
 , 马馨宇1, 秦朝朝3
, 马馨宇1, 秦朝朝3 1. 南京信息工程大学 江苏省大气海洋光电探测重点实验室, 南京 210044;
2. 江苏省大气环境与装备技术协同创新中心, 南京 210044;
3. 河南师范大学物理与材料科学学院, 河南 新乡 453007
摘要: 碘甲烷是一种有毒的甲基化试剂和土壤消毒剂,应用十分广泛,研究其基本的物理性质和使其降解的有效措施很有必要。使用密度泛函理论(density functional theory,DFT),在B3LYP/LANL2DZ水平上研究在外电场(0~0.04a.u.)作用下碘甲烷分子的解离特性以及多种物理性质。计算结果表明,在C—I键连线Z方向上,外电场从0逐渐增加到0.04a.u.时,分子体系能量逐渐减小,偶极矩单调增大.HOMO-LUMO能隙EG却呈现先增大后减小的变化趋势,C—I和C—H键键长逐渐增大,更加易于裂解。在外电场逐渐增强时,解离特性表现为:CH3I分子的C—I键方向扫描得到的势能曲线的束缚状态逐渐消失,势垒逐渐变小最后消失。计算发现,强度为0.04a.u.的外电场足以使CH3I分子发生C—I键断裂而降解。该结果为保护环境和对碘甲烷进行电场降解提供理论依据。
关键词: 碘甲烷外电场降解红外光谱
Methyl iodide is used primarily in the production of pharmaceutical intermediates and the organic synthesis for methylation reagents. It was considered as an alternative to soil disinfectants in the 1998 and 2002 Methyl Bromide Technical Options Committees Assessment. Methyl iodide is also widely used as a soil disinfectant to replace methyl bromide that severely destroyed the environment (prohibited by the Montreal Protocol Copenhagen Amendment). Methanol is toxic, corrosive, and carcinogenic, and methyl iodide naturally decomposes more slowly. At the same time, methyl iodide is widely used as a soil disinfectant but the possibility of entering the groundwater is very high. Inhalation of a large amount of methyl iodide inhibits the central nervous system and exerts strong stimulating effects on the skin and mucous membranes. Methyl iodine is easily decomposed by heat and produces toxic iodide fumes. It can be absorbed through the respiratory tract, skin, and digestive tract and causes poisoning. Domestic and foreign reports described how CH3I harmed people and animals and some poisoning incidents caused by CH3I[1, 2].
In recent years, the degradation kinetics of halogenated compounds containing CH3I has received unprecedented attention [3-7]. Molecules in the external electric field will have a series of physical and chemical changes. The characteristic of molecules in external electric field has become an important method for studying molecular properties. This method has been successfully applied to a number of fields to study the properties of molecules[8-12]. Applying a strong electric field to a molecule to break off the chemical bond is an effective method for the degradation of the molecule. However, there is no reported study on the dissociation of the CH3I molecule in the external electric field.
The molecular total energy, molecular dipole moment, molecular energy gap, molecular spectrum, and dissociation properties of the CH3I molecule in external electric field (0~0.04 a.u. (atomic unit)) were studied using density functional theory at the B3LYP/LANL2DZ level. The Gaussian 09[13]software was used. The result provides important theoretical support for the degradation of CH3I.
