A Middle Triassic kyphosichthyiform from Yunnan, China, and phylogenetic reassessment of early ginglymodians
XU Guang-Hui1,2,*, MA Xin-Ying1,2,3, WU Fei-Xiang1,2, REN Yi1,2,3通讯作者: xuguanghui@ivpp.ac.cn
收稿日期:2019-01-28网络出版日期:2019-07-20
| 基金资助: |
Corresponding authors: xuguanghui@ivpp.ac.cn
Received:2019-01-28Online:2019-07-20

摘要
铰齿鱼类是全骨鱼类中的一支,包括现生的雀鳝及其关系密切的化石类型。产自云贵地区中三叠世安尼期(~244 Ma)地层的拱鱼目(Kyphosichthyiformes)鱼类代表了铰齿鱼类最早的化石记录。根据最近在云南罗平关岭组二段发现的4块保存完好的鱼化石,命名了拱鱼目一个新属种,优美玉带鱼(Yudaiichthys eximius gen. et sp. nov.)。新的发现为重新研究拱鱼目和其他早期铰齿鱼类的系统发育关系提供了契机。分支分析结果表明,过去定义的拱鱼科(Kyphosichthyidae)和圣乔治鱼属(Sangiorgioichthys)都是并系类群。重新厘定后的拱鱼目分为拱鱼科和腊山鱼科(Lashanichthyidae fam. nov.); 其中,拱鱼科包括拱鱼属(Kyphosichthys)和富源鱼属(Fuyuanichthys); 苏氏圣乔治鱼(Sangiorgioichthys sui)和羊圈圣乔治鱼(S. yangjuanensis)归入新建的腊山鱼属(Lashanichthys gen. nov.), 和玉带鱼属一起组成腊山鱼科。圣乔治鱼属被移出拱鱼目,限定于产自圣乔治山地区中三叠世拉丁期地层的两个种(Sangiorgioichthys aldae和S. valmarensis); 该属和更进步的铰齿鱼类(半椎鱼目和雀鳝目)构成姐妹群关系。修订后的分支图为了解铰齿鱼类解剖特征的早期演化历史提供了新的见解。
关键词:
Abstract
Ginglymodi are a subgroup of holostean fishes, including living gars and their closely-related fossil taxa. The early Middle Triassic (Anisian, ~244 Ma) kyphosichthyiforms from Yunnan and Guizhou, China represent the earliest records of this clade. Here, we report the discovery of a new kyphosichthyiform fish, Yudaiichthys eximius gen. et sp. nov., on the basis of four well-preserved specimens from the second (upper) member of Guanling Formation in Luoping, eastern Yunnan. The new discovery stimulated a phylogenetic analysis to reassess the interrelationships of the Kyphosichthyiformes and their relationships with other early ginglymodians. Results of our analysis indicate that the previously defined family Kyphosichthyidae and the genus Sangiorgioichthys are paraphyletic. A revised Kyphosichthyiformes is proposed here, and it is divided into two families, Kyphosichthyidae and Lashanichthyidae fam. nov. The family Kyphosichthyidae is restricted to include two genera Kyphosichthys and Fuyuanichthys. The Chinese “Sangiorgioichthys” species are removed into a new genus Lashanichthys, which is recovered as a taxon sister to Yudaiichthys gen. nov., and both genera are grouped into the new family, Lashanichthyidae. Sangiorgioichthys is restricted to include two species (S. aldae and S. valmarensis) from the late Middle Triassic (Ladinian) of the Monte San Giorgio area. The genus is removed out of the Kyphosichthyiformes and is recovered as the sister taxon of the Semionotiformes-Lepisosteiformes clade. The revised topology provides new insights into the anatomical evolution during the earliest ginglymodian history.
Keywords:
PDF (21000KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
徐光辉, 马昕莹, 吴飞翔, 任艺. 云南中三叠世拱鱼目一新属种及早期铰齿鱼类系统发育关系再评估. 古脊椎动物学报[J], 2019, 57(3): 181-204 DOI:10.19615/j.cnki.1000-3118.190319
XU Guang-Hui, MA Xin-Ying, WU Fei-Xiang, REN Yi.
1 Introduction
Ginglymodi are a clade of holostean fishes, including living gars and their closely-related fossil taxa (Regan, 1923; Patterson, 1973, 1982; Olsen and McCune, 1991; Cavin and Suteethorn, 2006; Grande, 2010; Cavin, 2010; López-Arbarello, 2012; Near et al., 2012). Living gars (seven lepisosteid species) are confined to the freshwater environments of North and Central America and Cuba, although some taxa (e.g., Atractosteus spatula) occasionally venture into brackish and marine environments (Grande, 2010). Fossil representatives of this clade are taxonomically rich, including the Obaichthyidae, Lepidotidae, Semionotidae, Callipurbeckiidae, Macrosemiidae and Kyphosichthyidae; they are widely known from both freshwater and marine deposits in almost all continents except Australia and Antarctica, with the earliest record dating back to the early Middle Triassic (Anisian) of Southwest China (Schaeffer and Dunkle, 1950; Bartram, 1977; Schultze and M?ller, 1986; Olsen and McCune, 1991; Maisey, 1991; Wenz, 1999; Bürgin, 2004; Tintori and Lombardo, 2007; Lombardo and Tintori, 2008; Grande, 2010; López-Arbarello et al., 2011, 2016; Xu and Wu, 2012; Schr?der et al., 2012; Cavin et al., 2013, 2018; Gibson, 2013a,b; Chen et al., 2014; Deesri et al., 2014, 2016; Brito et al., 2017; Sun and Ni, 2018; Xu et al., 2018a).The phylogenetic interrelationships of Ginglymodi are controversial and accordingly the systematic classification of this clade remains unstable. Grande (2010) divided Ginglymodi into three orders, Macrosemiiformes, Semionotiformes and Lepisosteiformes, with the latter two together constituting the sister group of the former. Cavin (2010), Cavin et al. (2013) and Deesri et al. (2014, 2016) largely followed Grande’s (2010) classification of Ginglymodi but argued that Semionotiformes might be paraphyletic. López-Arbarello (2012) and López-Arbarello and Wencker (2016), followed by Sun and Ni (2018), Cavin et al. (2018), and Xu et al. (2018a), recovered Macrosemiidae as members of Semionotiformes, along with the Semionotidae and Callipurbeckiidae, and gave up the usage of Macrosemiiformes as a ginglymodian order. Recently, Sun and Ni (2018) named the order Kyphosichthyiformes and placed it at the base of Ginglymodi. However, López-Arbarello and Sferco (2018) challenged the monophyly of Kyphosichthyiformes, and recovered Ticinolepis (López-Arbarello et al., 2016) as the most basal ginglymodian. When described Fuyuanichthys (a sister taxon of Kyphosichthyis), Xu et al. (2018a) also noticed that Sun and Ni’s (2018) Kyphosichthyiformes was poorly defined and urgently needed a further revision.
