 ,1,2,*, 金剑
,1,2,*, 金剑 ,2,*, 刘晓冰2
,2,*, 刘晓冰2Physiological response of crop to elevated atmospheric carbon dioxide concentration: a review
LI Yan-Sheng ,1,2,*, JIN Jian
,1,2,*, JIN Jian ,2,*, LIU Xiao-Bing2
,2,*, LIU Xiao-Bing2通讯作者:
收稿日期:2020-04-15接受日期:2020-08-19网络出版日期:2020-08-28
| 基金资助: |
Received:2020-04-15Accepted:2020-08-19Online:2020-08-28
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (1292KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李彦生, 金剑, 刘晓冰. 作物对大气CO2浓度升高生理响应研究进展[J]. 作物学报, 2020, 46(12): 1819-1830. doi:10.3724/SP.J.1006.2020.02027
LI Yan-Sheng, JIN Jian, LIU Xiao-Bing.
自18世纪60年代工业革命开始, 人类活动特别是煤炭、天然气和石油等化石燃料的大量开发与利用使大气中CO2浓度迅速增加。研究表明, 工业革命前大气中的CO2浓度约为280 μmol mol-1, 在随后的200年(1760—1960年)大约增加了50 μmol mol-1 [1], 然而在刚过去的60年间(1960—2020年)却急剧增加了100 μmol mol-1, 达到目前的415 μmol mol-1左右(图1)。根据美国国家海洋和大气管理局全球温室气体监测系统的数据, 我们可以发现大气中CO2浓度的升高仍然在加速(图1), 所以联合国政府间气候变化专门委员会(IPCC)认为全球大气CO2浓度在2050年升高到550 μmol mol-1, 2100年达到700 μmol mol-1将是大概率事件[1]。在未来很长一段时间里人类的生产活动都将在高大气CO2浓度下完成。二氧化碳作为植物光合作用的底物, 其浓度的升高理论上有利于植物光合作用能力的提高, 从而促进植物(作物)生物量和产量的形成。然而, 在全球气候变化的大背景下, 高温、干旱和臭氧浓度升高等其他因子严重制约作物生产。所以解析植物(作物)生长对大气CO2浓度升高响应的生理生化机制, 最大程度利用大气CO2浓度升高所带来的“肥料效应”将有利于消减气候变化对作物生产带来不利影响, 也是保障不断增加的全球人口粮食安全的关键。
图1
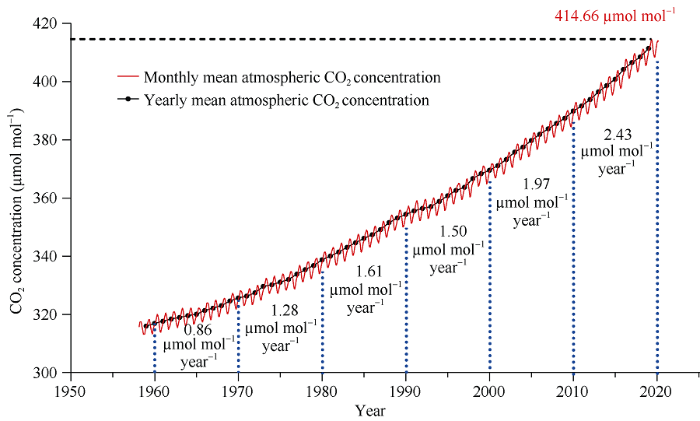 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图11958-2020年大气CO2浓度年平均和月平均变化
图中蓝色虚线内数字代表每10年间大气CO2浓度年平均值的年增加量; 黑色虚线代表目前大气CO2浓度最高值为2019年5月测量值。
Fig. 1Annual and monthly changes of atmospheric concentration of CO2 from 1958 to 2020
