10-24).
The male upper canine is much more robust, with a trapezoidal cross-section, grooved enamel, and enamel-free bands extended along the entire length of the rootless tooth, but the size has limited range of variation (Fig. 3: 1-9). The female upper canine is much more reduced and compressed, with an atypical triangular cross-section and it has a developed root with only the crown part is covered by enamel (Fig. 3: 10).
The upper incisors are few in number and not well preserved. The lower incisors are well represented. The most important characters of the i2 are the bended tooth body and the developed distal groove (Fig. 3: 12-13); whereas these two characters are absent in the i1 (Fig. 3: 11).
Fig. 3
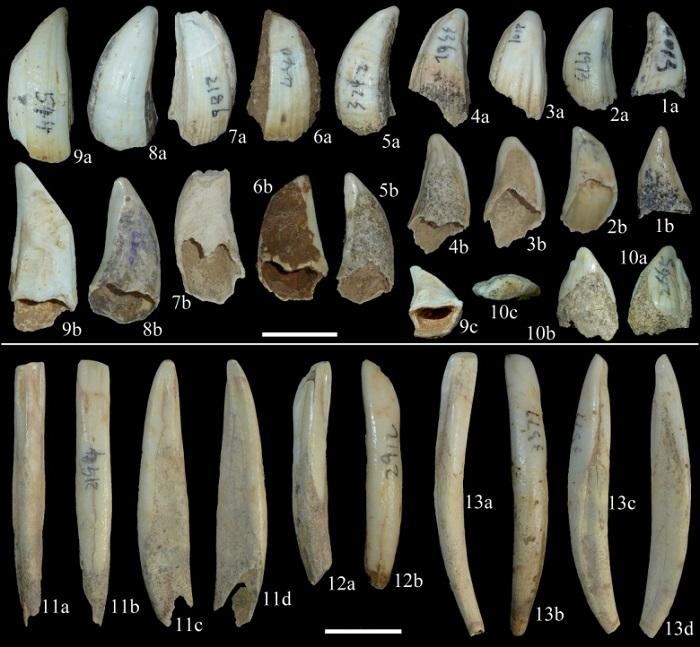 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 3Upper canines and lower incisors of Sus scrofa from Yangjiawan Cave 2
1, 6-7. male right upper canines (IVPP V 26768.1-3); 2-5, 8-9. male left upper canines (V 26768.4-9); 10. female left upper canine (V 26768.10); 11. left i1 (V 26768.11); 12-13. left i2s (V 26768.12-13). 1a-10a. inferior views; 1b-10b. superior views; 9c. basal view; 10c. crown view; 11a-13a. lingual views; 11b-13b. vestibular views; 11c, 13c. distal views; 11d, 13d. mesial views. Scale bars=2 cm
In form, the P2 (Fig. 4: 4) is similar to the P3, but prominently smaller. The P3 has a developed paracone, metacone and protocone as well as a prominent primocone (Fig. 4: 17). Both the P2 and P3 have two roots.
The P4 has three main cusps: a paracone and metacone at the buccal side, and a protocone at the lingual side. The protocone varies greatly in both shape and size, mainly lying at the contact point between the protocone precrista and the postcrista of the protopreconule; furthermore, the general shape and size of the tooth is also very variable (Fig. 4: 5-17). The P4 has four roots, but the two lingual roots commonly merge into one.
The M1 (Fig. 4: 19) is very similar to the M2 in shape, but much smaller. The M2 has four equally developed cusps: a paracone and metacone at the buccal side, protocone and hypocone (=tetracone: Fujita et al., 2000) at the lingual side, and a moderately developed tetrapreconule occurs at the central area surrounded by the four major cusps with the posterior cingulum (=pentapreconule: Fujita et al., 2000) being much more developed than the anterior one (Fig. 4: 20-34).