Here, we report the discovery of a new ginglymodian fish based on four specimens from the second (upper) member of Guanling Formation in Luoping, eastern Yunnan, China. They are nearly complete and well-preserved in thinly laminated micritic limestone, permitting a detailed description of the morphology of the taxon. Other macrofossils from the same fossiliferous horizons include plants, invertebrates, marine reptiles, two coelacanths (Wen et al., 2013), a stem actinopteran (Xu et al., 2014a), five saurichthyids (Wu et al., 2009, 2011, 2018), and many other neopterygians (Tintori et al., 2007, 2010; Sun et al., 2009, 2012, 2015, 2016; López-Arbarello et al., 2011; Lin et al., 2011; Lombardo et al., 2011; Xu and Wu, 2012; Wen et al., 2012, 2018; Tan and Jin, 2013; Xu et al., 2014b; Xu and Zhao, 2016; Xu and Ma, 2016; Ma and Xu, 2017; Sun and Ni, 2018). The whole fossil assemblage was named the Luoping biota (Zhang et al., 2009; Hu et al., 2011; Benton et al., 2013). The age of this biota (Pelsonian, Anisian, ~244 Ma) is well constrained by conodont studies (Zhang et al., 2009). The new discovery stimulated a phylogenetic analysis to reassess the phylogenetic relationships of early ginglymodian clades.
2 Material and methods
All specimens are curated at the fossil collections of the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Chinese Academy of Sciences in Beijing, China. They were mechanically prepared with sharp steel needles. For better contrast, the specimens were dusted with ammonium chloride (NH4Cl) when photographed. Illustrations were drawn under an Olympus SZX7 microscope with a camera lucida attachment. The relative position of fins and scale counts were expressed following Westoll (1944). The traditional actinopterygian nomenclature of Grande and Bemis (1998) and Grande (2010) are generally followed, for ease of comparison with most existing literature. The segmented and unbranched rays anterior to the principal ray in the ventral lobe of the caudal fin are termed as rudimentary rays, following the nomenclature used in other contributions (e.g., Alvarado-Ortega and Espinosa-Arrubarrena, 2008; López-Arbarello et al., 2011; Xu et al., 2018a, b), although they are also termed as procurrent rays by some authors (e.g., Arratia, 2013).To investigate the phylogenetic position of Yudaiichthys within Ginglymodi, we have incorporated it in a data matrix expanded from Xu et al. (2018a), with five additional Triassic ginglymodian species (Sangiorgioichthys aldae, S. valmarensis, “S”. sui, “S”. yangjuanensis, and Lophionotus chinleana). The genus Robustichthys from the Anisian Luoping biota was originally referred to the halecomorph order Ionoscopiformes (Xu et al., 2014b) but was recently recovered by Sun and Ni (2018) as a sister taxon to Ginglymodi. If Sun and Ni’s (2018) hypothesis is accepted, Robustichthys would be vital for understanding the origin of Ginglymodi. To test the possible relationships of Robustichthys with Ginglymodi, we also incorporated the genus in this analysis. Luoxiongichthys was not included because it is currently being restudied based on new material. The current data matrix includes 205 characters coded across 44 actinopteran taxa with Pteronisculus stensi?i selected for out-group comparison (electronic supplementary material). All characters were unordered and equally weighted. Tree searches were accomplished by the heuristic search algorithm (gaps treated as missing data; 10 000 random addition sequence replicates; 10 trees held at each iteration; and tree bisection-reconnection branch-swapping) in PAUP* 4.0b10 (Swofford, 2003).
3 Systematic paleontology
Neopterygii Regan, 1923Holostei Müller, 1844 (sensu Grande, 2010)
Ginglymodi Cope, 1872 (sensu Grande, 2010)
Kyphosichthyiformes Sun & Ni, 2018 (new usage)
Diagnosis A stem group of ginglymodians distinguished from other members of this clade by the following combination of features: frontal 1.5-2.0 times as long as parietal; single pair of extrascapulars separated by posterior portions of parietals (independently derived in macrosemiids); dermopterotic shorter than parietal (independently derived in macrosemiids); presence of one or two anterior infraorbitals (vs. single in Sangiorgioichthys, two in Ticinolepis and Semionotus, and three or more in most other ginglymodians); two to six suborbitals; maxilla ending below orbit (as in Ticinolepis but more anteriorly located in other ginglymodians); absence of preopercle/dermopterotic contact (reversal in Lashanichthys gen. nov.); presence of posttemporal/parietal contact (independently derived in macrosemiids); presence of median gular (vs. absent in other ginglymodians); presence of single complete row of elongated scales adjacent to ventral border of body lobe in caudal region (independently derived in several other neopterygians, e.g., Sangiorgioichthys and Pholidoctenus).
Content Kyphosichthyidae and Lashanichthyidae fam. nov.
Geographical distribution and age Luoping and Fuyuan, Yunnan, and Panxian and Xingyi, Guizhou; Pelsonian (Anisian)-Ladinian, Middle Triassic.
Remarks Sun and Ni (2018) named the family Kyphosichthyidae (and order Kyphosichthyiformes) which originally included three genera: Kyphosichthys, Sangiorgioichthys and Luoxiongichthys. However, the monophyly of this group has been challenged by subsequent studies (López-Arbarello and Sferco, 2018; Xu et al., 2018a). Our current studies confirm that Kyphosichthys is more closely related to Fuyuanichthys than to other ginglymodians (Xu et al., 2018a); both genera are placed in the Kyphosichthyidae sensu stricto here. Additionally, our analysis resolved the previously defined Sangiorgioichthys (López-Arbarello et al., 2011; Chen et al., 2014) as paraphyletic; this genus is restricted to include two species, S. aldae (Tintori and Lombardo, 2007) and S. valmarensis (Lombardo et al., 2012) from the Ladinian (late Middle Triassic) of the Monte San Giorgio area and is removed out of the Kyphosichthyiformes. The “Sangiorgioichthys” species from the Anisian (early Middle Triassic) of Yunnan and Guizhou, closely related to the new ginglymodian described herein, are removed into a new genus and family within the Kyphosichthyiformes (see Discussions below). In addition, our studies indicate that Luoxiongichthys lacks the diagnostic features of the Kyphosichthyiformes revised here and should be removed out of this order.
Kyphosichthyidae Sun & Ni, 2018 (new usage)
Emended diagnosis Body depth 42%-68% of standard length; preorbital portion nearly equal to or slightly longer than orbit in length; presence of two anterior infraorbitals, 1.5-2.0 times deeper than long; single infraorbital between lacrimal and posteroventral infraorbital; two or three suborbitals; mandibular length 42%-44% of head length; quadrate almost fully covered by infraorbital; and six to seven pairs of branchiostegal rays.
Content Kyphosichthys and Fuyuanichthys.
Geographical distribution and age Luoping and Fuyuan, Yunnan, and Xingyi, Guizhou; Pelsonian (Anisian)-Ladinian, Middle Triassic.
