 ,1, 黄峰1, 沈青山1, 温彦涛2, 郭志刚2, 景晓亮2, 张春晖
,1, 黄峰1, 沈青山1, 温彦涛2, 郭志刚2, 景晓亮2, 张春晖 ,1
,1The Influence of Low-Temperature and Long-Time Cooking on the Quality of Pork Products
WANG JingFan ,1, HUANG Feng1, SHEN QingShan1, WEN YanTao2, GUO ZhiGang2, JING XiaoLiang2, ZHANG ChunHui
,1, HUANG Feng1, SHEN QingShan1, WEN YanTao2, GUO ZhiGang2, JING XiaoLiang2, ZHANG ChunHui ,1
,1通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-06-19接受日期:2020-10-14网络出版日期:2021-02-01
| 基金资助: |
Received:2020-06-19Accepted:2020-10-14Online:2021-02-01
作者简介 About authors
王静帆,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1429KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王静帆, 黄峰, 沈青山, 温彦涛, 郭志刚, 景晓亮, 张春晖. 低温长时蒸煮对猪肉品质的影响[J]. 中国农业科学, 2021, 54(3): 643-652 doi:10.3864/j.issn.0578-1752.2021.03.017
WANG JingFan, HUANG Feng, SHEN QingShan, WEN YanTao, GUO ZhiGang, JING XiaoLiang, ZHANG ChunHui.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】蒸煮是中式肉制品最常用的一种加工方法。中式传统蒸煮通常在沸水中加热30 min至2 h,这种热加工方法存在肉制品水分损失高、出品率低的缺点,且易造成嫩度及色泽不佳。低温长时蒸煮(Low-temperature long-time cooking,LTLT cooking)通常指在50—65℃对原料肉进行从几小时到几天不等的恒温热处理,近年来在肉品加工中应用广泛[1]。目前已有研究表明,低温长时烹饪能显著改善肉制品嫩度、持水性及感官品质,提高出品率[2]。探讨55℃和60℃不同低温长时加热条件下,肌纤维及结缔组织蛋白变化情况对肉品品质、微观结构的影响,揭示低温长时加热对猪肉品质的改善机理,为应用低温长时蒸煮加工猪肉提供理论基础。【前人研究进展】有研究表明,低温长时蒸煮过程中,肉的持水性、嫩度、色泽等品质的变化可能由肌原纤维结构变化、肌纤维及结缔组织中蛋白质变性和水解等一系列复杂作用导致[2]。肌球蛋白是肌原纤维中主要组分之一,肌球蛋白热稳定性较差,其40℃即开始变性[3]。肌内结缔组织的主要组分是胶原蛋白,由原胶原分子通过共价交联聚合而得[4]。加热温度升高至58℃左右,胶原蛋白热变性,其结构由完整的三螺旋结构变为随机卷曲状结构(无定形)[5]。然而在低温长时蒸煮过程中,尽管未达到蛋白变性温度,但是随着加热时间延长,有些蛋白质含量也会逐渐降低。在常规加热过程中,肌肉结构的收缩主要分为两阶段,其中横向收缩主要发生在40—60℃时,增大了肌纤维与肌内膜之间的间隙,肌肉大量失水;60—70℃时肌内结缔组织(intramuscular connective tissue,IMCT)与肌纤维协同收缩造成肌肉纵向收缩,随温度升高,收缩作用增强,进一步增大蒸煮损失[6]。低温长时蒸煮因温度低造成的有限纵向收缩可能正是其较常规加热蒸煮损失更少的原因[7]。目前,关于肌内结缔组织在加热过程中的水分作用机制存在一定争议。有****认为蛋白变性使肌原纤维结构的硬度增加及胶原纤维收缩,对肌纤维中的水分产生一种挤出作用,导致了蒸煮损失的形成,降低了肉的嫩度[8];但是也有****认为肌内结缔组织可以在一定程度上防止肌纤维水分的蒸发和外渗,即结缔组织含量越丰富的肌肉,其持水力也越强[1],且有研究表明在59℃时,肌束膜溶解凝胶化,有助于提高肉制品的保水作用[9]。【本研究切入点】虽然近年来关于低温长时蒸煮的研究十分广泛,但是不同加热条件下,肌肉微观结构如肌纤维及肌内结缔组织的变化对肉品持水性等品质的影响机制尚不完全明确。关于胶原蛋白变性温度前后,低温长时蒸煮过程中,猪肉蛋白质、微观结构及持水性等品质变化的相关研究工作仍需进一步开展。【拟解决的关键问题】结合前人研究基础[10]及预试验结果,基于57℃开始出现胶原纤维收缩[11],不同加热时间猪肉肌纤维及结缔组织变性情况不同[12],本研究以猪背最长肌(Longissmus dorsi)为研究对象,选取55℃和60℃为不同加热温度,4、8和24 h为不同加热时间,研究肉制品持水性等品质、结构、肌纤维及结缔组织蛋白变性情况,为应用低温长时蒸煮加工猪肉提供理论基础。1 材料与方法
试验于2019年在农业部农产品加工综合性重点实验室进行。1.1 材料与试剂