The p2 (Fig. 4: 1) and p3 (Fig. 4: 2) are similar in form, but the former is prominently smaller; both of them have developed protoconid and hypoconid. Both the p2 and p3 have two roots. The crown of the p4 consists of four major cuspids: paraconid, protoconid, metaconid and hypoconid, and a hypoendocristid developed at the lingual side of the hypoconid; at the buccal side, two vertical ridges occur at the anterior and posterior corners respectively (Fig. 4: 3). Normally the p4 has two roots, but the posterior one is occasionally forked distally; anatomical study shows that the p4 has three root canals (Ide et al., 2013).
The m1 (Fig. 4: 18) is very similar to the m2 in shape but relatively smaller. The m2 has four main cuspids: protoconid and hypoconid at the buccal side, metaconid and entoconid at the lingual side. Furthermore, the pentaconid at the distal end and the hypopreconulid at the central part surrounded by the four main cuspids are also quite prominent (Fig. 4: 35-43). Both the m1 and m2 have four roots. The m2s are less variable in dimensions than the M2s.
The M3s of suid fossils unearthed from YJW Cave 2 generally have three lobes (Fig. 4: 44-52). The crown shape is triangular with a rounded right angle near the protocone. Some specimens are also found that they bend toward the lingual side in the zone of the second lobe to the third lobe. Taking the left M3 as an example, the paracone is the highest among the cusps. The heights of the protocone and metacone are similar, while the size and height of the hypocone are the smallest among the four main cusps. A hypoectoconule usually develops between the protocone and the hypocone. The hypopreconule that develops between the first and the second lobe is somewhat large; it may sometimes be as large as the protocone and the metacone. The pentacone is large. A pentaectoconule may develop between the hypocone and the pentacone, although it is sometimes missing. The pentapreconule is generally slightly smaller than the hypopreconule with the surrounding tip fully developed. The variability of pentacone is relatively large, and some specimens split at the top. Several small cusps are attached around it. Each cusp of the M3 is relatively complicated with many deep grooves and small cristae developed, as well as some cusplets developed. In terms of shape, the M3 of the pig fossils of YJW Cave 2 is relatively close to S. peii and S. scrofa. However, the third lobe of the M3 of S. peii is generally composed of a large cusp, and there are fewer small cusps develop with fewer cristae developing in the first and second lobe. The variability is mainly manifested in the total size and the development of the third lobe. Therefore, the M3 of the suid fossils of YJW Cave 2 should not be referred to S. peii but are likely closer to S. scrofa instead. The M3 normally has five roots, corresponding to each main cusp and the talon respectively, but occasionally has extra rootlets.
More than half of the m3s have four lobes with a complex variability in the fourth lobe (Fig. 4: 53-62). Taking the left m3 as an example, the protoconid is generally lower than the metaconid, and it is almost the same height as the hypoconid. The entoconid is generally lower than the hypopreconulid but higher than the protoconid. The hypopreconulid is similar in size to the pentapreconulid but higher than the latter. The variability of the hypoectoconulid is great in size and division at the top. There are several deep grooves around the pentapreconulid when the crown is not abraded yet, which separates it from the surrounding cusps. The pentaectoconulid varies greatly in size and is not consistently present. The pentaconid is usually lower than the hypoconid but higher than the hexaconid with a deep groove separating them from each other. The variability of the fourth lobe mainly lies in the expanding of the heptaconid. Other variability is found in the size of heptaconid and the existence of the small cusps and cristid. In some specimens, a tendency to bend to the lingual side from the third lobe is also observed (Fig. 4). The m3 has five roots, corresponding to each main cuspid and the talonid respectively.