Lashanichthyidae fam. nov.
Diagnosis Preorbital portion less than orbit in length; presence of single anterior infraorbital, deep and narrow; two to four infraorbitals between lacrimal and posteroventral infraorbital; three to six suborbitals; quadrate almost fully covered by suborbital; mandibular length nearly half of head length; interopercle nearly trapezoidal (vs. triangular in other ginglymodians); and eight or nine pairs of branchiostegal rays.
Content Lashanichthys gen. nov. and Yudaiichthys gen. nov.
Type genus Lashanichthys gen. nov.
Geographical distribution and age Luoping, Yunnan and Panxian, Guizhou; Pelsonian, Anisian, Middle Triassic.
Lashanichthys gen. nov.
Etymology The generic epithet ‘Lashan’ refers to the Lashan sub-distinct and Baila hills in Luoping County, Yunnan, and the Greek suffix ‘-ichthys’ means fish.
Type species Lashanichthys sui (López-Arbarello et al., 2011).
Other species L. yangjuanensis (Chen et al., 2014).
Geographical distribution and age Luoping, Yunnan and Panxian, Guizhou; Pelsonian, Anisian, Middle Triassic.
Diagnosis Nasal narrow and curved; frontal 1.8 times as long as parietal; presence of two to four supraorbitals; seven or eight infraorbitals, including two or three between lacrimal and posteroventral infraorbital; three or four suborbitals, separated by posteroventral infraorbital into two or three dorsal ones and single ventral one; presence of preopercle/dermopterotic contact; supramaxilla nearly half of length of maxilla (excluding anteromedial process); opercle 2.2-3.0 times as deep as subopercle (excluding anterodorsal process); median gular nearly circular, 0.4 times as long as lower jaw; 19-21 principal caudal rays.
Yudaiichthys gen. nov.
Etymology The generic epithet ‘Yudai’ refers to the Yudai Lake in Luoping County, Yunnan.
Type species Yudaiichthys eximius gen. et sp. nov.
Diagnosis Same as for the type and only known species.
Yudaiichthys eximius gen. et sp. nov.
(Figs. 1-7)
Fig. 1
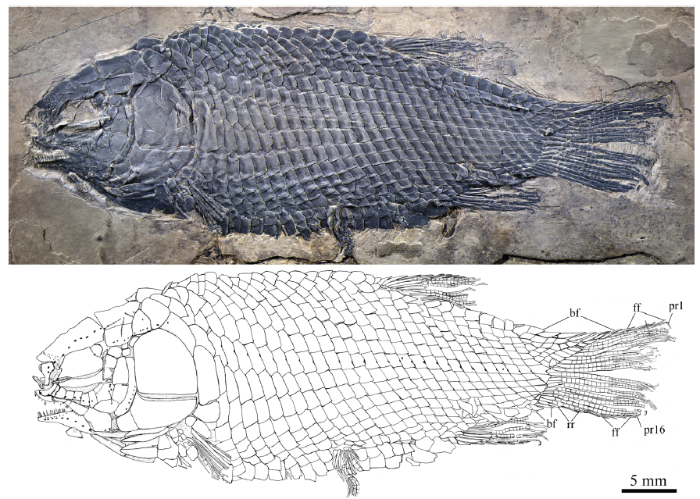 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 1Holotype of Yudaiichthys eximius gen. et sp. nov., IVPP V 20431
Abbreviations: bf. basal fulcrum; ff. fringing fulcrum; pr. principal fin ray; rr. rudimentary ray
Fig. 2
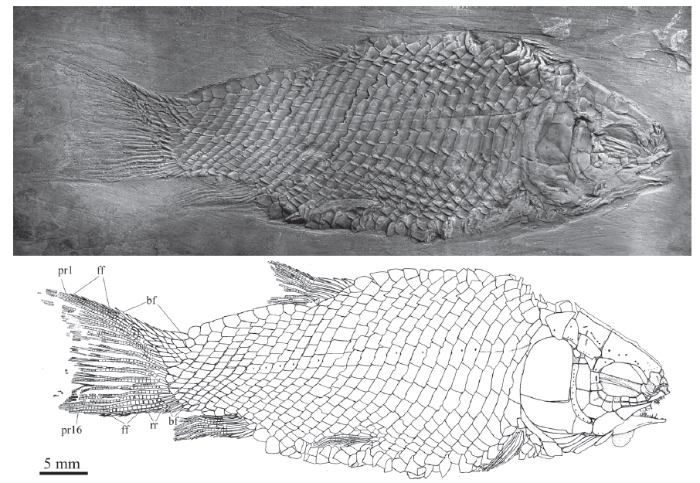 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 2Yudaiichthys eximius gen. et sp. nov., IVPP V 20432
For abbreviations see
Fig. 3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 3Yudaiichthys eximius gen. et sp. nov.
A. IVPP V 20434; B. IVPP V 20433
Fig. 4
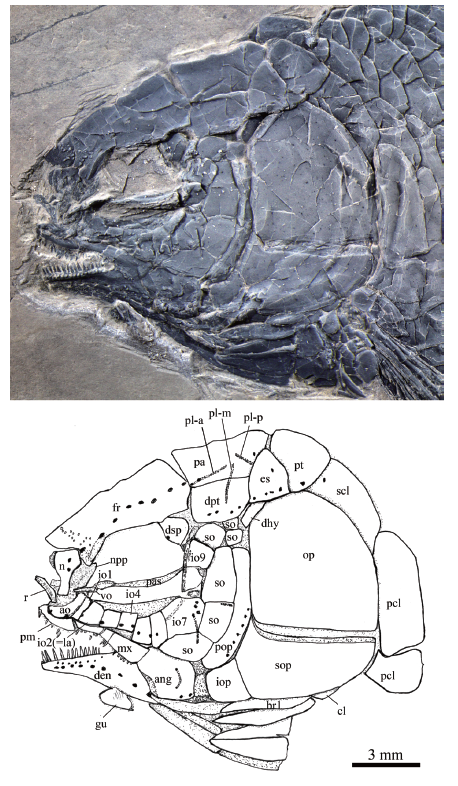 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 4Skull and pectoral girdle of Yudaiichthys eximius gen. et sp. nov., IVPP V 20431
Abbreviations: ang. angular; ao. antorbital; br. branchiostegal rays; cl. cleithrum; den. dentary;dhy. dermohyal; dpt. dermopterotic; dsp. dermosphenotic; es. extrascapular; fr. frontal; gu. gular;io. infraorbital; iop. interopercle; la. lacrimal; mx. maxilla; n. nasal; npp. nasal process of premaxilla;op. opercle; pa. parietal; pas. parasphenoid; pcl. postcleithrum; pl-a. anterior pit-line; pl-m. middle pit-line; pl-p. posterior pit-line; pm. premaxilla; pop. preopercle; pt. posttemporal; qj. quadratojugal; r. rostral;scl. supracleithrum; so. suborbital; sop. subopercle; su. supraorbital; vo. Vomer
Fig. 5
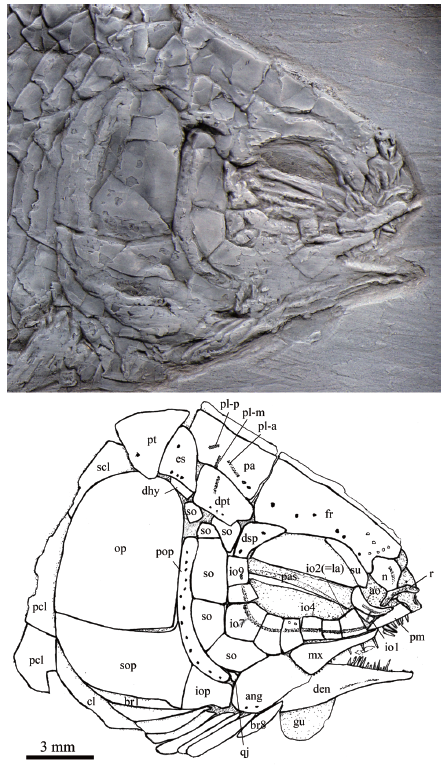 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 5Skull and pectoral girdle of Yudaiichthys eximius gen. et sp. nov., IVPP V 20432
For abbreviations see
Fig. 6
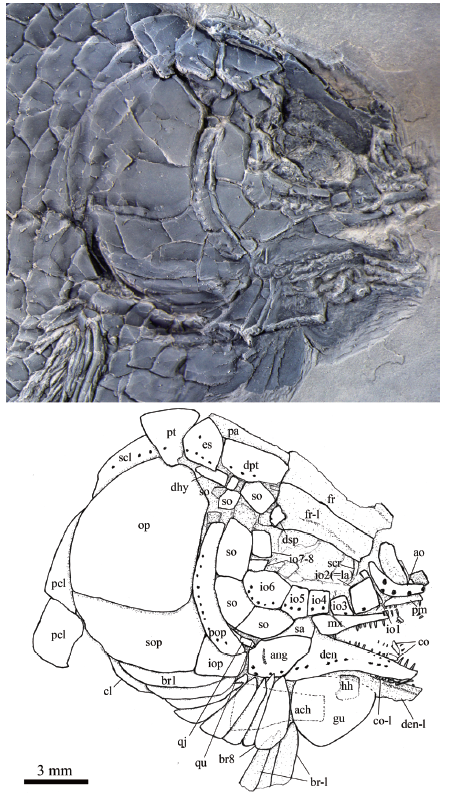 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 6Skull and pectoral girdle of Yudaiichthys eximius gen. et sp. nov., IVPP V 20434