The data between the two blue dashed lines represent the increases of CO2 concentration per 10 years. The black dashed line represents the highest atmospheric CO2 concentration measured in May 2019. All the data in this figure are from
大气CO2浓度升高促进全球植物生产能力的推断主要基于目前陆地覆盖面积占比80%以上的C3植物的光合作用未达到其饱和CO2浓度, 仍然存在较大的提升空间[2]。此外, 虽然C4植物光合作用受到环境CO2浓度变化的影响不明显, 大气CO2浓度仍然会通过提高水分利用效率促进其在干旱等环境胁迫条件下生产能力的提高。已有的研究证明了大气CO2浓度升高对植物生产能力的促进, 例如Jablonski等[3]利用Meta分析方法系统总结了79种植物生殖生长指标对大气CO2浓度升高的响应, 结果表明大气CO2浓度升高到500~800 μmol mol-1, 植物(作物)的开花数量平均增加了19%, 籽实数量平均增加了16%~18%, 或者单个籽实重量平均增加4%。同时他也发现大气CO2浓度升高对栽培品种产量的促进作用比野生类型更大(21% vs. 4%)。
然而, 通过田间开放式大气CO2浓度升高处理(free air CO2 enrichment, FACE)研究发现, 植物(作物)长期生长在高CO2浓度下存在光合适应现象(photosynthesis acclimation), 即植物在高CO2浓度下生长的初始期出现的光合作用增强会逐渐减弱, 甚至消失[4,5]。目前关于植物光合适应现象的产生具体机制还不清楚。不同种类植物(作物),甚至同种作物不同品种的光合作用对大气CO2浓度升高的响应都存在显著差异。为此, 本文将从作物光合生理角度入手, 系统总结国内外关于大气CO2浓度升高对作物生长影响的研究进展, 分析相关的生理机制, 为准确评估大气CO2浓度升高对作物产量和品质形成的影响提供参考。
1 大气CO2浓度升高促进C3植物光合作用的生理基础
1.1 光合作用的关键酶——Rubisco
植物光合作用是植物利用叶绿素吸收光能驱动对大气中CO2分子进行固定并与H2O分子结合形成碳水化合物的过程。在C3植物中核酮糖-1,5-二磷酸羧化酶/加氧酶(ribulose-1,5-bisphosphate carboxylase/oxygenase, Rubisco)是催化CO2固定的关键限速酶, 它的活性决定了光饱和点的最大光合速率[6]。Rubisco最大的特点是与CO2分子的结合不具有专一性, 氧气(O2)分子会与CO2分子竞争Rubisco结合位点发生光呼吸反应(photorespiration)。光呼吸对于C3植物来说是有害的, 因为光呼吸会限制光合作用底物Rubp的再生从而限制卡尔文循环对CO2的固定(图2)。光呼吸被称为乙醇酸途径(或者C2循环), 该反应会将2分子的2-磷酸乙醇酸(2PG)转变为1分子的3-磷酸甘油(3PGA), 1分子的CO2和1分子$NH_{4}^{+}$。在典型的C3植物中, 由光呼吸产生的$NH_{4}^{+}$最终以消耗ATP的方式被重新固定, 释放到空气中的CO2占参与该循环总C量的25% [7]。由于CO2分子是利用浓度差通过气孔扩散进入C3作物叶片细胞内, 不像C4植物叶片具备可以富集CO2能力的特殊“花环结构”, 所以C3植物叶片胞间CO2浓度(Ci)较低, 只相当于大气CO2浓度的70%左右(≈280 μmol mol-1), 还未达到Rubisco催化光合反应的饱和点[8]。通常C3作物的光合C固定能力(A)的变化可以用Ci的一元二次方程即A/Ci响应模型进行估计[9]。