Fig. 4
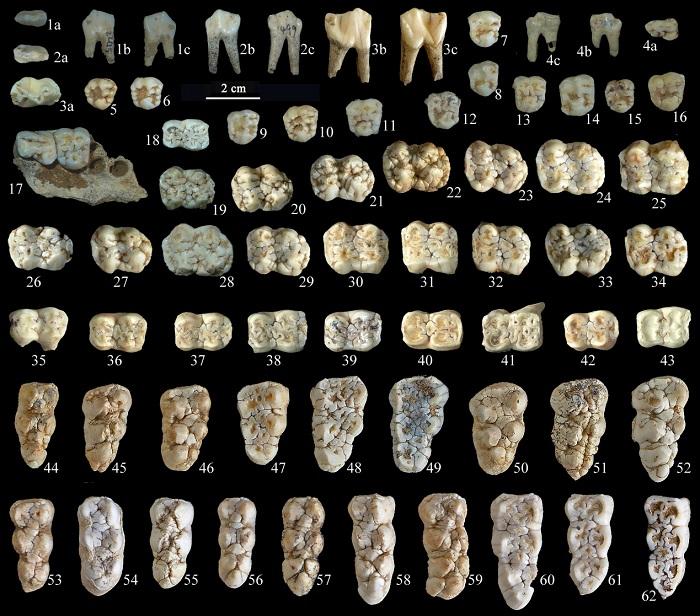 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 4Selected cheek teeth of Sus scrofa from Yangjiawan Cave 2
1. left p2 (IVPP V 26768.38); 2. left p3 (V 26768.39); 3. left p4 (V 26768.40); 4. left P2 (V 26768.41); 5-16. right P4s (V 26768.42-53); 17. partial left maxilla with P3-4 (V 26768.54); 18. right m1 (V 26768.55); 19. left M1 (V 26768.56); 20-34. left M2s (V 26768.57-71); 35-43. left m2s (V 26768.72-80); 44-52. left M3s (V 26768.81-89); 53-62. left m3s (V 26768.90-99) 1a, 2a, 3a, 4a, 5-62. occlusal views; 1b, 2b, 3b, 4b. buccal views; 1c, 2c, 3c, 4c. lingual views
The variability of the pig fossils of YJW Cave 2 is mainly manifested in the total size and the situation of the third and fourth lobes. The crown shape of S. peii is characterized by a rectangular crown shape, a talonid with a pair of unseparated conids, fewer small cuspids and only a small number of individuals developing the fourth lobe (Chen, 2004). Therefore, the m3s from YJW Cave 2 should not belong to S. peii, but are closer to those of S. scrofa. Previous study shows that only S. lydekkeri, S. scrofa and S. australis have four lobes in m3s (Han, 1987), which means the atypical verrucosic type suids in southern China shared some characters with the boreal suid species during the Early Pleistocene.
Concerning the significance of the m3 in taxonomy, it’s still open to debate; the study on the pig remains from Dadiwan Neolithic site shows that the m3 is the most variable tooth among the cheek teeth. It not only varies greatly in size, but also in the development of the talonid (Qi et al., 2006). The present study also demonstrates that the m3 has quite high values of coefficient of variation (Figs. 5, 6; Table 1).
Table 1
Table 1Data of Sus xiaozhu, S. peii and S. scrofa (mm)
| n | Variation | Average | SD | CV | R2 | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| S. xiaozhu (Han, 1987) | M3 | L | 37 | 16.3?22.0 | 19.4 | 1.37 | 7.05 | 0.65 | <0.01 |
| W | 11.4?16.6 | 13.8 | 1.19 | 8.62 | |||||
| m3 | L | 25 | 16.2?26.8 | 21.6 | 2.76 | 12.78 | 0.85 | <0.01 | |
| W | 8.3?14.3 | 11.3 | 1.33 | 11.73 | |||||
| S. peii (Han, 1987) | M3 | L | 30 | 34.6?42.0 | 38.4 | 1.94 | 5.05 | 0.07 | 0.17 |
| W | 21.3?25.5 | 23.6 | 0.96 | 4.07 | |||||
| m3 | L | 58 | 35.0?44.3 | 38.5 | 1.96 | 5.09 | 0.37 | <0.01 | |
| W | 16.2?22.9 | 19.0 | 1.13 | 5.94 | |||||