试验材料:购于北京幸福荣耀超市(农大南路店),取6头4℃条件下冷却24 h的猪左侧胴体的背最长肌(约2.5 kg)。试剂:天狼猩红染色试剂盒,北京中科万邦生物科技有限公司;磷酸氢二钾、磷酸二氢钾、无水乙醇、二甲苯、氯胺T、一水柠檬酸、乙酸钠等(均为分析纯),国药集团化学试剂有限公司;溴酚蓝,美国Amersco公司。
1.2 仪器与设备
MesoMR23-060H-I低场核磁共振分析及成像系统,上海纽迈电子科技有限公司;CR-400色差仪,日本柯尼卡美能达公司;TA-XTPlus质构分析仪,英国Stable Micro Systems公司;T6紫外可见分光光度计,北京普析通用仪器有限责任公司;TESTO735型温度仪,德国德图仪器有限公司;HHS型电热恒温水浴锅,上海博讯实业有限公司;RW20数显形顶置式均质机,德国艾卡仪器设备有限公司。1.3 方法
1.3.1 样品制备 6条猪背最长肌去除可见脂肪及结缔组织,使用前在4℃条件下平衡30 min,分割前用厨房用纸擦干,每条背最长肌均切出6块75 g左右的肉块,放入聚乙烯蒸煮袋中抽真空。将肉块对应放入不同加热温度55℃和60℃的水浴锅中,加热4、8和24 h,每个处理为6块肉样,加热结束后的肉块在冰水中浸泡5 min以终止反应。1.3.2 蒸煮损失的测定 蒸煮前擦干肉块表面水分并准确称重(精确至0.01 g),蒸煮完成终止反应后,轻轻擦干肉样表面水分并准确称重。按照如下公式计算肉样的蒸煮损失率。
$ 蒸煮损失(\%) =\frac{M_{1}-M_{2}}{M_{1}} \times 100$
式中,M1为蒸煮前肉样质量(g),M2为蒸煮后肉样质量(g)。
1.3.3 剪切力的测定 参考WANG等[13]的方法,将蒸煮后的肉样顺肌纤维方向切成1 cm×1 cm×4 cm大小的肉条,4℃静置过夜,以TA-XTPlus质构分析仪进行测定。探头型号BSW,探测器从阻力点降低25 mm,测前速度5 mm?s-1,进刀速度10 mm?s-1,测后速度10 mm?s-1,每块肉样至少平行测量3次,结果为6块肉样重复测量结果的平均值。
1.3.4 色泽的测定 用CR-400色差仪测定肉样中心的亮度值L*、红度值a*和黄度值b*。每块肉样平行测定5次,结果为6块肉样重复测量结果的平均值。参照WANG等[14]的方法,总色差按照如下公式计算。
$\Delta E=\sqrt{\Delta L^{* 2}+\Delta \mathrm{a}^{* 2}+\Delta b^{* 2}}$
1.3.5 体积收缩率的测定 体积收缩率的测定参照KONG等[15]的方法,并稍作修改。肉样的长度a、宽度b和高度c均通过Image-Pro Plus 6.0软件进行测量。
$纵向收缩率 (\%)=\frac{a_{1} \times b_{1}-a_{2} \times b_{2}}{a_{1} \times b_{1}} \times 100$
$横向收缩率(\%)=\frac{b_{1} \times c_{1}-b_{2} \times c_{2}}{b_{1} \times c_{1}} \times 100$
$体积收缩率(\%)=\frac{a_{1} \times b_{1} \times c_{1}-a_{2} \times b_{2} \times c_{2}}{a_{1} \times b_{1} \times c_{1}} \times 100$
式中,a1、b1和c1分别为蒸煮前肉样的长度、宽度和高度(cm);a2、b2和c2分别为蒸煮后肉样的长度、宽度和高度(cm)。纵向为顺肌纤维方向;横向为垂直于肌纤维方向。
1.3.6 水分分布及组成测定 参照WANG等[14]的方法,使用低场核磁共振(low field nuclear magnetic resonance,LF-NMR)分析系统测定T2弛豫时间,采用CPMG脉冲序列进行波谱测定。测定条件如下:共振频率,23.39 MHz;采样频率,286.1954 kHz;采样点数384 996;重复时间3 000 ms;回波个数2 000;90脉冲时间为17 μs;180脉冲时间为35 μs;累加次数为4次。反演得到T2反演图谱。
1.3.7 氢质子密度成像测定(magnetic resonance imaging,MRI) 将试验样品放入永磁场的射频线圈中心,使用低场核磁共振分析及成像系统测定样品的氢质子密度,分析水分的空间分布情况。采用MSE成像序列进行测定,主要参数设置为:中心频率23.319 MHz,回波时间20 ms,重复时间3 000 ms,视野宽度为:横向50 mm,纵向50 mm,重复扫描4次。经过滤波处理及伪彩处理得到最终图像。
1.3.8 组织结构观察 参照LI等[16]的方法,采用天狼星红染色法对肉样切片进行组织学观察,取样后,将组织放入包埋模中,进行梯度乙醇(70%、80%、90%、95%、100%)脱水,二甲苯透明后,浸蜡包埋,冷冻切片后进行4 μm切片,烤片后按照试剂盒说明书进行天狼星红染色,倒置显微镜下观察。
1.3.9 蛋白质表面疏水性测定 参照MITRA等[17]的方法,取2 g肉样加入20 mL的20 mmol?L-1磷酸缓冲液(pH 6),用高速均质机在20 600 r/min下均质30 s,双缩脲法测定均质后样品蛋白浓度,用磷酸缓冲液将蛋白浓度调整为5 mg?mL-1。取1 mL稀释后的蛋白溶液加入200 μL的溴酚蓝(BPB,1 mg?mL-1)溶液,充分混合。以磷酸缓冲液中直接加入BPB溶液作为对照组。对照组和样品组均置于室温下震荡10 min,然后在4℃条件下离心(2 000×g)15 min。取上清液以磷酸缓冲液稀释10倍,磷酸盐缓冲液为空白,在595 nm处测定吸光度。以结合态BPB为指标测定表面疏水性。