当Ci较低时,光合作用的另一底物Rubp含量充足, 光合反应速率受到CO2浓度的限制, 而不是Rubisco的最大羧化效率限制(Vc, max)。例如Zhao等[10]报道, 在温室条件下当大气CO2浓度从360 μmol mol-1升高到720 μmol mol-1时, 棉花叶片的净光合速率增加46%, 而Vc, max却无显著变化。如果Ci持续增加, A/Ci响应曲线将会达到拐点, Rubp的再生速率(Jmax)成为限制因素并决定光合C固定能力[11]。在黄瓜上的研究表明, 半自然条件下,当大气CO2浓度升高到800 μmol mol-1, 叶片的电子传递速率变大, 促进Jmax的提高最终增加光合C的固定能力[12]。但也有研究认为大气CO2浓度升高对Jmax的提高很可能是短期效果。通过3年连续FACE处理发现, Jmax是大气CO2浓度升高对水稻光合C固定能力促进的主要限制因素[13]。由于不同植物本身的Vc,max和Jmax差别较大, 这也造成了不同植物光合作用对大气CO2浓度升高的差异。理论上大气CO2浓度升高后, 光合反应受到Vc,max限制的树木和草的光合速率增幅要大于受到Jmax限制的灌木、豆科作物和其他非豆科C3作物的光合速率增幅。通过Meta分析方法总结不同种类植物光合C固定能力对大气CO2浓度升高的响应, 结果表明树木平均增加46%, 草类平均增加39%, 灌木平均增加21%, 豆科作物平均增加19%, 增幅最小的是非豆科C3作物仅为13% [14]。虽然可以从功能类型或者分类角度总结植物光合C固定能力对大气CO2浓度升高响应的变化趋势, 我们仍然需要对同种植物内部存在的较大变异进行深入研究, 此外环境和遗传因素同样对大气CO2浓度升高产生的“肥料效应”有着巨大影响。图2
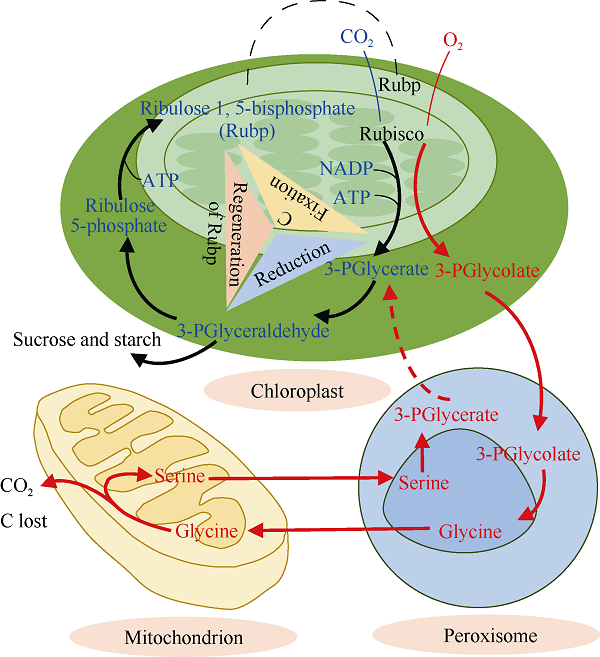 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2C3植物卡尔文循环(黑色线条循环)和光呼吸(红色线条循环)
示意图参考
Fig. 2Calvin cycle (black lines) and photorespiration (red lines)
This schematic diagram is modified according to
1.2 叶片气孔对大气CO2浓度升高的响应
植物叶片与大气之间的气体交换和蒸腾作用都是通过气孔完成的, 气孔是由一对保卫细胞(guard cells)围成的空腔结构。气孔对CO2浓度敏感是保卫细胞的固有属性, 测量气孔导度(Gs)评估大气CO2浓度升高对气孔的影响是公认的有效方法。气孔导度可以用Ball等[15]的模型进行预测:${{G}_{\text{s}}}\text{= }{{G}_{\text{0}}}\text{+m}\frac{Ah}{\text{C}{{\text{O}}_{2}}}$
在该模型中, A代表植物光合C同化能力; h代表相对湿度; CO2代表叶片表面大气CO2浓度; G0代表y轴截距; m代表直线的斜率, 其中G0和m具有专一性, 在不同的物种中不同。该公式表明植物叶片气孔导度与大气CO2浓度成反比关系, 但也有研究认为气孔导度与胞间CO2浓度的关系更大[16]。不管叶片表面CO2浓度还是胞间CO2浓度变化对气孔导度影响的重要性大小如何, 目前绝大多数试验表明大气CO2浓度升高植物叶片Gs下降, 但是不同试验方式, 不同物种叶片Gs下降幅度差别也很大[17,18]。