| S. scrofa (YJW Cave 2) | M2 | L | 106 | 15.3?28.1 | 23.3 | 1.71 | 7.35 | 0.17 | <0.01 |
| W | 11.7?21.4 | 18.7 | 1.53 | 8.18 | |||||
| m2 | L | 117 | 20.1?29.2 | 23.0 | 1.38 | 6.01 | 0.41 | <0.01 | |
| W | 13.1?18.5 | 15.3 | 0.93 | 6.09 | |||||
| M3 | L | 76 | 29.1?41.0 | 35.1 | 2.47 | 7.04 | 0.54 | <0.01 | |
| W | 19.3?23.0 | 20.2 | 1.40 | 6.92 | |||||
| m3 | L | 133 | 32.1?49.1 | 38.7 | 2.89 | 7.48 | 0.30 | <0.01 | |
| W | 13.0?20.8 | 17.1 | 1.17 | 6.84 |
新窗口打开|下载CSV
3.2 Data analysis
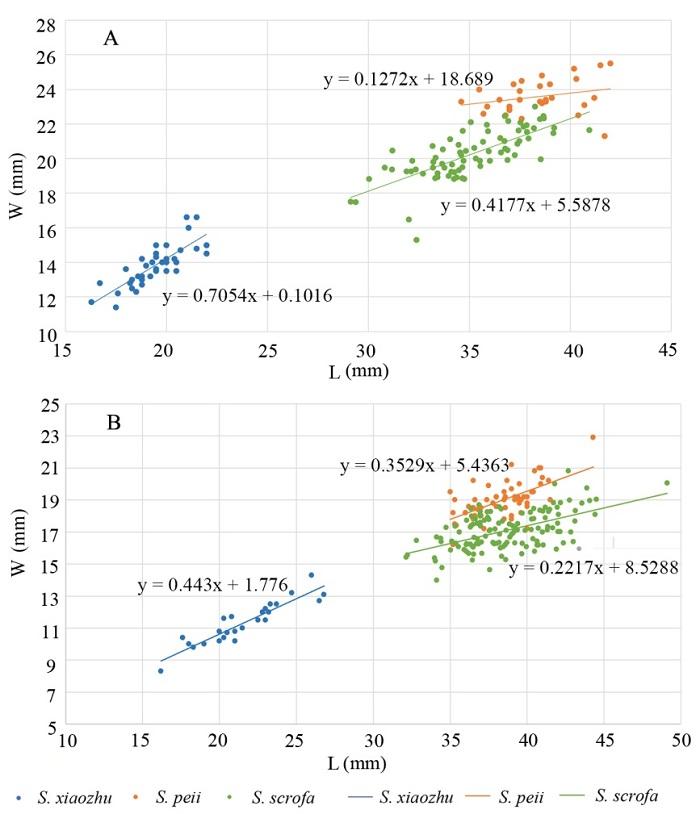
In this study, the data of Sus peii and S. xiaozhu from the Gigantopithecus Cave by Han (1987) is used for comparison. A scatter plot was drawn (Fig. 5), and a regression analysis with 95% confidence was performed to obtain R2 and P values. The data of length and width were studied for coefficients of variation.Sus xiaozhu was named by Han et al. (1975) based on the small pig fossils from Bijiashan, of Liuzhou, Guangxi. It is very small, with short upper and lower dentitions, a small M3 talon and a m3 talonid with one cuspid. The M3 of S. xiaozhu is extremely small so that it can be clearly distinguished from the pig fossils of YJW Cave 2 and S. peii only in terms of measurement data. The M3 distribution of S. peii and the suid specimens from YJW Cave 2 slightly overlap; the latter is generally smaller in width than the S. peii, with the length variation range greater than that of S. peii (Fig. 5B). It is worth mentioning that the M3 length-width regression analysis of S. peii failed the P value test, which indicates that its regression equation is invalid, and the variation is great. The P value of the other two is less than 0.05. The R2 value of S. xiaozhu is the biggest which indicates that the variability of S. xiaozhu is small, when the variability of the other two is relatively large (Table 1). The m3 of S. xiaozhu is also extremely small so that it can be distinguished from the S. peii and the pig fossils from YJW Cave 2 in size. The overlap between S. peii and the m3 fossils from YJW Cave 2 is larger. But in general, the data distribution of S. peii is still slightly wider with the variation range of the length smaller than that of the specimens of YJW Cave 2 (Fig. 5B). The P value of the regression equation of the m3 of all three species is less than 0.05, while the R2 value of S. xiaozhu is still greater than the other two, indicating that its variability is smaller (Table 1).