1 Theoretical methodThe Hamiltonian of the radiation process is given as
| $H = {H_0} + {\rm{ }}{H_{{\rm{int}}}}.$ | (1) |
| ${H_{{\rm{int}}}} = - \mu \cdot F$ | (2) |
Based on the model proposed by Grozema et al.[14-15], excitation energy under the action of field Eexc, electric field strength F with the variation amounts Δμ, Δα of the electric dipole moment and the polarization rate satisfies the relational expression
| ${E_{{\rm{exc}}}}\left( F \right) = {E_{{\rm{exc}}}}\left( 0 \right) - \Delta \mu \cdot F - 1/2 \cdot \Delta \alpha \cdot {F^2}.$ | (3) |
2 Results and discussion2.1 Molecular stable structure without external electric fieldTheoretical calculations show that the CH3I molecule has the C3 V symmetry. In the present work, we optimize the structure of CH3I with different methods. The experimental data[16] and optimization data are listed in Table 1. By comparing the calculated data with the experimental data, we can see that the structure parameters(the bond lengths and the bond angle) calculated at the B3LYP/LANL2DZ leved are the closest to the experimental data. Therefore, the dissociation and spectral characteristics of CH3I in the external electric field are calculated at the B3LYP/LANL2DZ level. The calculated stable structure is shown in Fig. 1. The X-axis, Y-axis, and Z-axis are delined according to the Dicker coordinate system, and the Z-axis is along the the C-I bond.
Table 1
| Table 1 Optimized parameters of the structure of CH3I at different levels, together with the experimental data |
Fig. 1
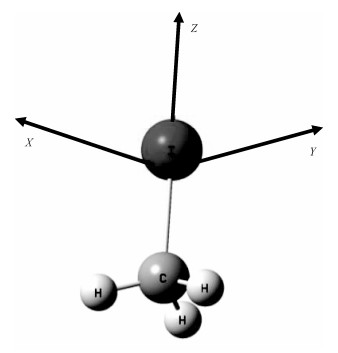 | Download: JPG larger image |
Fig. 1 Optimized geometry of the ground-state CH3I | |
2.2 Effect of external electric fields on bond length and total energyThe CH3I molecule was optimized and calculated at the B3LYP/LANL2DZ level when different electric fields (0~0.04 a.u.) were applied in the Z-axis (along the C—I bond). The stable structures of methyl iodide at different field strengths were obtained. The calculated bond length, and dipole moments of the CH3I molecule are given in Table 2. It can be seen in Table 2 that the total energy decreases gradually with the increase of the external electric field (0~0.04 a.u.). The C—I and C—H bond lengths increase gradually with the increase of the electric field (0~0.04 a.u.) in the Z-axis. The results show that the chemical bond length increases gradually with the external electric field and the modecule becomes more and more prone to dissociation. With the increase of the external field from 0 to 0.04 a.u. along the Z-axis, the dipole moment increases.
Table 2
| Table 2 Calculated molecular total energies, bond distances (C—I and C—H), and dipole moments of CH3I in different external electric fields |
2.3 Effect of external electric fields on the molecular orbital energiesIt is well known that many properties of a molecule are determined by the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). The HOMO and LUMO energies of the CH3I molecule are obtained at the B3LYP /LANL2DZ level. The HOMO energy EH, the LUMO energy EL, and the energy gap EG are shown in Table 3. EG is given as
| ${E_{\rm{G}}} = ({E_{\rm{L}}} - {E_{\rm{H}}}) \times 27.2\ {\rm{eV}}.$ | (4) |
| Table 3 Calculated LUMO energy EL, HOMO energy EH, and HOMO-LUMO energy gap EG for CH3I in different external fields |
It can be seen in Fig. 2 that with the increase of electric field the HOMO -LUMO energy gap EG gradually increases first and then decreases sharply. It is shown that at the beginning the methyl iodide molecule is hard to be excited to the excited state. When the electric field strength is greater than 0.02 a.u., molecule are getting more and more easily excited. It is also shown that the ability of methyl iodide to participate in chemical reactions weaks and then becomes strong.