$\text { 结合态 } B P B(\mu \mathrm{g})=\frac{O D_{(\text {control })}-O D_{(\mathrm{sample})}}{O D_{\text {(control) }}} \times 200$
式中:OD(control)为空白组吸光度;OD(sample)为样品组吸光度。
1.3.10 胶原蛋白含量及热溶解性测定 参照STARKEY等[18]的方法进行测定。总胶原蛋白含量:分别取冷冻干燥的肉样粉末0.1 g,加入3 mL 3.5 mmol?L-1 H2SO4,在105℃烘箱中消化16 h。消化后的样品加水至50 mL后过滤。取1 mL滤液加入3.75 mL水和0.25 mL 1.5 mol?L-1 NaOH。取0.5 mL溶液测定羟脯氨酸含量。
热溶性胶原蛋白含量:分别取冷冻干燥的肉样粉末1.5 g,加入10 mL蒸馏水后,80℃水浴加热2 h,加热过程中每30 min振荡一次。加热后的样品在室温下振荡(1 500×g)15 min后过滤。上清液中加入3 mL 3.5 mmol?L-1 H2SO4,在105℃烘箱中消化16 h,消化后的样品加水至10 mL。1 mL稀释后的样品加入1 mL NaOH,取0.5 mL溶液测定羟脯氨酸含量。
羟脯氨酸含量的测定:0.5 mL待测溶液中加入0.25 mL氧化剂(50 mmol?L-1氯胺T,156 mmol?L-1一水柠檬酸,375 mmol?L-1 NaOH,661 mmol?L-1乙酸钠溶于29%正丙醇)混匀后室温下放置20 min,然后加入0.25 mL显色剂(246 mmol?L-1对二甲氨基苯甲醛溶于35%高氯酸和65%异丙醇)。样品混匀后在60℃水浴中保温15 min。加热结束后流水冷却3 min以终止反应,在558 nm处测定吸光度。
$总胶原蛋白含量(mg \cdot \mathrm{g}^{-1} )=m \times 7.25 \times \frac{250}{1000 \times n}$
$\text { 热溶性胶原虫白含量 }\left(\mathrm{mg} \cdot \mathrm{g}^{-1}\right)=m \times 7.25 \times \frac{400}{1000 \times n}$
$\text { 胶原蛋白溶解度 }=\frac{\text { 热溶解性胶原蛋白含量 }}{\text { 总胶原蛋白含量 }}$
式中,m为羟脯氨酸含量(μg);n为取样质量(g)。
1.4 数据分析
本试验利用IBM SPSS Statistics 19统计分析软件进行显著性分析(P<0.05),不同加热时间处理组间采用邓肯氏多重比较分析法,不同加热温度处理组间采用独立样本T检验分析法,除特殊说明外,指标测定均为6次重复测定结果,表示为“平均值±标准误”。2 结果
2.1 不同加热处理对肉品品质的影响
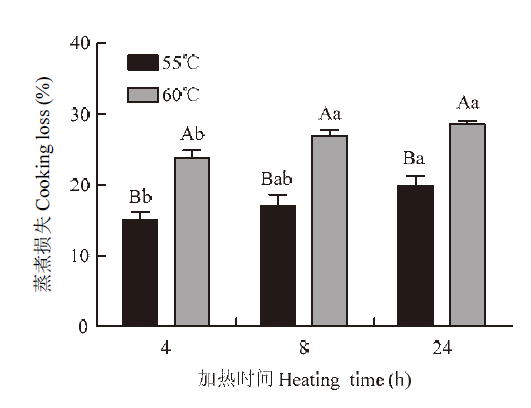
2.1.1 蒸煮损失 由图1可知,在相同加热时间下,55℃组肉样蒸煮损失率均显著低于60℃组(P<0.05)。在相同加热温度下,随加热时间的延长,肉样蒸煮损失均呈增加趋势。与加热时间4 h组相比,24 h组蒸煮损失显著增大(P<0.05)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同加热处理对猪肉蒸煮损失的影响
不同大写字母表示相同加热时间下,不同加热温度之间差异显著(P<0.05);不同小写字母表示相同加热温度下,不同加热时间之间差异显著(P<0.05)。下同
Fig. 1Effects of heating temperature and time on cooking loss
Different capital letters indicate significantly differences among different heating temperatures and the same heating time (P<0.05); Different lowercase letters indicate significantly differences among same heating temperature and different heating time (P<0.05). The same as below
2.1.2 剪切力 剪切力变化主要受肌纤维和肌内结缔组织蛋白的变性收缩影响[19,20]。猪肉加热时肌节收缩导致水分流出,剪切力因此增加,嫩度降低[9]。本试验中,随着蒸煮损失增加,剪切力未产生显著变化(图2),可能是由于加热导致肌内结缔组织溶解,含量降低,溶解性增强。
图2
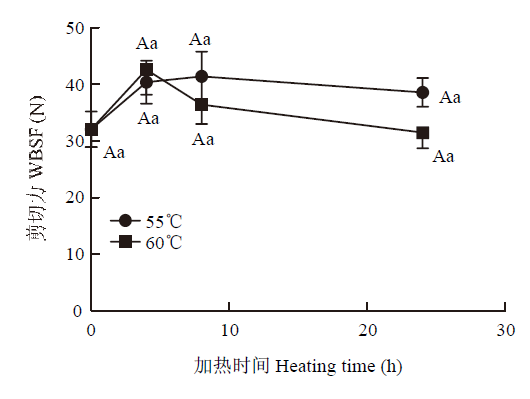 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同加热处理对猪肉剪切力的影响
Fig. 2Effects of heating temperature and time on shear force
2.1.3 色泽 色泽是肉制品重要的食用品质之一,肉品的色泽与其持水性具有一定的相关性[9],可以反应肉制品的熟度[21]。由表1可知,加热温度和时间对肉制品的色泽有重要影响。在相同加热时间条件下,60℃组样品b*值均显著高于55℃组(P<0.05),b*值越高说明肉色越黄。b*值升高可能是由脂肪氧化和蛋白变性导致[22],这与蛋白表面疏水性的结果一致。