Fig. 5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 5Scatter plots of length versus width of the third molars of Sus xiaozhu and S. peii from Liucheng Gigantopithecus Cave and S. scrofa from Yangjiawan Cave 2
A. M3; B. m3. The data of S. xiaozhu and S. peii are from Han, 1975
The earliest record of S. scrofa in southern China is the Yanjinggou area. Colbert and Hooijer (1953) provided some length measurement data of the teeth in this article. The range of the M3 is 33.5-41.5 mm. The range of the length of M3 of S. peii given by Han is 34.6-42.0 mm. The length of the M3 of YJW Cave 2 is 29.1-41.0 mm. The variation range of the m3 from Yanjinggou is 39.0-41.0 mm. The length of the m3 of S. peii is 35.0-44.3 mm, and the length of the m3 unearthed in YJW Cave 2 is 32.1-49.1 mm. The data of S. scrofa from Yanjinggou is closer to that of YJW Cave 2. Albarella et al. (2015), when studying Eurasian S. scrofa, once calculated the average length of the m3 and the first lobe width of S. scrofa in South and Southeast Asia. The length is 37.1 mm and the width is 17.1 mm. The average length and width of the S. peii unearthed in the Gigantopithecus Cave were 38.5 mm and 19 mm, respectively. The average m3 length and the first lobe width of the suid fossils from YJW Cave 2 were 38.7 mm and 17.1 mm. On average, the suid fossils from YJW Cave 2 are closer to S. scrofa.
In this study, the data of the length and width of the three species was used to calculate the coefficient of variation. It is found that the coefficient of variation of the M3 of the S. xiaozhu and the suid fossils in Yangjiawan is similar, which are both bigger than the data of S. peii in terms of length or width. The variability of S. peii is smaller, which is different from the variability of the length-width combination. The variability of the m3 of S. xiaozhu is large, indicating that the length or width of the m3 of S. xiaozhu is volatile. The overall variability of suid fossils in YJW Cave 2 is larger than that of S. peii.
Among the molars of S. scrofa from YJW Cave 2, the M2s (Fig. 6D) and m3s (Fig. 6B) are among the most variable teeth in dimensions (Table 1).
As regard to the relatively smaller fossil species S. liuchengensis, it has simpler and smaller teeth, and its M3 with reduced talon and m3 with no more than three lobes (Han, 1987). Concerning the species S. australis, it was regarded as a synonym of S. peii by Chen (2004). Moreover, the S. scrofa from YJW Cave 2 has the typical scrofic type of male lower canine, which is different from those of the Early Pleistocene Sus species of southern China. The fossil species S. lydekkeri is a boreal taxon which mainly appeared in northern China during the Early to Middle Pleistocene, which can be distinguished from S. scrofa mainly by its relatively larger size and atypical scrofic male lower canine (Young, 1932), i.e. intermediate between the scrofic and verrucosic conditions (Fujita et al., 2000; Chen, 2004; Dong, 2008); therefore, it was treated as a subspecies of S. scrofa by recent authors (van der Made, personal communication; Fujita et al., 2000). It worth mentioning that the skull material of S. lydekkeri from Nihewan Basin is likely a young adult male rather than a female as originally identified by Liu et al. (2017) , but its relatively narrower posterior face is an exception, which falls into the range of the intermediate type and agrees well with its species features.
Through morphological comparison and data comparison, this study concludes that the fossil of suid teeth unearthed in YJW Cave 2 should belong to S. scrofa. Although suid fossils are very common in the Pleistocene mammalian fauna in southern China, they are far less diversified compared with their counterparts in Southeast Asia at the species level (Hardjasasmita, 1987; Frantz et al, 2016), and the majority species of Sus in Southeast Asia show a verrucosic type of cross section in the lower canine of the male (Hardjasasmita, 1987), which is a crucial feature to separate them from those of southern China; the verrucosic type of male lower canine has never been reported for the Chinese Pleistocene Sus species. Furthermore, compared with the Early Pleistocene suid fauna of southern China, the Middle-Late Pleistocene ones are much less diversified.