Abbreviations: ach. anterior ceratohyal; co. coronoid; hh. hypohyal; qu. quadrate; sa. supra-angular;scr. sclerotic ring. For other abbreviations see
Fig. 7
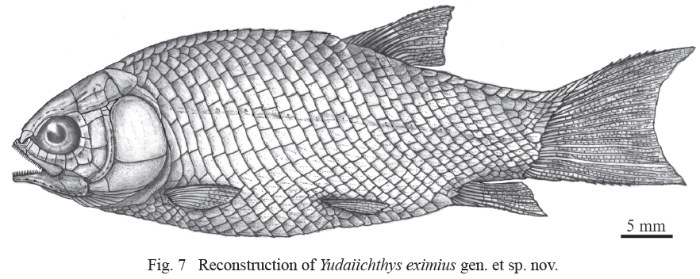 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 7Reconstruction of Yudaiichthys eximius gen. et sp. nov.
Etymology The specific epithet ‘eximius’ means graceful.
Holotype IVPP V 20431, a nearly complete, laterally compressed specimen.
Paratype IVPP V 20432-20434.
Locality and horizon Luoping, Yunnan, China; second (upper) member of Guanling Formation, Pelsonian, Anisian, Middle Triassic.
Diagnosis Nasal relatively broad and hourglass-shaped; frontal 1.5 times as long as parietal; single supraorbital, tapering at both ends; nine (eight, occasionally) infraorbitals, including four (three, occasionally) between lacrimal and posteroventral infraorbital; six suborbitals arranged in two vertical rows (four in anterior row and two in posterior one); presence of dermohyal; absence of preopercle/dermopterotic contact; opercle twice as deep as subopercle (excluding anterodorsal process); absence of supramaxilla; median gular pear-like, half of length of lower jaw; eight pairs of branchiostegal rays; eight dorsal rays; seven anal rays; 16 principal caudal rays; scales with weakly serrated posterior margin only in anterior flank region; and scale formula of D18/P8, A16-17, C25/T28.
4 Description
General morphology and size Yudaiichthys has a blunt snout, a fusiform body and a slightly forked caudal fin (Figs. 1-3). The dorsal fin inserts slightly posterior to the origins of pelvic fins. The greatest body depth lies midway between the posterior margin of the opercle and the origin of the dorsal fin. The holotype (Fig. 1) has a head length of 15 mm, a body depth of 19 mm, and a standard length (SL, the length from the tip of the snout to the posterior extremity of the caudal peduncle) of 47 mm. The largest known specimen (Fig. 3A) has a SL of 56 mm. The anterior portions of the frontals, anterior circumorbital bones and lower jaws are ornamented with tubercles, whereas the other cranial bones are largely smooth on their surfaces (Figs. 4-6). The general body form (Fig. 7) is reconstructed mainly based on the holotype (Fig. 1) and IVPP V 20432 (Fig. 2).Snout The canal-bearing bones of the snout consist of a median rostral and paired nasals and antorbitals. The rostral is a small curved bone that encloses the ethmoid sensory commissure and has a tube-like middle portion with a pair of short lateral extensions (Figs. 4, 5). The nasals are hourglass-shaped, more expanded posterodorsally than anteroventrally (Fig. 4). Each nasal has a nearly straight ventral margin, concave lateral and median margins and a convex dorsal margin. A short anterior portion of the supraorbital sensory canal extends into the nasal from the frontal, indicated by two pores on the posterior half portion of this bone. The antorbitals are L-shaped bones (Figs. 4-6). The horizontal branch of each antorbital extends forwards, contacting the rostral anteriorly, the nasal dorsally, the premaxilla medially, and the anterior infraorbital posteriorly. The dorsal branch of the antorbital extends posterodorsally and does not contact the lateral margin of the frontal (Fig. 5). A small gap exists between the antorbital and the supraorbital, and accordingly the circumorbital ring is not closed (Fig. 4). The antorbital is slightly displaced in V 20432 (Fig. 5), causing a false contact of this bone with the supraorbital. The conjunction of the ethmoid commissural canal and the infraorbital canal is located at the middle portion of the antorbital.