Table 1
表1
表1不同加热处理对猪肉色泽的影响
Table 1
| 55℃ | 60℃ | |||||
|---|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | |
| L* | 81.28±0.73Aa | 81.59±0.54Aa | 81.61±0.50Aa | 82.63±0.36Aa | 82.79±0.51Aa | 82.80±0.42Aa |
| a* | 6.18±0.42Aa | 6.57±0.37Aa | 6.64±0.20Aa | 5.50±0.21Aa | 5.64±0.42Aa | 5.77±0.26Ba |
| b* | 6.00±0.30Ba | 6.05±0.32Ba | 6.14±0.16Ba | 6.75±0.11Ab | 6.79±0.06Ab | 7.30±0.17Aa |
| ?E | 27.56±1.80Aa | 27.87±1.26Aa | 27.88±1.22Aa | 28.97±0.90Aa | 29.14±1.26Aa | 29.19±1.05Aa |
新窗口打开|下载CSV
2.1.4 体积收缩率 由图3可知,较加热温度而言,加热时间对纵向收缩率的影响更大,随着加热时间的延长,60℃组纵向收缩率(顺肌纤维方向)显著增加(P<0.05)。加热过程中,与蒸煮损失结果一致,体积收缩主要发生在加热前期;且与横向收缩相比,纵向收缩变化更为显著。
图3
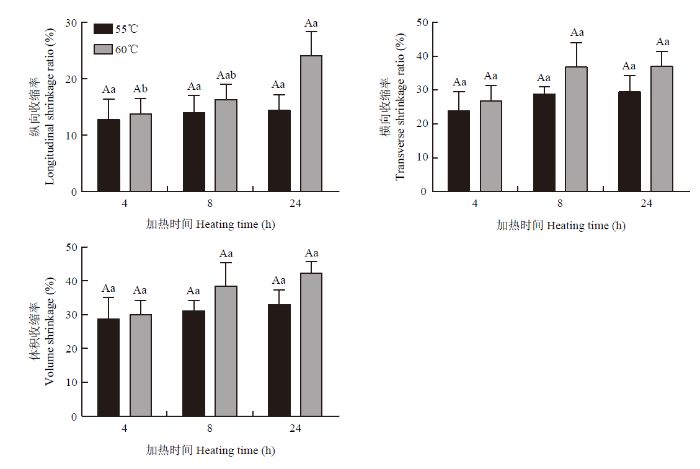 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同加热处理对猪肉体积收缩率的影响
Fig. 3Effect of heating temperature and time on area shrinkage
2.1.5 水分分布及组成 不同温度-时间组合下肉样的水分分布和组成如表2所示。通过低场核磁共振技术可以定量分析肌肉中的结合水(T21)、不易流动水(T22)和自由水(T23)的水分分布和组成[23]。T2弛豫时间表示氢质子在肉样中所处环境,积分面积A表示肉样在相应弛豫时间段的信号量。从显著性分析结果来看,加热温度对水分组成和分布的影响大于加热时间。与55℃组相比,60℃组弛豫时间T22显著减小(P<0.05),这可能与不同温度下加热过程中肌原纤维蛋白质的热变性程度不同有关。
Table 2
表2
表2不同加热处理下肉品水分分布和组成
Table 2
| T21 (ms) | T22 (ms) | T23 (ms) | A21 (%) | A22 (%) | A23 (%) | |
|---|---|---|---|---|---|---|
| 55℃-4h | 0.56±0.18Aa | 36.59±1.90Ab | 207.87±17.17Aa | 2.66±0.01Bb | 96.08±0.00Ba | 0.97±0.00Aa |
| 55℃-8h | 0.57±0.15Aa | 34.91±1.33Aab | 217.70±17.34 Ba | 3.00±0.00Bab | 95.89±0.00Aa | 1.11±0.00Aa |
| 55℃-24h | 0.58±0.12Aa | 33.73±1.48Aa | 222.98±20.32 Ba | 3.22±0.00Ba | 95.85±0.00Aa | 0.93±0.00Aa |
| 60℃-4h | 0.40±0.02 Aa | 32.22±1.80Ba | 215.61±23.17Ac | 3.46±0.00Ab | 95.69±0.00Aa | 0.86±0.00Aa |
| 60℃-8h | 0.43±0.01 Aa | 29.38±1.82Bb | 250.13±19.93Ab | 3.86±0.00Aa | 95.32±0.00Ba | 0.77±0.00Ba |
| 60℃-24h | 0.37±0.05Ba | 29.36±1.29Bb | 280.90±24.19Aa | 3.91±0.00Aa | 95.55±0.00Aa | 0.50±0.00Bb |
新窗口打开|下载CSV
2.2 不同加热处理对肉品结构影响
2.2.1 肉品水分分布 通过核磁共振成像技术将氢质子信号转换为氢质子密度加权伪彩图,可以直观反应水分在肉样中的空间分布情况。图谱中的蓝色区域代表氢质子信号强度较低,代表结合水;红色区域代表氢质子信号强度较高,代表不易流动水和自由水[24]。由图4可知,相同温度下,加热至8 h时,红褐色大幅度减少,表明在低温长时蒸煮的前期,猪肉水分流失更为严重,不易流动水和自由水大量流失,与失水形成蒸煮损失主要发生在加热前期结果一致。图4
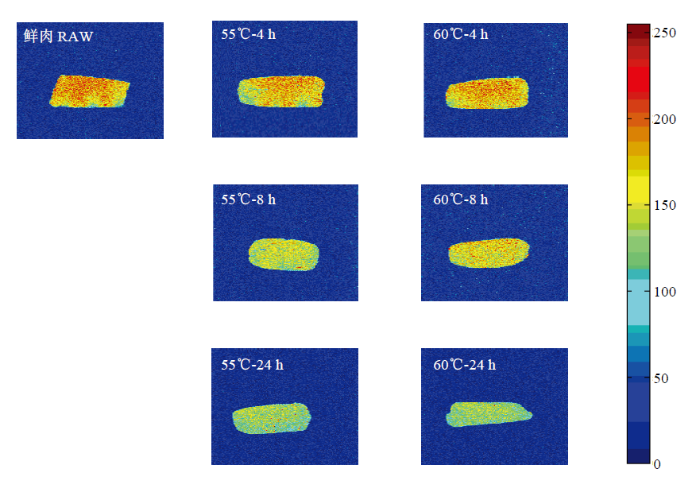 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4不同加热处理肉样的氢质子密度成像测定
Fig. 4Proton density weighted pseudo color images of pork after different temperature and time heating