在美国伊利诺大学FACE试验平台, 正常大气CO2浓度条件下三叶草(Trifolium repens)叶片Gs在不同季节无显著差异(P > 0.05); 当大气CO2浓度升高到600 μmol mol-1时, 整个生长季叶片Gs平均降低25%, 同时秋季叶片Gs显著(P < 0.05)低于春季[19]。Sanz-Saez等[20]报道在FACE条件下大气CO2浓度升高后大豆品种HS93-4118和Loda叶片Gs连续3年均表现出显著下降的趋势。通过连续2年的FACE试验表明, 在生殖生长期虽然大气CO2浓度升高到600 μmol mol-1条件下大豆Gs下降, 但光合速率可以维持相对较高水平, 一定程度上缓解了高温(+4oC)条件下Gs降低所造成的光合C同化能力降低, 从而保证大豆的产量形成[21]。
C4植物可以利用其特殊的维管束鞘花环结构富集CO2, 通常胞间CO2浓度可以达到大气CO2浓度的3~8倍[22]。所以有研究者认为大气CO2浓度升高对玉米光合速率的影响很小或者可以忽略不计, 但是有研究报道大气CO2浓度升高后叶片Gs下降有利于提升C4植物的水分利用效率, 从而促进C4植物光合速率和生物量的增加[23,24]。Cunniff等[25]利用4种C3作物和4种C4作物在生长箱中进行对比研究, 结果表明C4作物的产量在高大气CO2浓度下有所增加, 但是增幅(10%~15%)小于C3作物; 同时该研究还发现只有一种C4作物的光合速率显著增加。目前, 更多研究倾向于大气CO2浓度升高对C4植物碳水化合物积累能力的影响是水分利用效率提高所带来的间接效果[17,26-27]。Maroco等[28]在生长箱条件下将大气CO2浓度从350 μmol mol-1升高到1000 μmol mol-1时发现玉米叶片的气孔导度下降71%, 水分利用效率提高225%, 最终地上部生物量显著增加20%。长期FACE试验结果表明大气CO2浓度升高到550 μmol mol-1, 玉米和高粱的叶片气孔导度下降20%以上, 整株水分利用效率显著提高, 同时5~25 cm耕层土壤含水量增加31%, 25~55 cm耕层土壤含水量增加11%, 而产量却无明显变化[29]。
大气CO2浓度升高引起植物叶片Gs下降从而提高水分利用效率并不是C4植物的专一响应, C3植物中同样有这种现象。所以, 大气CO2浓度升高可能会提高作物抵抗轻、中度干旱胁迫对产量形成带来的不利影响, 大气CO2浓度升高后C4作物的增产潜能同样很大, 特别是在半干旱地区。
2 光合适应现象
植物长期生长在高大气CO2浓度下出现的光合适应现象内在机制虽然还不是很清楚, 目前有两种假说对这方面进行解释。第一, “源-库”调节假说: “源端”光合产物合成速率变大, 不能即时运往“库端”造成的负反馈, 以及“库强”不足造成的负反馈(图3)。第二, N素抑制假说: N素缺乏抑制光合C固定对大气CO2浓度升高的持续响应。图3
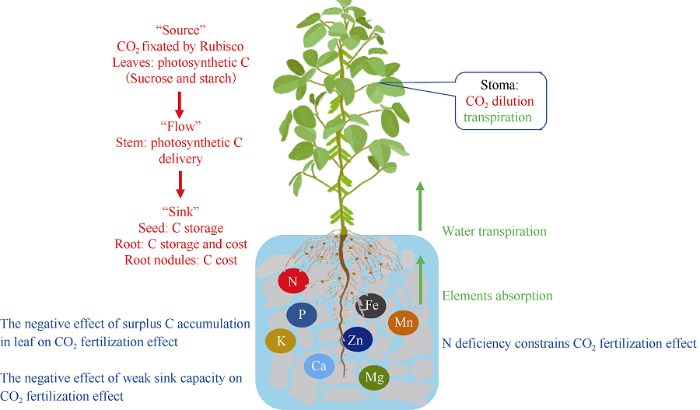 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3大气CO2浓度升高条件下C3作物(大豆)光合适应现象相关假说
Fig. 3Hypothesis of the photosynthetic acclimation under elevated atmospheric CO2 concentration in C3 crop (soybean)
2.1 “源-库”调节理论