Skull roof The skull roofing bones include pairs of frontals, parietals, dermopterotic, and extrascapulars (Figs. 4-6). The frontals are roughly rectangular with a constriction above the orbit. The medial suture between frontals is slightly sigmoid (Figs. 4, 5). Each frontal is nearly one and half the length of the parietal, being the largest element on the skull roof. The posterior margin is nearly straight and the anterior margin is concave for the contact of the nasal. The lateral margin sutures the supraorbital anteriorly and the dermosphenotic posteriorly with a posterior-middle portion contributing to the orbital margin (Fig. 5). The course of the supraorbital canal is indicated by a longitudinal line of eight pores on each frontal (Fig. 4).
The parietals are nearly trapezoidal, contacting the frontal anteriorly, the dermopterotic anterolaterally, the extrascapular posterolaterally, and the posttemporal posteriorly (Figs. 4-6). The length is nearly twice its width. Three pit-lines are present (Figs. 4, 5). The anterior pit-line originates at the middle portion of the parietal, extends anteriorly, and ends near the posterior end of the supraorbital sensory canal. The middle pit-line originates slightly posterior to the posterior end of the anterior pit-line in the parietal, and ends near the temporal sensory canal in the dermopterotic. The posterior pit-line extends posterolaterally and ends near the posterior margin of the parietal.
The paired dermopterotic is trapezoidal, two-thirds as long as the parietal, to which it is sutured medially with a nearly straight margin. The temporal sensory canal runs longitudinally through the dermopterotic, indicated by a line of four or five pores parallel to the lateral margin of this bone (Fig. 4).
A single pair of extrascapulars is present. They are roughly triangular, tapering medially. The left is separated from the right by the posterior portions of the parietals with the supratemporal commissural canal running transversely through both the extrascapulars and parietals (Figs. 4, 5).
Circumorbital bones As shown in V 20432 (Fig. 5) and V 20433 (Fig. 3B), there is a single supraorbital flanking the anterior portion of the frontal. It is elongated and slightly curved, tapering at both ends.
Nine infraorbitals are commonly present (Figs. 4, 5); among them, the first (anteriormost) (= anterior infraorbital of Wenz, 1999) is narrow and deep, tapering dorsally. The second (= lacrimal) is relatively large and trapezoidal, forming the ventral half of the anterior orbital margin. The third and fourth are nearly square, and the fifth is trapezoidal, slightly deeper than long. The sixth is pentagonal, having a depth one and half its length. The seventh is also pentagonal, being the largest element of the infraorbital series. The last two (eighth and ninth) infraorbitals are relatively small and quadrangular. The infraorbital sensory canal passes longitudinally through the anterior three infraorbitals near their ventral margin, the fourth to sixth at their middle portions, and then extends upwards and runs dorsoventrally through the last three ones near their anterior margins. An intraspecific variation is discernable in V 20434, in which eight infraorbitals are present (Fig. 6). The fifth infraobital is relatively broad and pentagonal in this specimen, probably representing a fusion of fifth and sixth infraorbitals in other specimens (Figs. 4, 5).
The dermosphenotic is trapezoidal, contacting the dermopterotic and suborbital posteriorly, the frontal dorsally, and the last infraorbital ventrally. The dermosphenotic tapers anteriorly with a pointed anterior tip, lacking a contact with the supraorbital. Two or three pores are visible near the orbital margin of this bone (Fig. 5).
Sclerotic bones are partly discernible near the orbital rim of the holotype and V 20434 (Fig. 6). They are thin and curved and their number cannot be determined because of poor state of preservation.
Suborbitals and dermohyal There are six suborbitals, and their size and shape slightly vary in different specimens (Figs. 4-6). The anterior four suborbitals are relatively large, the upper three are rectangular or trapezoidal and the bottom is pentagonal. Two pit-lines are occasionally present, with a horizontal one parallel to the dorsal margin of the third suborbital and a vertical one in the upper portion of the bottom one (Fig. 4). Additionally, there are two suborbitals between the preopercle and dermopterotic (Figs. 4-6). They are small and either rectangular or trapezoidal. Posteriorly, there is an elongate, trapezoidal bone, which extends posterodorsally and contacts the dermopterotic and extrascapular anterodorsally and the opercle posteroventrally (Figs. 4, 5). This bone is unknown in other Triassic ginglymodians. It is labeled here as the dermohyal according to its position similar to that in living gars (Grande, 2010).
Jaws The upper jaw is composed of a premaxilla and a maxilla. A supramaxilla is absent. The paired premaxilla contacts its counterpart medially, having a long oral region that is half the total length of the maxilla. Each premaxilla has a deep, posterodorsally directed nasal process that is sutured to the frontal dorsally (Fig. 4). It is unknown if there is a foramen for the olfactory nerve in the nasal process of the premaxilla because of the overlapping of the nasal and antorbital. About ten large, conical teeth are present along the oral margin of the premaxilla.
The maxilla is elongate, having a peg-like, medially-directed anterior process (Figs. 4, 5). Excluding this process, the length of the maxilla is about three times its maximum depth. The posterior end of the maxilla is located below the middle portion of the orbit. The dorsal and posterior margins of the maxilla are nearly straight or slightly convex, and the oral margin is slightly concave. A row of 18 conical teeth are present along the full length of the oral margin of the maxilla. The anterior several teeth are nearly equal in length, and the posterior ones gradually reduce in length posteriorly.
The lower jaw is wedge-shaped and relatively long, being about half of the head length (Figs. 4-6). It bears a prominent coronoid process with the maximal height 32%-34% of its length. The dentary is proportionally long, accounting 3/4 the length of the lower jaw. It deepens posteriorly with a triangular posteroventral process flanking the anteroventral edge of the angular. 16 or 17 conical teeth are present along the oral margin of the anterior half of the dentary. They are nearly as large as those on the premaxilla. The angular is trapezoidal, forming most of the posterior portion of the lower jaw. The supra-angular is relatively small and sub-circular in lateral view, contacting the angular ventrally and the dentary anteriorly (Fig. 6). No independent retroarticular is discernable. The angular and dentary carry the mandibular canal forward from the preopercle, with a dorsoventrally directed pit-line (= Wenz’s (1967) posterior mandibular pit-line) in the posterior portion of the angular (Fig. 4, 6). Medially, only two coronoid bones are discernible (Fig. 6). Both are small and elongate, bearing small conical teeth at their oral margins.
Palatal elements and hyoid arch A possible left vomer is exposed in the holotype (Fig. 4). It is a small elongate bone that contacting the parasphenoid posteriorly. Two conical teeth are preserved on the oral margin of the vomer. They are about half the size of those in the premaxilla. Only the anterior part of the parasphenoid is discernable through the orbit (Figs. 4, 5). Teeth are absent on the ventral margin of this bone. The pterygoid elements are sutured to each other and it is difficult to identify the boundaries between them.
The quadratojugal is a small splint-like bone which tapers posterodorsally. It contacts the quadrate medially and rests on the anterior edge of the ventral portion of preopercle (Fig. 6). A small part of quadrate is discernable through the gap between the quadratojugal and the suborbital in V 20434, and its complete shape is still unknown.