2.2.2 组织结构变化 天狼星红染色结果如图5所示。天狼星红染色法将肌内结缔组织染为红色,较粗的为肌束膜,包裹在肌纤维外层的为肌内膜。相同加热温度下,随着加热时间延长,肉样中肌原纤维收缩,肌纤维直径减小,肌纤维之间的空隙也逐渐增大,这与体积收缩率的结果相一致。相同加热时间下,60℃组的肌内结缔组织的溶解现象更明显(图5)。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5不同加热处理肉样的组织切片
Fig. 5Sections of muscle tissue in meat during different temperature and time heating
2.3 不同加热组合处理对肉品蛋白质变性影响
2.3.1 蛋白质表面疏水性 蛋白质表面疏水性是常用的测量蛋白变性程度的指标,溴酚蓝与疏水基团定向结合,结合态溴酚蓝含量越高,即蛋白质的表面疏水性越高,变性越严重。由图6可知,蛋白表面疏水性受加热时间和温度的显著影响(P<0.05)。加热4 h和8 h时,60℃组显著高于55℃组(P<0.05),加热24 h时,60℃组与55℃组蛋白质表面疏水性无显著性差异。图6
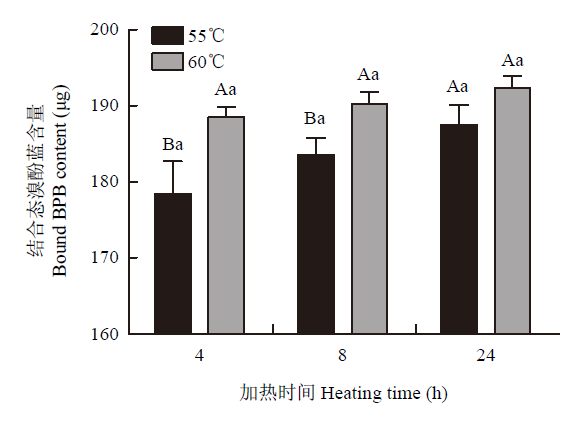 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6不同加热处理对蛋白质表面疏水性的影响
Fig. 6Effect of heating temperature and time on protein surface hydrophobicity
2.3.2 胶原蛋白含量及热溶解性 由表3可知,随着加热时间的延长和温度的升高,肉制品中总胶原蛋白含量显著减少(P<0.05),热溶性胶原蛋白含量显著增加(P<0.05),胶原蛋白的溶解度逐渐增加,而8 h组与24 h组则无显著差异,这与蒸煮损失主要形成于加热前期的结果一致。加热前期,60℃组总胶原蛋白含量显著低于55℃组,表明在加热前期,温度对胶原蛋白变性的影响较大;而24 h组则无显著差异,表明通过延长加热时间,可以降低总胶原蛋白含量对肉制品持水性、嫩度等品质的影响。
Table 3
表3
表3不同加热处理对胶原蛋白的影响
Table 3
| 指标 Index | 55℃ | 60℃ | ||||
|---|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | 4 h | 8 h | 24 h | |
| 总胶原蛋白含量 Total collagen content (mg?g-1) | 1.34±0.09Aa | 0.74±0.05Ab | 0.61±0.03Ab | 0.80±0.05Ba | 0.55±0.02Bb | 0.49±0.06Ab |
| 热溶性胶原蛋白含量 Heat soluble collagen content (mg?g-1) | 0.10±0.07Ab | 0.12±0.01Aab | 0.13±0.00Aa | 0.10±0.05Bb | 0.14±0.00Aa | 0.13±0.00Aa |
| 胶原蛋白溶解度 Collagen solubility | 0.09±0.01Bb | 0.19±0.01Aa | 0.17±0.01Aa | 0.17±0.00Ab | 0.17±0.02Ab | 0.29±0.04Aa |
新窗口打开|下载CSV
3 讨论
3.1 加热处理影响肉品持水性
蛋白质在低温长时加热过程中发生热变性,肌纤维及肌内结缔组织收缩,体积收缩增大,部分可溶性蛋白和水分以蒸煮损失形式渗出[25],肉品持水性下降。在低温长时蒸煮过程中,蛋白质受热变性,分子构象发生变化,蛋白质亚基之间的氢键断裂,蛋白质结构舒展,起初包裹在内部的疏水残基暴露出来[26],降低了蛋白质的水合能力,表面疏水性逐渐增高,蛋白质变性程度增加。肌内结缔组织中的胶原蛋白发生不可逆的改变,由于氢键断裂,蛋白质和水分的相互作用降低,肌原纤维结构变得松散,热溶解性逐渐增大,胶原纤维收缩,肌纤维直径减小,肌肉发生体积收缩,尤其是纵向收缩,从而增加肌原纤维内的水分流失[20]。核磁共振分析结果与蛋白质变性情况一致,即蛋白质的热收缩将主要存在于肌原纤维间隙中的不易流动水挤出[27],所以MRI图中肉样中的红色区域逐渐减少,不易流动水含量减少,A22减小;由于水-蛋白质氢键键能较大而滞留的不易流动水中,氢质子受束缚较大,自由度较小,所以T22左移减小。由于蛋白质热变性,肌纤维结构被破坏,体积收缩加剧,蒸煮损失逐渐增大,肉品持水性下降。本研究中加热温度对肉制品蒸煮损失的影响大于加热时间,55℃与60℃组间蒸煮损失的差异可能与胶原蛋白的变性主要发生在57℃有关[2]。当加热温度处于50—60℃时,随着温度的升高,肌纤维直径显著减小,肌纤维的横向收缩更明显;而当加热温度高于60℃时,顺肌纤维方向的收缩较为显著,这可能与肌球蛋白和胶原蛋白的变性温度不同有关,肌球蛋白的变性温度为50—60℃,胶原蛋白的变性温度为60—70℃[5,27]。60℃组蛋白变性程度更大,肉样组织结构受损更严重。加热后期,55℃组的肉样结构完整性更好,持水性也更强,表明完整的肌束膜结构可以在一定程度上降低肉样的汁液损失。
失水形成蒸煮损失的过程主要集中在前4 h,这与CHRISTENSEN等[11]和ZIELBAUER等[7]的研究结果一致。可能是由于肉制品的温度上升主要在前4 h,达到蛋白质的热变性温度,蛋白表面疏水性增加,肌内结缔组织热溶解性增强,肌内结缔组织收缩,蒸煮损失增大。总胶原蛋白含量的减少,可能与加热过程中蒸煮损失的增大有关[28];热溶性胶原蛋白含量的增大,可能与胶原蛋白受热变性不断降解有关。研究结果表明,在加热过程中热不溶性胶原蛋白对肉制品的持水性等品质的影响可能更大。而随加热时间延长,不同温度下蛋白质变性情况逐渐相同,即在低温长时加热过程中,延长加热时间可以改善较低温度加热下蛋白变性不完全的影响。