有研究人员认为光合适应现象可以用“源-库”调节理论解释[30,31]。“源-库”关系变化对植物叶片光合速率的调节较为复杂, 涉及植物内部生理生化反应以及整株形态水平上的变化, 但我们仍然可以通过不同指标进行评估。植物叶片碳水化合物合成能力增加后会改变植物的地上部/地下部的比值, 引起整株水平上的C平衡变化[32]。生长箱模拟条件下大气CO2浓度升高后小麦的叶片和茎秆会积累更多的碳水化合物而不是将增加的碳水化合物运输到“库端”籽粒中[33]。碳水化合物特别是非结构性碳水化合物(蔗糖、果糖及淀粉等)在叶片中的积累会抑制光合相关基因的表达, 导致光合C固定能力下降[34]。Zhu等[30]利用FACE研究发现, 当大气CO2浓度增加200 μmol mol-1后水稻和小麦叶片中淀粉和可溶性糖含量显著增加, 但与光合作用降低之间的相关关系不显著。此时光合作用的下调很可能是由非结构性碳水化合物积累引起己糖激酶表达上调, 最终加速了叶片衰老速度引起的[30,35-36]。水稻叶片碳水化合物合成能力在高大气CO2浓度下迅速增加, 但是在午后碳水化合的转运能力成为关键的限制因素, 最终表现出光合速率下调[37]。树木中也有类似研究报道, 例如杨树在高大气CO2浓度下光合C固定能力提高55% [38]。这主要得益于杨树较强的光合C输出能力, 白杨可以将90%以上白天合成的光合C转运出“源端”, 同时把剩余的光合产物以淀粉的形式储存起来, 这样就可以避免光合适应的发生, 最大限度保证大气CO2浓度升高对光合C固定能力的促进作用[39]。此外, “源-库”平衡改变后叶片磷酸丙糖浓度变化与叶片最大光合速率密切相关, 当蔗糖在叶片迅速积累后, 磷酸丙糖和Vc,max均表现出降低[40]。除了碳水化合物在“源端”大量积累导致的光合作用的下调, “库强”大小也是限制植物光合作用对大气CO2浓度升高的重要因素。相同作物, 当大气CO2浓度升高后, “源端”-叶片的光合产物供应能力大幅度提高, 理论上“库强”较大的品种光合C固定能力增幅更大[41]。Ainsworth等[42]利用不同大豆品种为试验材料, 分析了FACE条件下大气CO2浓度升高后库强在“源-库”调节中所发挥的作用, 结果表明“库强”较小(分支少, 结荚少)的大豆品种光合适应现象明显, 叶片Vc,max和Jmax均表现出下降。在长期FACE试验条件下, 通过改变环境因子限制“库强”的方式同样被证明会使植物产生光合适应现象[14,17]。豆科作物对大气CO2浓度升高响应高于非豆科作物, 不仅因为共生固氮作用可以使其一定程度上避免N胁迫, 还因为共生固氮系统进行固氮作用时消耗较多的碳水化合物, 是除去茎、根和籽粒以外的重要碳库。研究表明豆科植物共生生物固氮系统进行固氮时地上部分的光合碳以蔗糖形式运输到根部, 来满足氮固定过程中所需要的能量[4,43]。光合产物蔗糖对根瘤固氮的影响最早是在rug4基因突变豆科植物中发现, 由于此基因突变导致蔗糖合成能力下降, 豆科植物体内来自根瘤共生生物固氮的氮素比例显著降低[44]。有研究者对大豆进行暗处理, 发现6 h后根瘤固氮酶活性显著降低60%, 但如果在暗处理同时淋浇蔗糖溶液, 根瘤固氮酶活性则会明显提高[45]。根瘤不具备蔗糖合成能力却广泛存在蔗糖转化酶(sucrose invertase, INV)和蔗糖合成酶(sucrose synthetase, SUS), 这两种酶在植物体内主要功能是蔗糖不同部位转运过程中的卸载和分解, 这从另一角度证明了根瘤是重要的碳库。
2.2 氮素抑制假说