The outlines of the hypohyal and anterior ceratohyal can be traced in V 20434 (Fig. 6), although both are overlapped by the gular and branchiostegal rays. The former is nearly square, and the latter is elongate and widens posteriorly, being nearly half the length of the lower jaw.
Opercular series The preopercle is roughly crescent-shaped, contacting two suborbitals and the quadratojugal anteriorly, a suborbital dorsally, and the rest of opercular bones posteriorly. Indicated by a vertical line of at least 13 pores near the posterior margin of the preopercle, the preopercular sensory canal extends dorsoventrally through this bone (Fig. 5). The opercle is nearly trapezoidal, 1.1-1.3 times as deep as its maximum length. The anterior margin is nearly straight, and the dorsal, ventral and posterior margins are convex. The subopercle is sickle-shaped, bearing a short triangular anterodorsal process that inserts between the preopercle and opercle. This process is one-fifth the depth of the opercle. Excluding this process, the subopercle is about half the depth of the opercle. The interopercle is relatively small and nearly trapezoidal, having slightly convex anterior and ventral margins, and nearly straight dorsal and posterior margins.
Branchiostegal rays and gular There are eight pairs of branchiostegal rays (Figs. 4-6). They are elongate and plate-like, gradually increasing in length and width posteriorly.
The median gular is broad and nearly half the length of the lower jaw. It is pear-shaped, tapering towards the anterior tip (Fig. 6).
Paired girdles and fins A posttemporal, a supracleithrum, a cleithrum and two postcleithra are present at each side of the pectoral girdle (Figs. 4, 5). The posttemporal is sub-triangular with its median tip nearly reaching the midline. The lateral line pierces the anterolateral portion of the posttemporal and extends posteroventrally into the supracleithrum which is an anteriorly inclined bone, slightly deeper than the first lateral line scale. The cleithrum is large and curved, with its anterior portion overlapped by the opercle, subopercle and branchiostegal rays. There are two postcleithra associated with the cleithrum. Both are roughly trapezoidal; the ventral is nearly half the depth of the dorsal.
The pectoral fins insert low on the body, and each is composed of eight distally segmented rays. The first is unbranched, preceded by two basal fulcra and a series of fringing fulcra. The remaining rays are branched distally.
The pelvic girdles are not exposed. The pelvic fins insert at the 8th vertical scale row, and each is composed of five or six distally segmented and branched rays, preceded by two basal fulcra and a series of fringing fulcra.
Median fins The dorsal fin originates above the 18th vertical scale row. It is short-based, composed of nine distally segmented rays (Figs. 1-3). The first ray is unbranched, preceded by four basal fulcra and six fringing fulcra; the remaining rays are branched distally.
The anal fin, slightly smaller than the dorsal fin, originates below the 16th or 17th vertical scale row (Figs. 1, 2). It has seven distally segmented rays. The first ray is unbranched, preceded by two or three basal fulcra and six fringing fulcra, and the remaining rays are branched distally.
The caudal fin is abbreviated heterocercal with a slightly forked profile (Figs. 1, 2). There are 16 principal rays; among them, seven or eight are located below the level of the lateral line. The marginal principal rays are unbranched, and the middle ones are branched up to three times. In addition, there are seven basal fulcra in the dorsal lobe, and three basal fulcra and two rudimentary rays in the ventral one. Fringing fulcra are present in both lobes.
Scales The body is fully covered with rhombic scales (Figs. 1-3). The scales are smooth, arranged in 28 vertical rows along the lateral line. In addition, there are five inverted vertical rows of scales posterior to the hinge line in the caudal region; among them, eight scales are present in the last inverted vertical row. The scales in the anterior flank region are twice as deep as they are long with a serrated posterior margin. The number and size of serrations vary in different specimens; they are weak in V 20431 (Fig. 1) and V 20432 (Fig. 2), but relatively strong in V 20433 and V 20434 (Fig. 3). The scales gradually become shorter dorsally, ventrally and posteriorly. The terminal scales adjacent to the caudal fin are notably longer than deep with a posterodorsal process. The ridge scales anterior to the dorsal fin have a posterodorsally directed spine. A dorsal peg and an anterodorsal extension are present on some scales exposed in the anterior flank region (V 20433).
5 Phylogenetic results
The phylogenetic analysis resulted in five most parsimonious trees (tree length = 521 steps, consistency index = 0.4760, retention index = 0.7509), the strict consensus of which is presented in Fig. 8. Ticinolepis occupies a basal position among the Ginglymodi (consistent with López-Arbarello and Sferco, 2018; Xu et al., 2018a), followed successively by the Kyphosichthyiformes, Sangiorgioichthys, Semionotiformes and Lepisosteiformes. Yudaiichthys is recovered as the sister taxon to Lashanichthys; both genera consist of a clade sister to the Kyphosichthyidae (including Kyphosichthys and Fuyuanichthys) within the Kyphosichthyiformes.Fig. 8
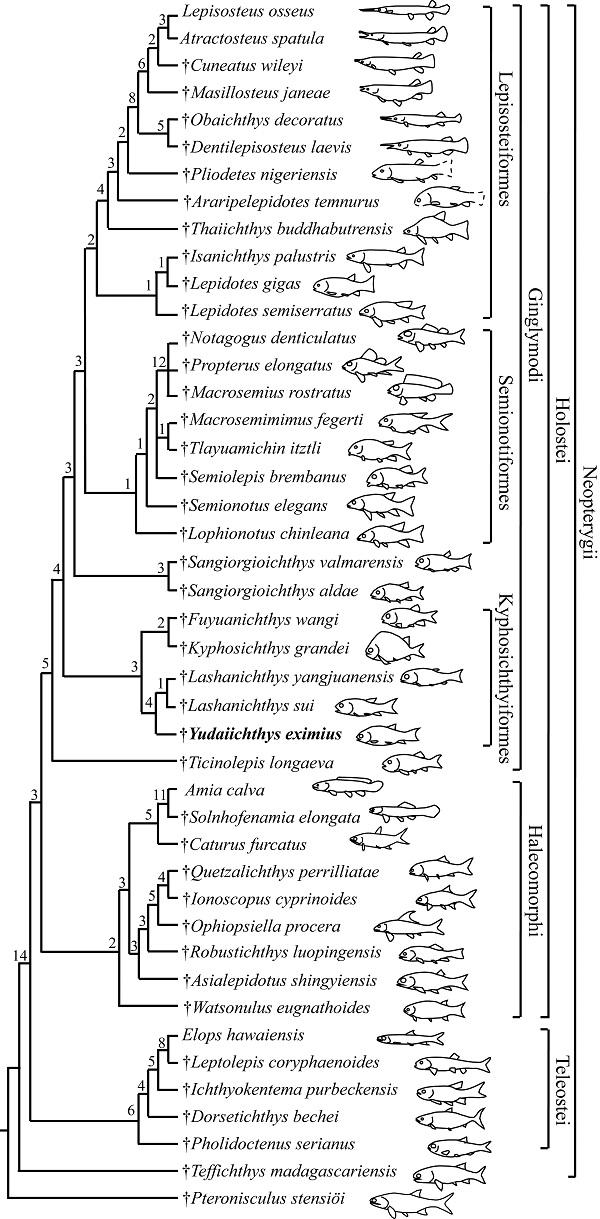 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 8Strict consensus of five most parsimonious trees (TL= 521, CI= 0.4760, RI= 0.7509), illustrating the phylogenetic position of Yudaiichthys within the Neopterygii Digits above nodes indicate Bremer decay indices
The Kyphosichthyiformes are unambiguous ginglymodians, possessing several synapomorphies of this clade (those uniquely derived are identified with an asterisk), e.g., presence of anterior infraorbitals (*); six or more infraorbitals between the antorbital and the dermosphenotic (*); orbital length no longer than the pre-orbital length (independently derived in some ionoscopiforms, and reversal in Lashanichthyidae); and presence of no more than nine pairs of branchiostegal rays (independently derived in some stem-neopterygians). The order is positioned above Ticinolepis (the most basal ginglymodian) as it shares six derived features with all remaining ginglymodians: 1) presence of a relatively long parietal (independently derived in some other neopterygians); 2) a subrectangular dermopterotic (independently derived in some ionoscopiforms); 3) a lacrimal deeper than long (independently derived in some ionoscopiforms); 4) presence of an olfactory foramen in the nasal process of the premaxilla (independently derived in amiiforms, and secondarily lost in macrosemiids); 5) absence of a presupracleithrum (independently derived in some other neopterygians); and 6) presence of a posterodorsally directed spine on the predorsal ridge scale (secondarily lost in most lepisosteiforms).