3.2 加热处理影响肉品剪切力和色泽
在本研究中,剪切力先上升后下降。剪切力的上升主要是由肌内结缔组织的热收缩导致[29];剪切力的下降则是由于当加热温度处于55—65℃时,随着加热时间的延长,肌球蛋白发生解离,肌纤维强度逐渐减弱[20];胶原蛋白变性,原胶原分子间的氢键发生断裂,肌束膜和肌内膜等肌内结缔组织完整性被破坏,并逐渐溶解。在低温长时蒸煮过程中,加热时间及温度对肉制品的嫩度均无显著影响。优化低温长时蒸煮加工参数时,可以从微生物安全角度对肉制品进行评估调控,进而适当降低加热温度以提高肉制品的出品率。在蒸煮过程中,胶原蛋白的热变性与肉制品的嫩度等品质均相关。较55℃而言,60℃组热溶性胶原蛋白含量无显著差异,而胶原蛋白溶解度逐渐增加,这与PURSLOW[28]提出的观点一致,即肌内结缔组织中的胶原蛋白分为两部分,一部分对热较为敏感,另一部分热稳定性更强。色泽是影响消费者满意度的重要指标之一,与持水性有一定相关性,肉样中水分分布结构的变化会影响肉样中的光学散射,进而影响肉品的色泽[9]。L*值与肉样的结构特征具有一定的相关性,a*值和b*值的变化主要与肌红蛋白的状态有关,结构蛋白热变性使肌纤维直径减小、肌节收缩、粗细肌丝重叠部分增加,导致光在肉样中的散射值增加,即亮度值增加[9]。蛋白变性情况对色泽也有影响,随着温度升高,肉样中的肌红蛋白受热变性,3种肌红蛋白中,高铁肌红蛋白比例略有增加;氧合肌红蛋白比例显著减少,肉品颜色发生改变[29,30]。
4 结论
1)猪肉在55、60℃低温长时蒸煮过程中,蒸煮损失、体积收缩率增大,不易流动水含量减少,肌纤维及肌内结缔组织被破坏,肌原纤维收缩,肌内结缔组织逐渐溶解,蛋白变性,表面疏水性逐渐增高,胶原蛋白含量减少,热溶解度逐渐增强。2)肉制品的水分流失及蛋白质热变性的显著变化主要集中在加热前期,加热温度对肉品食用品质和蛋白变性程度影响更为显著(与55℃组相比,60℃组蒸煮损失、体积收缩率、b*值更大,T22和A22显著减少)。可以进一步研究两种温度下胶原蛋白变性情况差异对肉品食用品质的影响。
3)低温长时蒸煮过程中,胶原蛋白含量减少,热溶解性逐渐增加;加热时间延长会改善较低温度下蛋白质变性不完全的情况,降低肌内结缔组织对肉品持水性、嫩度的影响。可以进一步研究肌内结缔组织不同含量样品及热不溶性胶原蛋白对肉品品质的影响。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
URLPMID:29730528 [本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 2]
URLPMID:22063748 [本文引用: 1]
DOI:10.1016/j.meatsci.2014.05.022URL [本文引用: 5]

The colour, water-holding capacity (WHC) and tenderness of meat are primary determinants of visual and sensory appeal. Although there are many factors which influence these quality traits, the end-results of their influence is often through key changes to the structure of muscle proteins and their spatial arrangement. Water acts as a plasticiser of muscle proteins and water is lost from the myofibrillar lattice structure as a result of protein denaturation and consequent reductions in the muscle fibre volume with increasing cooking temperature. Changes in the myofilament lattice arrangement also impact the light scattering properties and the perceived paleness of the meat. Causes of variation in the quality traits of raw meat do not generally correspond to variations in cooked meat and the differences observed between the raw muscle and cooked or further processed meat are discussed. The review will also identify the gaps in our knowledge and where further investigation would beneficial. (C) 2014 Elsevier Ltd.