Fig. 2
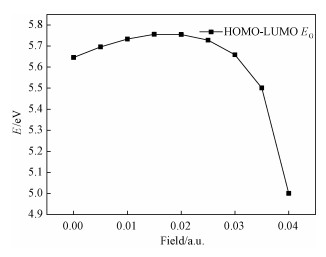 | Download: JPG larger image |
Fig. 2 Variations in the HOMO-LUMO energy gap of CH3I in external fields | |
2.4 Effect of external electric fields on IR spectraThe IR spectra of CH3I were calculated at the B3LYP/LANL 2DZ level. The calculated IR spectra mainly correspond to six kinds of vibration peaks, as shown in Table 4. Frequency of 481.67 cm-1 is attributed to the CI str vibration, frequency of 890.77 cm-1 is attributed to the CH3 rock vibration, frequency of 1 282.34 cm-1 is attributed to the CH3 s-deform vibration, frequency of 1 431.46 cm-1 is attributed to the CH3 s-str vibration, frequency of 2 981.21 cm-1 is attributed to the CH3 s-str vibration, and frequency of 3 110.33 cm-1 is attributed to the CH3 d-str vibration. As shown in Table 4, the calculated symmetries and vibration frequencies are in agreement with the experimental results[17], with errors of no more than 10%.
Table 4
| Table 4 Calculated IR parameters for CH3I including vibrational frequencies and symmetries, together with the experimental data | ||||||||||||||||||||||||||||||
In Table 4, str denotes the stretching vibration, and deform denotes the bending vibration. The IR spectra of the CH3I molecule under different external electric fields were calculated by applying different intensity of electric fields (0~0.04 a.u.) in the direction of the C—I bond. The external electric field has significant influences on the IR spectra of the CH3I molecule.
2.5 Effect of external electric field on molecular potential energy curveWithout external electric field, we carried out energy scanning for CH3I along the C—I bond direction at the B3LYP /LANL2DZ level to obtain the potential energy curve. The same method was used to calculate the potential energy curves along the C—I bond direction with different electric fields along the Z-axis (the C—I bond direction). The potential energy curves without and with the field are plotted in Fig. 3. It is shown that the potential energy curves of the CH3I molecule are gradually repulsive in the external electric fields (0~0.04 a.u.). When the external electric field is 0.04 a.u., the potential energy curve of CH3I changes from "bound" to "repulsive". The CH3I molecule will be degraded at the strength of 0.04 a.u.. The electrochemical degradation of the CH3I molecul will be important for the protection of the environment.
Fig. 3
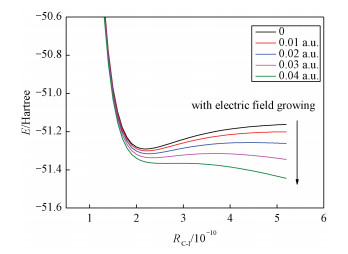 | Download: JPG larger image |
Fig. 3 Variation of the potential energy curve along the C—I bond of CH3I in external field | |
3 ConclusionsIn the present work, the dissociation characteristics and spectral characteristics of CH3I in external electric field were calculated using the principle of density functional theory. It is found that the results obtained at the B3LYP/LANL2DZ level are the closest to the experimental data. Therefore, the B3LYP/LANL2DZ method was used to calculate the configuration, IR spectrum, and dissociation potential energy curve of CH3I in different electric fields. When a series of external electric fields are applied along the Z-axis (the C—I bond), the energy of the molecular system gradually decreases. The dipole moment decreases, indicating that the polarity is constantly increasing. HOMO -LUMO energy gap EG increases first and then decreases sharply, indicating that at the beginning, the methyl iodide molecule is hard to be excited into the excited state. When the electric field strength is greater than 0.02 a.u., the excitation of the molecule to the excited state will become more and more simple. It is also shown that the ability of methyl iodide to participate in chemical reactions weakens and then becomes strong. The C—I and C—H bond lengths monotonously increase. The external electric field also has effects on the positions and intensities of the IR spectra of the CH3I molecule.The potential energy curve of the CH3I molecule along the C—I bond changes gradually from "bound" to "repulsive". It is found that the electric field of 0.04 a.u. is sufficient to cause the C—I bond breakage of CH3I, which provides an important scientific basis for the degradation of CH3I.