有理论认为这种光合适应现象是由于叶片Rubisco酶蛋白含量下降引起, 而不是Rubisco酶的催化能力发生了改变[46,47]。研究表明Rubisco酶每秒大约只能羧化3.3个Rubp, 其效率之低在酶促反应中极为罕见。植物为了弥补Rubisco酶催化效率低下的问题不得不合成大量的Rubisco酶蛋白来完成对CO2的固定。据统计植物叶片中氮有50%用来合成Rubisco酶, 所以Rubisco酶也成为世界上存量最多的单一蛋白[48,49]。当大气CO2浓度升高之后, Rubisco的催化效率显著提高, 植物减少了Rubisco酶蛋白的合成量, 最终抑制了光合作用对大气CO2浓度升高的持续响应[50]。利用Meta分析Vc, max对大气CO2浓度升高的响应表明, 在大田条件下植物叶片Vc,max平均下降10% [14]。在不同的研究中均发现作物叶片Rubisco酶含量在高大气CO2浓度下表现出下降的现象, 并最终大幅提高叶片氮的利用效率[50]。例如, 在开顶式气室模拟条件下, 当大气CO2浓度从330 μmol mol-1升高到660 μmol mol-1后水稻叶片Rubisco酶蛋白含量降低22%左右, 大豆叶片Rubisco酶蛋白含量则降低8%~17% [51]。利用FACE研究表明黑麦草(Lolium perenne)叶片Rubisco酶蛋白含量在高大气CO2浓度下降低25%, 同时Vc,max降低30%, 这也间接证明了Rubisco酶催化效率的升高[52]。大气CO2浓度升高导致叶片氮利用效率的升高最终使得植物叶片氮浓度表现出显著降低的趋势[6]。很多研究认为大气CO2浓度升高后C3作物植株体内氮素浓度下降是抑制产量持续增加的主要原因[53,54,55]。那么如果提高供氮水平是否会缓解叶片氮浓度降低呢?有研究发现, 在开顶式气室模拟条件下高CO2浓度下增施硝态氮, 黄瓜的产量可以显著增加同时还能维持叶片中氮的浓度不降低[56]。然而, 更多研究证明增施氮肥不能有效抑制CO2升高条件下植物叶片氮浓度的降低, 例如高CO2浓度条件下大麦、小麦和水稻籽粒中氮含量会降低14%左右[57]。不过值得注意的是豆科植物植株氮浓度在高大气CO2浓度下降低程度小于非豆科植物[58]。例如, 草地生态系统中的豆科植物在高CO2条件下并没有发现叶片氮含量和C︰N有明显的变化, 而非豆科植物叶片氮浓度则明显降低[4]。在农田生态系统中, 高大气CO2浓度下大豆生长早期叶片浓度下降, 但在共生生物固氮发挥作用之后这种叶片氮含量下降的现象也随即消失[59]。利用开顶式气室对比24个大豆品种叶片氮浓度对大气CO2浓度升高的响应发现, 当大气CO2浓度从390 μmol mol-1升高到550 μmol mol-1后大豆叶片氮浓度平均降低3.2%, 表明即使是具有固氮能力的豆科植物依然受到叶片氮浓度下降的制约[60]。
此外, 大气CO2浓度升高条件下, 植物蒸腾速率下降引起氮素吸收能力降低的理论也受到研究人员广泛关注, 这种理论认为, 高CO2浓度条件下, 植物不再需要较大的蒸腾作用换取气孔的打开进而增加对CO2的吸收和固定[61]。植物气孔导度的下降会使蒸腾速率降低, 进一步导致质流的下降, 限制植物对可移动的氮(硝态氮)的被动吸收[62]。虽然植物对大气CO2浓度升高的响应依赖于环境和物种, 但是气孔导度和蒸腾作用的下降在很多研究中都有报道[63,64,65]。这个理论的支撑点是可移动元素从土壤运转到植物的地上依赖于质流, 通过蒸腾作用来实现。许多的研究者讨论了蒸腾作用对高CO2下植物营养元素吸收的潜在作用大小[55,66-67]。然而, 也有研究者认为质流作用对氮素吸收的影响并不重要, 因为植物体内的蒸腾作用变化与质流作用主导的元素吸收变化并不匹配, 其次, 质流作用只是植物对氮的被动吸收, 作用远没有植物根系的主动吸收重要[68,69,70]。