Cherin et al. (2018) proposed that S. scrofa and the Early-Middle Pleistocene S. lydekkeri are the species of Sus with the most numerous plesiomorphic characters, which were followed by the monophyletic group of suines with verrucosic lower canines, including the Pliocene S. arvernensis, S. strozzii, and the verrucosic pigs from insular Southeast Asia; on the other hand, they took the verrucosic canine as a plesiomorphic character, which agrees well with the proposal by Groves (1981) that scrofic canine is a derived character. Furthermore, some authors (Groves, 1981; Groves and Grubb, 1993) divided the living species of the genus Sus into two groups based on the lower canine morphology: the “scrofic group” includes S. scrofa only, and the “verrucosic group” consists of the other species. Cherin et al. (2018) even proposed that the position of S. strozzii in their phylogenetic tree is the first cladistic evidence of the affinity between the European fossil species and the far separated insular Southeast Asian verrucosic pigs. The present authors suggest that the phylogenetic significance of the lower canine should be urgently re-evaluated, and other teeth should also be taken into equal considerations as some authors already proposed that the molar shape in Eurasian wild boar populations is biogeographically structured into clearly defined Western and Eastern clusters (Evin et al., 2015).
Concerning the living form of the wild boar in China, its classification is still under debate; the traditional scenario is to put all of the living wild pig into the species S. scrofa, but different subspecies were designated for different regions; in Central China, the wild pig used to be named as S. scrofa chirodonta (Heude, 1888) or S. scrofa moupinensis (Milne-Edwards, 1871) (Allen, 1940; Groves,1981), both of which are currently resumed to species level, i.e. S. chirodontusHeude, 1888 and S. moupinensis Milne-Edwards, 1871 by recent authors (Keuling et al., 2017).
4 Study on intraspecific variation of Sus scrofa
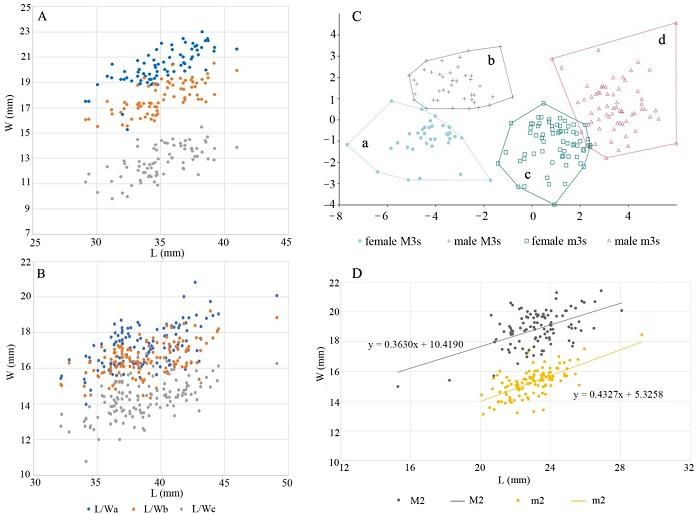
The suid fossils from YJW Cave 2 have been identified as S. scrofa mainly based on the characters of the canine teeth, which excludes the possibility of the co-existence of another suid species. Whereas significant attention should be paid to the great variation of the third molars in both size and form (Fig. 6).Fig. 6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 6Scatter plots of length versus width and linear discriminant analysis (LDA) of selected teeth of Sus scrofa from Yangjiawan Cave 2
A, B. total length versus lobe’s width of M3 (A) and m3 (B); C. linear discriminant analysis of the third molars: the data points of M3 separate into two distinct clusters, which probably represent females (a) and males (b) respectively, the dots of m3 also form two distinct groups, which probably represent females (c) and males (d) respectively; D. total length versus width of M2 and m2 L/Wa. total length versus first lobe’s width; L/Wb. total length versus second lobe’s width; L/Wc. total length versus third lobe’s width
In this study, the length (L) and widths of the first lobe (Wa), the second lobe (Wb) and the third lobe (Wc) of the third upper and lower molars of S. scrofa from YJW Cave 2 were analyzed by scatter diagram. The length of the M3 is between 29.1-41.0 mm, while the widths are fairly variable. As we can see from Fig. 6, the data points show a trend of being divided into two groups, especially in the second lobe of M3 (Fig. 6A). In this case, we believe that it may represent sexual dimorphism. Provisional groups were obtained based on the separation (Fig. 6A, B), a further verification was conducted by using PAST 3.26 (Hammer et al., 2001) to perform linear discriminant analysis (LDA) on the length versus the widths of the first, second and third lobes of the third molars of S. scrofa respectively. Linear discriminant analysis is an effective method for feature extraction. It can bring