6 Phylogenetic discussion
6.1 Taxonomic revision of the Kyphosichthyiformes
Sun and Ni (2018) listed three features to support the monophyly of their named Kyphosichthyiformes (= Kyphosichthyidae, including Kyphosichthys, Sangiorgioichthys and Luoxiongichthys), i.e., 1) a triangular suborbital laterally covering the quadrate; 2) infraorbitals at the ventral orbital rim subtriangular, broader ventrally, and about twice deeper than long; and 3) absence of a foramen for the olfactory nerve in the nasal process of the premaxilla. However, the first feature is absent in Kyphosichthys and Luoxiongichthys; the second is only present in Kyphosichthys; and the last is plesiomorphic for Holostei (Grande, 2010; Xu and Ma, 2018; López-Arbarello and Sferco, 2018). Thus, the above three features should not be considered as kyphosichthyiform synapomorphies (Xu et al., 2018a).The Kyphosichthyiformes revised here include four genera (Kyphosichthys, Fuyuanichthys, Yudaiichthys and Lashanichthys) in two families, and their monophyly is supported by four synapomorphies: 1) presence of a dermopterotic slightly shorter than the parietal (independently derived in macrosemiids); 2) a pair of extrascapulars separated by posterior extensions of parietals (independently derived in macrosemiids); 3) absence of a preopercle/dermopterotic contact (reversal in Lashanichthys, and independently derived in Sangiorgioichthys aldae and several semionotiforms); and 4) presence of a posttemporal/parietal contact (independently derived in macrosemiids). Luoxiongichthys should be removed out of the Kyphosichthyiformes because it lacks the above synapomorphies of this order.
Within the Kyphosichthyiformes, the sister taxon relationship between Yudaiichthys and Lashanichthys are strongly supported by five derived features: 1) pre-orbital length no longer than orbital length (reversal); 2) the depth/length ratio of the anterior infraorbital larger than 2.5 (independently derived in a few semionotiforms); 3) presence of two to four infraorbital bones between the lacrimal and the infraorbital at the posteroventral corner of the orbit (independently derived in crownward ginglymodians); 4) presence of a mandible nearly half of the head in length (reversal); and 5) presence of a trapezoidal interopercle (*). Yudaiichthys and Lashanichthys are easily distinguished from other kyphosichthyiforms by these features. As such, they are grouped into a new family (Lashanichthyidae fam. nov.) of Kyphosichthyiformes rather than into the previous, broadly inclusive Kyphosichthyidae (Sun and Ni, 2018).
The Kyphosichthyidae is restricted here to include the type genus Kyphosichthys and the recently reported Fuyuanichthys, which was originally classified in the Ginglymodi without reference to a particular family or order (Xu et al., 2018a). The current analysis confirm the sister-taxon relationships between both genera (Xu et al., 2018a), supported by three derived features: 1) infraorbital bones at the middle portion of the orbit deeper than the orbital radius (independently derived in Sangiorgioichthys, a few semionotiforms and most lepisosteiforms); 2) presence of a quadrate laterally covered by infraorbitals (independently derived in some lepisosteiforms); and 3) absence of suborbitals extending anteriorly below the orbit (independently derived in some semionotiforms).