References
| [1] | Schwartz M D, Obamwonyi A O, Thomas J D, et al. Acute methyl iodide exposure with delayed neuropsychiatric sequelae:report of a case[J]. Am J Ind Med, 2005, 47(6): 550-556. DOI:10.1002/(ISSN)1097-0274 |
| [2] | Nair J R, Chatteriee K. Methyl iodide poisoning presenting as a mimic of acute stroke:a case report[J]. J Med Case Rep, 2010, 4(1): 1-4. DOI:10.1186/1752-1947-4-1 |
| [3] | Liu Y Z, Thomas G, Gregor K. Optical control of the vibrational excitation of the polyatomic ions via strong field multi-photon ionization[J]. Acta Phys Sin, 2014, 63(24): 244208. |
| [4] | Schiermeier Q. Chemists poke holes in ozone theory[J]. Nature, 2007, 449(7161): 382-383. DOI:10.1038/449382a |
| [5] | Hobe M V. Revisiting ozone depletion[J]. Science, 2007, 318(5858): 1878-1879. DOI:10.1126/science.1151597 |
| [6] | Liu Y Z, Long J Y, Zhang X Y, et al. Probing ultrafast dissociation dynamics of chloroiodomethane in the B band by time-resolved mass spectrometry[J]. Chinese Phys Lett, 2017, 34(3): 033301. DOI:10.1088/0256-307X/34/3/033301 |
| [7] | Liu Y Z, Gerber T, Radi P, et al. Switching the vibrational excitation of a polyatomic ion in multi-photon strong field ionization[J]. Chemical Physics Letters, 2014, 610-611: 153-158. DOI:10.1016/j.cplett.2014.07.016 |
| [8] | Li X X, Zhang Z P, Long Z W, et al. Ground state properties and spectral properties of borospherene B40 under different external electric fields[J]. Acta Phys Sin, 2017, 66(10): 103102. |
| [9] | Wu Y G, Li S X, Hao J X, et al. Properties of ground state and spectrum of CdSe in different external electric gelds[J]. Acta Phys Sin, 2015, 64(15): 153102. |
| [10] | Bhattachryya P K. Effect of external electric field on ground and singlet excited states of phenylalanine:a theoretical study[J]. Computational and Theoretical Chemistry, 2015, 1057: 43-53. DOI:10.1016/j.comptc.2015.01.017 |
| [11] | Shen H J, Shi Y J. Geometey configuration and invalidity of Dimer C60 fullerene molecule in applied external electric field[J]. Chin J Chem Phys, 2005, 18(3): 351-356. |
| [12] | Zhang G Q, Xie S J. Temperature and electric field effect on proton transfer in adenine-thymine[J]. Bull Korean Chem Soc, 2014, 35(12): 3532-3534. DOI:10.5012/bkcs.2014.35.12.3532 |
| [13] | Frish M J, Trucks G W, Schlegel H B, et al. Gaussian 09, Revision C.01[CP]. Walling ford: Gaussian Inc, 2010. |
| [14] | Kjellberg P, He Z, Pullerits P. Bacteriochlorophyll in electric field[J]. J Phys Chem B, 2003, 107(49): 13737-13742. DOI:10.1021/jp035642y |
| [15] | Grozema F C, Telesca R, Jonkman H T, et al. Excited state polarizabilities of conjugated molecules calculated using time dependent density functional theory[J]. J Chem Phys, 2001, 115(21): 10014-10021. DOI:10.1063/1.1415085 |
| [16] | Sutton L E. Tables of molecular parameters-carbon compounds[EB/OL]. (1958)[2017-07-18]. http://www.wiredchemist.com/chemistry/data/carbon-compounds. |
| [17] | Shimanouchi T. "Vibrational and/or electronic energy levels" in NIST Chemistry WebBook[EB/OL].(1968-07-11)[2017-07-18].http://webbook.nist.gov/cgi/cbook.cgi?ID=C74884&Units=SI&Mask=800. |