objects of the same category together and separate objects of different categories as much as possible. By projecting the data into different classifications, four groups were obtained. The data points of the M3 (Fig. 6C: a, b) and m3 (Fig. 6C: c, d) were divided into two groups respectively, indicating that the same anatomical object can indeed be divided into two groups. It’s reasonable to think that it was resulted from the gender difference. Therefore, it’s possible to use only the length versus the widths of the first, second and third lobes of the third molars to finish the analysis of the sexual dimorphism of S. scrofa (Fig. 6C).
5 Conclusion
The Late Pleistocene suid fossils in YJW Cave 2 are significantly larger in size than S. xiaozhu, while overlapping with S. peii to a certain extent and being the closest to S. scrofa. In terms of morphological characteristics, the crown folds of the cheek teeth are more complicated than that of S. peii. In terms of crown measurement, it is narrower than S. peii and is close to S. scrofa. Moreover, the male lower canines of the fossil suid from YJW Cave 2 demonstrate a typical scrofic type. Therefore, the suid fossils from YJW Cave 2 should be classified as S. scrofa. The large number of S. scrofa teeth unearthed in YJW Cave 2 provided materials for studying intraspecific variation. After the study of canine teeth and 209 upper and lower third molars, the results display an obvious sexual dimorphism.Acknowledgements
We would like to thank the following institutions and people for their helps: The Cultural Relics Bureau of Jiangxi; Bureau of Culture, Radio, Television and Press and Publication of Pingxiang; Bureau of Culture, Radio, Television and Press and Publication of Shangli County for strong supports to the field survey and excavations; Mr. Yang Zhangqian and his family for providing fossil clues and various conveniences; Mr. Yang Jingbo and his family for providing convenient living conditions; Dr. Wang Qiang for the pre-contact work; Dr. Zhao Keliang, Dr. Ge Junyi, and Mr. Yang Qingjiang for participating partial field work and the sampling; Liu Yuchun, Li Yan, Xiang Fei, Peng Wei, Li Wentao, Zhou Yunsong, Liu Guijun and Luo Na et al. from Pingxiang Museum for digging; Cui Xindong for providing assistance in data analysis; Cai Jiachen for photo processing; Prof. Van der Made J. for sharing bibliography and fruitful discussions, and Prof. Dong Wei and Dr. Hou Sukuan for critical reviews of the manuscript. This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDB26000000) and the National Natural Science Foundation of China (41572003) as well as the Special Fund for Fossil Excavation and Preparation, CAS.参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
DOIURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 1]

We measured crania and mandibles of Ryuku wild pigs from Iriomote Island. Sex and age were determined by observation of lower teeth. From the present data, the growth pattern was established for some items. For several parts of the cranium and mandible, the relative growth coefficient was compared. The results obtained here are summarized as follows: (1) Although sexual dimorphism was already shown in the younger group, a significant difference in profile length and length from the angle was not seen in the adult group. (2) As the animal grew, the proportion of length to width became larger in the skull. (3) The visceral cranium grew more rapidly in length than the cerebral cranium. On the other hand, growth of the cerebral cranium contributed to width in cranial development. (4) The body of the mandible was shown to grow more rapidly in length than the ramus of the mandible. (5) The growth pattern of some items was related to that of their associated muscles. These basic data are expected to be compared with data from other population of the same subspecies and Japanese wild pig. The comparisons will contribute to the establishment of origin and phylogeny of this animal.