6.2 Phylogenetic reassessment of Sangiorgioichthys
The genus Sangiorgioichthys was named on the basis of the type species S. aldae from the Ladinian of the Monte San Giorgio area (Tintori and Lombardo, 2007). López-Arbarello and her colleagues named the second species S. sui from the Anisian of Yunnan, China and placed the genus as a semionotiform incertae sedis (López-Arbarello et al., 2011; López-Arbarello, 2012; López-Arbarello and Wencker, 2016) or a ginglymodian incertae sedis (López-Arbarello and Sferco, 2018). Lombardo et al. (2012) and Chen et al. (2014) named the other two species, the Ladinian S. valmarensis from the Monte San Giorgio area and the Anisian S. yangjuanensis from Guizhou, respectively, and considered them as semionotiforms incertae sedis. Recently, Sun and Ni (2018) recovered S. aldae as a sister taxon to a clade composed of S. sui and Kyphosichthys and placed them into their named Kyphosichthyidae (Kyphosichthyiformes). For the first time, we incorporated all four Sangiorgioichthys species in a phylogenetic analysis, the results of which support that the Chinese “Sangiorgioichthys” species (S. sui and S. yangjuanensis; removed into the new genus Lashanichthys, see above) are members of Kyphosichthyiformes, but the European Sangiorgioichthys species (S. aldae and S. valmarensis) should be removed out of Kyphosichthyiformes because they lack all of the synapomorphies of this order and share four derived features with the Semionotiformes-Lepisosteiformes clade: 1) presence of a lacrimal closely related to the supraorbital (*); 2) presence of a quadrate-mandibular articulation under the orbit (independently derived in Fuyuanichthys); 3) presence of a maxilla ending anterior to the orbit (*); and 4) absence of a well-developed supramaxillary process on the maxilla (independently derived in some other neopterygians). Consequently, the genus Sangiorgioichthys is here restricted to include S. aldae and S. valmarensis; the sister taxon relationships between both species are supported by four derived features: 1) presence of an anteriorly tapered dermopterotic (reversal); 2) middle infraorbital bones below the orbit deeper the orbital radius; 3) suborbitals separated by the posteroventral infraorbital into dorsal and ventral portions (independently derived in Lashanichthys); and 4) presence of teeth on only the anterior one third or less of the dentary.6.3 Phylogenetic position of Robustichthys
Results of our analysis support that Robustichthys is an ionoscopiform halecomorph (Xu et al., 2014b; Xu and Shen, 2015; Xu and Ma, 2018; contra Sun and Ni, 2018). Two other recent analyses (López-Arbarello and Sferco, 2018; Ebert, 2018) also recovered Robustichthys as a halecomorph although their proposed positions of this genus within Halecomorphi are somewhat different from the one we suggested. Our current studies confirm that Robustichthys is phylogenetically distant from Ginglymodi, because it lacks the important synapomorphies of Ginglymodi listed above. The three characters listed by Sun and Ni (2018) supporting the sister group relationships of Robustichthys with Ginglymodi are either the one miscoded in Robustichthys (nasals very narrow, separated medially) or those widely distributed in neopterygians (presence of a well-developed posteroventral process of the dentary, and series of denticles along the ridge between the branchial and lateral surfaces of the cleithrum). A detailed description and revision of Robustichthys are out of scope for this contribution and will be presented in the future.7 Ecological implications
The Kyphosichthyiformes are so far composed of five species in two families with a geological range of about 4 Ma (~244-240 Ma) from the Middle Triassic marine deposits of Yunnan and Guizhou, China. All kyphosichthyiforms have a relatively small-sized body with a maximum standard length no more than 125 mm. Among them, Lashanichthys is the most common actinopterygian in the Anisian Luoping ichthyofauna, which probably swam in a schooling behavior, with over 30 specimens preserved in a square meter of slab. Yudaiichthys and other kyphosichthyiforms have a significantly lower numerical abundance than Lashanichthys, lacking schooling evidences in the past years of field collections. The Kyphosichthyiformes appear an endemic group of ginglymodian fishes with a distribution confined to the eastern Paleotethys. No kyphosichthyiforms have been known from the Middle Triassic of Europe in the western Paleotethys. Further studies are needed to clarify if this is caused by sampling and/or taphonomic biases.The Kyphosichthyiformes show noteworthy differences on body shape and teeth distribution between lashanichthyids and kyphosichthyids. The body is elongated fusiform in the Lashanichthyidae and relatively deep and short in Fuyuanichthys (Xu et al., 2018a). In Kyphosichthys, the body is notably deep and short with a strikingly arched hump anterior to the dorsal fin; a similar morphotype is present in several other ginglymodians (e.g., Gibson, 2013a). As implicated by functional and ecological studies on extant fishes (Drucker and Lauder G V, 2002; Ruehl et al., 2011), kyphosichthyids are likely not fast swimmers but have better performance in precise maneuvering among structurally complex habitats than the Lashanichthyidae, which could be adapted to simple habitats. Additionally, lashanichthyids have a larger mouth gap than kyphosichthyids. The teeth are present along almost the full length of the maxilla (excluding the anteromedial process) in lashanichthyids and Kyphosichthys, but are absent in that of Fuyuanichthys. These differences indicate that the Kyphosichthyiformes have a broad ecomorphological diversity.
8 Conclusion
The new kyphosichthyiform Yudaiichthys eximius from the early Middle Triassic (Anisian) of China represents one of the oldest records of Ginglymodi and provides an important addition to our understanding of the earliest morphological and ecological diversifications of this clade. Yudaiichthys possesses diagnostic features of the Kyphosichthyiformes revised in this contribution, but it is easily distinguished from other kyphosichthyiforms with a unique combination of features (e.g., presence of a dermohyal, presence of a relatively large number of suborbitals, and absence of a supraorbital/dermosphenotic contact). Results of a phylogenetic analysis recover Yudaiichthys as a sister taxon of the coeval Lashanichthys (previously referred to the genus Sangiorgioichthys); both genera are grouped into the new family, the Lashanichthyidae, within the Kyphosichthyiformes. Sangiorgioichthys, restricted to include two species (S. aldae and S. valmarensis) from the late Middle Triassic (Ladinian) of the Monte San Giorgio area, is removed out of the Kyphosichthyiformes and is recovered as the sister taxon of the Semionotiformes-Lepisosteiformes clade. The previous placement of Robustichthys near the ginglymodian base is rejected. The revised topology in and around the Kyphosichthyiformes provides new insights into the anatomical evolution during the earliest ginglymodian history.Acknowledgements
We thank Chang M. M., Wen W. and Hu S. X. for constructive suggestions and discussions, Cavin L. for valuable comments on a previous version of this manuscript, Grande L. and Maisey J. for granting access to comparative fossil materials in the Field Museum of Natural History (Chicago) and American Museum of Natural History (New York) respectively, and Liu T. L. and He B. T. for their help with field trips in Yunnan Province.Supplementary material can be found on the website of Vertebrata PalAsiatica (
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 4]
[本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 2]
DOIPMID [本文引用: 1]

The great anatomical diversification of paired fins within the Actinopterygii (ray-finned fishes) can be understood as a suite of evolutionary transformations in design. At a broad taxonomic scale, two clear trends exist in the morphology of the anteriorly situated pectoral fins. In comparing basal to more derived clades, there are general patterns of (i) reorientation of the pectoral fin base from a nearly horizontal to more vertical inclination, and (ii) migration of the pectoral fin from a ventral to mid-dorsal body position. As yet, the functional significance of these historical trends in pectoral fin design remains largely untested by experiment. In this paper we test the proposal that variation in pectoral fin structure has an important influence on the magnitude and orientation of fluid forces generated during maneuvering locomotion. Using digital particle image velocimetry for quantitative wake visualization, we measure swimming forces in ray-finned fishes exhibiting the plesiomorphic and apomorphic pectoral fin anatomy. Our experiments focus on rainbow trout (Oncorhynchus mykiss), a lower teleost with pectoral fins positioned ventrally and with nearly horizontally inclined fin bases, and bluegill sunfish (Lepomis macrochirus), a relatively derived perciform fish with more vertically oriented pectoral fins positioned mid-dorsally on the body. In support of hypotheses arising from our prior wake studies and previously untested models in the literature, we find that the pectoral fins of sunfish generate significantly higher forces for turning and direct braking forces closer to the center of mass of the body than the pectoral fins of trout. These results provide insight into the hydrodynamic importance of major evolutionary transformations in pectoral fin morphology within the Actinopterygii.
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
[本文引用: 10]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 3]
DOIURL [本文引用: 6]
DOIURL [本文引用: 6]
DOIURL [本文引用: 4]
DOIURL
DOIURL
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 16]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL
[本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 3]
DOIURL [本文引用: 11]
[本文引用: 3]
DOIURL [本文引用: 2]