URL [本文引用: 1]

畜禽的肉品质受多种因素的影响,其中与肌肉组织学特性关系最为密切.骨骼肌是主要的可食性肉,它由肌纤维、结缔组织和肌内脂肪组成.本文综述了肌纤维类型、肌纤维直径、肌纤维密度、肌纤维面积比例、肌节长度、结缔组织特性、肌内脂肪含量与分布等肌肉组织学特性与畜禽肉品质的关系.
URL [本文引用: 1]

畜禽的肉品质受多种因素的影响,其中与肌肉组织学特性关系最为密切.骨骼肌是主要的可食性肉,它由肌纤维、结缔组织和肌内脂肪组成.本文综述了肌纤维类型、肌纤维直径、肌纤维密度、肌纤维面积比例、肌节长度、结缔组织特性、肌内脂肪含量与分布等肌肉组织学特性与畜禽肉品质的关系.
DOI:10.1016/j.meatsci.2011.03.002URL [本文引用: 2]

The relationship between water-protein interactions and heat-induced protein denaturation in low temperature long time (LTLT) treated pork Longissimus dorsi was investigated by combining low-field NMR T(2) relaxometry with DSC measurements and measures of shrinkage of porcine Longissimus dorsi heated to 53 degrees C, 55 degrees C, 57 degrees C and 59 degrees C for either 3 or 20 h. Water within the myofibrils, measured by NMR T(21) relaxation times, was affected by both temperature and holding time during LTLT treatment between 53 degrees C and 59 degrees C. The changes in NMR T(21) relaxation times were associated with decreased fiber diameter and increased cooking loss, revealing a relationship between transverse shrinkage, water-protein interactions and cooking loss. DSC measurements revealed a concomitant decrease in Delta H(68 degrees C) which suggests impact of collagen denaturation on the retention of water within the meat during LILT treatment. Furthermore, a decrease in Delta H(75 degrees C) suggested that prolonged cooking (20 h) resulted in actin denaturation leading to decreased T(21) relaxation times and higher cooking loss. (C) 2011 Elsevier Ltd.
DOI:10.1016/j.meatsci.2010.12.035URL [本文引用: 1]

The effect of low temperature long time (LILT) heat treatment at 48 degrees C, 53 degrees C, 58 degrees C, and 63 degrees C for T(c) (time to reach a core temperature equal to the water bath), T(c) + 5 h holding time, and T(c) + 17 h holding time was studied in Longissimus dorsi and Semitendinosus muscles from slaughter pigs and sows. Meat toughness (Warner-Bratzler Shear Force), cooking loss and color (Minolta L*a*b*-values) was measured and in the cooking loss the amount of heat-soluble collagen and activity of cathepsin B + L was determined. Decreasing shear force and increasing cooking loss during LTLT treatment was observed between 53 degrees C and 58 degrees C. Furthermore, increasing temperature from 53 degrees C to 58 degrees C and increasing time from T(c) to T(c) + 17 h increased the solubility of collagen. Residual activity of cathepsin B + L in LTLT treated pork was mainly affected by temperature, showing the highest activity at 58 degrees C and 63 degrees C. (C) 2011 Elsevier Ltd.
[本文引用: 1]
DOI:10.1016/j.meatsci.2018.10.001URLPMID:30336963 [本文引用: 2]

The pork steaks were stored at 4 degrees C for 7days to investigate the effect of oxygen concentrations (0%, 20%, 50% and 80%) in modified atmosphere packaging and air packaging on water holding capacity (WHC) of raw meat and cooked meat. The ultrastructure of muscle, and oxidation of lipids and proteins were also studied. The results showed that purge loss of meat increased with increasing oxygen concentration (P<.05). The extent of oxidation of lipids and proteins was greater in meat packaged under 50% and 80% oxygen. It suggested that the decreased WHC of meat was closely associated with oxidation degree during postmortem storage. Notable sarcomere shortening was consistent with the decreased WHC of meat packaged with 50% and 80% oxygen. It is plausible that the higher drip loss of meat in air packaging was induced by other factors other than the oxidation of protein.
[本文引用: 1]
DOI:10.1016/j.meatsci.2018.12.001URLPMID:30562641 [本文引用: 1]

The combined effects of aging and low temperature, long time heating (LTLT) on meat toughness were investigated. Pork loins were heated at 53 or 58 degrees C for up to 20h, and shear force values, cooking loss, moisture content, collagen solubility, electrophoresis of myofibrillar proteins were determined. Structural changes in perimysium were also observed by light microscopy and scanning electron microscopy (SEM). Results showed that aging and LTLT cooking independently affected meat toughness, and higher temperature or longer time were required to decrease toughness of one-day aged meat to the same level as in 10-day aged meat. Collagen solubilization is suggested as the main reason for the tenderization effect of LTLT. Myofibrillar proteolysis might not occur during LTLT cooking, and will not be contributing to meat tenderness.