DOIURLPMID [本文引用: 1]

BACKGROUND: The mechanisms that underlie the diversification of tropical animals remain poorly understood, but new approaches that combine geo-spatial modeling with spatially explicit genetic data are providing fresh insights on this topic. Data about the diversification of tropical mammals remain particularly sparse, and vanishingly few opportunities exist to study endangered large mammals that increasingly exist only in isolated pockets. The chimpanzees of Cameroon represent a unique opportunity to examine the mechanisms that promote genetic differentiation in tropical mammals because the region is home to two chimpanzee subspecies: Pan troglodytes ellioti and P. t. trogolodytes. Their ranges converge in central Cameroon, which is a geographically, climatically and environmentally complex region that presents an unparalleled opportunity to examine the roles of rivers and/or environmental variation in influencing the evolution of chimpanzee populations. RESULTS: We analyzed microsatellite genotypes and mtDNA HVRI sequencing data from wild chimpanzees sampled at a fine geographic scale across Cameroon and eastern Nigeria using a spatially explicit approach based upon Generalized Dissimilarity Modeling. Both the Sanaga River and environmental variation were found to contribute to driving separation of the subspecies. The importance of environmental variation differed among subspecies. Gene-environment associations were weak in P. t. troglodytes, whereas environmental variation was found to play a much larger role in shaping patterns of genetic differentiation in P. t. ellioti. CONCLUSIONS: We found that both the Sanaga River and environmental variation likely play a role in shaping patterns of chimpanzee genetic diversity. Future studies using single nucleotide polymorphism (SNP) data are necessary to further understand how rivers and environmental variation contribute to shaping patterns of genetic variation in chimpanzees.
[本文引用: 1]
DOIURLPMID [本文引用: 2]

The Suidae are a family of Cetartiodactyla composed of 17 species classified in a minimum of five extant genera that originated at least 20 million years ago. Their success is evident in the multitude of habitats in which they are found as both natural and feral populations in tropical Island Southeast Asia, the high plateau of the Himalayas, Siberia, North Africa, the Pacific Islands, Australia, and the Americas. Morphological and molecular analyses of these species have revealed numerous aspects of their biology, including the ease with which many lineages have and continue to hybridize. This trait has made them an ideal model for evolutionary biologists. Suid species have also shared a deep history with humans, from their association with early hominids in Africa to their domestication. Here we review the current knowledge of this fascinating group and provide a comprehensive evolutionary history from the Oligocene to the present day.
[本文引用: 9]
[本文引用: 1]
[本文引用: 10]
[本文引用: 2]
[本文引用: 1]
[本文引用: 7]
[本文引用: 1]
[本文引用: 4]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]

Sus scrofa holds the largest share of mammalian fossils. Primates and carnivores are also rich. Rodents and perissodactylas are rare. Proboscideans include Stegodon orientalis and Elephas maximus. Insectivores and chiropterans are absent. There are some extinct species, such as Ailuropoda baconi, Stegodon orientalis and Megatapirus augustus. In faunal composition, Yangjiawan Cave 2 belongs to Late Pleistocene Ailuropoda-Stegodon fauna, which is similar to the Yangjiawan Cave 1 and Fuyan Cave. However, the number of species of Yangjiawan Cave 2 is less than the latter two, especially on the micromammals. In addition, the dental measurements of Ailuropoda baconi and the presence of Elephas maximus also indicate that the time of Yangjiawan Cave 2 is similar to Yangjiawan Cave 1 and Fuyan Cave. The presence of Elephas maximus, Megatapirus augustus and Cervus(Rusa)unicolor in Yangjiawan Cave 2 fauna implies that the climate in Jiangxi were warm and humid in the Late Pleistocene.]]>
DOIURL [本文引用: 1]
[本文引用: 1]