URLPMID:28941693 [本文引用: 1]
DOI:10.1016/j.meatsci.2015.02.011URLPMID:25768395 [本文引用: 1]

Meat tenderness is known to be affected by sarcomere length (SL), proteolysis and collagen content (CC). Sixty lambs were slaughtered and the Longissimus muscle was sampled. Samples for shear force (SF), SL, proteolysis indicators (desmin degradation, particle size: PS) and CC were taken after the allotted ageing periods (1, 7, and 14 days). PS explained a large part of the variation in shear force (approximately 34%) when modelled across ageing periods. Other factors (CC, SL) combined with proteolysis indicators (PS, desmin degradation) explained just under 40% of the variation in shear force. Within ageing periods SL explained a small, but significant, part of the variation in shear force after 14 days of ageing (8%) and at day 1 of ageing desmin degradation explained 17% of the variation in shear force. Methods to improve the tenderness of lamb longissimus muscle should focus on increasing the extent of post-mortem proteolysis, when processing conditions are sufficient to prevent muscle fibre shortening.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/s0309-1740(99)00157-6URLPMID:22061287 [本文引用: 3]

The structural changes in beef semitendinosus caused by cooking were studied by performing tensile tests of the isolated meat components (i.e. single muscle fibres and perimysial connective tissue) and related to the toughness of the whole meat. Whole meat toughness was found to increase in two separate phases upon cooking from 40-50 degrees C, and again from 60 to 80 degrees C with a decrease in meat toughness between 50 and 60 degrees C, in agreement with previous studies. The changes in whole meat toughness at temperatures below 60 degrees C were found to correspond to changes in the mechanical properties of the perimysial connective tissue, whereas changes of whole meat toughness at temperatures above 60 degrees C were found to correspond to increased breaking strength of single muscle fibres. The myofibrillar component explained approximately 47% of the variation in whole meat toughness upon cooking whereas inclusion of the connective tissue component increased the goodness of fit.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.meatsci.2006.05.020URLPMID:22063224 [本文引用: 1]

By combining simultaneous nuclear magnetic resonance (NMR) T(2) relaxometry and differential scanning calorimetry (DSC) on pork samples heated to nine temperature levels between 25 and 75 degrees C, the present study investigates the relationship between thermal denaturation of meat proteins and heat-induced changes in water characteristics. Principal component analysis (PCA) on the distributed (1)H NMR T(2) relaxation data revealed that the major changes in water characteristics during heating occur between 40 and 50 degrees C. This is probably initiated by denaturation of myosin heads, which however, could not be detected in the DSC thermograms obtained directly on the meat. In contrast, the DSC thermograms revealed endothermic transitions at 54, 65 and 77 degrees C, probably reflecting the denaturation of myosin (rods and light chain), sarcoplasmic proteins together with collagen and actin, respectively. Simultaneous modelling of DSC and NMR data by partial least squares regression (PLSR) revealed a correlation between denaturation of myosin rods and light chains at approximately 53-58 degrees C and heat-induced changes in myofibrillar water (T(2) relaxation time approximately 10-60ms) as well as between actin denaturation at approximately 80-82 degrees C and expulsion of water from the meat. Accordingly, the present study demonstrates a direct relationship between thermal denaturation of specific proteins/protein structures and heat-induced changes in water mobility during heating of pork.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1016/j.meatsci.2011.11.024URLPMID:22154568 [本文引用: 1]

This paper describes the influence of different factors on sous-vide cooked pork. Pork cheeks were cooked at different combinations of temperature (60 degrees C or 80 degrees C), time (5 or 12h) and vacuum (vacuum or air packaged). Weight losses were lower and moisture content higher in samples cooked for a shorter time (P=0.054) and at a lower temperature (P<0.001). Samples cooked at 60 degrees C showed more lightness (L*) and redness (a*) (P<0.001). Lipid oxidation showed an interaction between cooking time and temperature (P=0.007), with higher TBARs values for samples cooked for 12h at 60 degrees C and lower for those cooked for 12h at 80 degrees C. Samples cooked at 80 degrees C for 12h showed lower (P<0.05) values for most textural parameters than all the other types of samples. Vacuum packaging showed no influence on any of the studied variables. For the treatments evaluated, cooking temperaturextime combination seems to be more important than vacuum packaging in the textural and colour parameters of pork cheeks.
URLPMID:22209093 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.foodchem.2013.04.034URLPMID:23790834 [本文引用: 2]

The objectives of this study, were to examine the relationship between duck meat tenderness, actomyosin dissociation and endogenous enzyme activities when heating the duck breast muscle, to the internal temperature of 30, 40, 50, 60, 70, 80, 90 degrees C. The shear force increased in the temperature range of 30-50 degrees C and 70-90 degrees C and decreased from 50 to 70 degrees C, which was negatively related with liberated actin (P<0.05). The activity of cathepsin B and L was stable while heating the meat to a temperature below 50 degrees C, then it decreased rapidly with temperature increase. The calpain activity kept decreasing with the temperature increase. There was no significant change in the cathepsin D activity below 70 degrees C but it declined rapidly thereafter, and its activity was strongly correlated with actomyosin dissociation (P<0.05). The results suggest that actomyosin dissociation and cathepsin D, could contribute to the tenderness of duck meat during the cooking process.
DOI:10.1016/j.meatsci.2018.03.026URLPMID:29636208 [本文引用: 2]

Variations in the quantity and thermal stability of collagen in intramuscular connective tissue (IMCT) play a role in variations in cooked meat tenderness. This review is focussed on sources of variability, especially in the perimysial IMCT, and challenges some of the accepted ideas about its denaturation behaviour, its contribution to cooking shrinkage at high temperatures and the concept of IMCT as an immutable
[D].
[本文引用: 2]
[D].
[本文引用: 2]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