大气CO2浓度升高引起的植物体内氮浓度的降低也被归因于土壤中氮素可利用性的降低[58,71]。长期FACE研究表明, 大气CO2浓度升高条件下农田土壤中的$\text{NH}_{\text{4}}^{\text{+}}-N$降低了7.9%,$\text{NO}_{\text{3}}^{-}-N $则增加了26.7%, 而C3植物更趋向于吸收利用$\text{NH}_{\text{4}}^{\text{+}}-N$而不是$\text{NO}_{\text{3}}^{-}-N $, 所以高CO2浓度下土壤中$\text{NO}_{\text{3}}^{-}-N $上升的同时还发现植物中的$\text{NO}_{\text{3}}^{-}-N $利用下降26.7%[72]。然而, 有研究认为, 在氮供应充足的情况下植物组织中氮素浓度的下降并不是土壤中N的可利用性的降低, 而是植物本身碳库和氮库平衡变化导致的氮素吸收下调, 例如硝酸还原酶活力的下调等, 其中的生理机制需要进一步研究[73]。
所以, 如何协调高CO2浓度作物对氮素的吸收利用, 最大程度发挥“CO2肥料效应”将是未来很长一段时间的研究重点。
3 大气CO2浓度升高对作物品质的影响
植物对大气CO2浓度升高的响应不仅包括生物量或产量的变化, 还包括籽实品质的变化, 特别是与人类健康息息相关的籽实营养品质变化。满足全球70亿人口40%碳水化合物需求的水稻和小麦均是C3作物, 受益于大气CO2浓度的升高, 这两种作物籽粒中的糖及其他碳水化合物含量增幅潜力很大,特别是蔗糖、果糖、葡萄糖、麦芽糖等这些易于人类消化吸收的非结构性碳水化合物浓度预计增加10%~45%[74,75]。此外, 与碳贮存和能量代谢密切相关的脂肪浓度在大气CO2浓度升高后也会出现升的趋势[76], 这种现象在脂肪含量较高的作物中表现明显。利用开顶式气室研究表明, 当大气CO2浓度升高到550 μmol mol-1后, 4个大豆品种成熟籽粒脂肪浓度增加8.3%~13.0% [77]。同时脂肪酸组分发生变化, 其中油酸(18:1)浓度显著增加, 亚油酸(18:2)浓度显著降低, 这种变化趋势可以增加大豆油的保存时间, 降低亚油酸对人类心血管带来的负面影响[77,78,79,80]。H?gy等[81]利用FACE研究发现油菜籽中的脂肪浓度在大气CO2浓度升高条件下未发生改变, 但脂肪酸组分发生了改变, 显著变化体现在油酸(18:1)浓度的增加1.3%, 而亚麻酸(18:3)、二十碳烯酸(20:1)和神经酸(24:1)分别降低4.1%、2.2%和5.3%。
越来越多的研究表明大气CO2浓度升高后作物籽实中除碳水化合物以外的营养物质的浓度有下降的风险。例如, 大气CO2浓度升高到550 μmol mol-1后,C3作物小麦和水稻籽粒蛋白质浓度下降幅度趋同, 均在7.8%左右; C4作物玉米下降5%左右, 高粱则基本保持不变[82]。豆科作物由于具有共生生物固氮能力, 所以被认为可以避免籽粒蛋白质浓度的下降[83]。利用开顶式气室, 以24个大豆品种为材料分析大气CO2浓度升高到550 μmol mol-1后大豆籽粒中氮浓度变化, 结果表明大豆籽粒中氮浓度依然有下降的趋势, 平均降低6.2%左右, 如果按照氮浓度和粗蛋白浓度之间的换算方法, 意味着大豆籽粒中粗蛋白浓度下降6.2%左右, 小于非豆科C3作物[84]。大气CO2浓度升高导致作物籽实蛋白质浓度的降低给许多贫困国家和地区人群带来健康隐患, 特别像印度等国家日常蛋白质摄入主要来自于C3谷物, 未来面临的营养风险变得更加严峻[85]。游离氨基酸在作物籽粒灌浆过程中具有合成蛋白质的功能, 当蛋白质浓度降低时我们可以推断游离氨基酸的浓度也会下降。半自然条件下的研究发现大气CO2浓度升高到550 μmol mol-1大豆籽粒在灌浆盛期和成熟期游离氨基酸浓度均呈降低趋势[77]。在籽粒灌浆盛期谷氨酰胺和精氨酸浓度的降低表明大气CO2浓度升高后作物合成代谢速度变快, 功能性蛋白需求量变大, 游离氨基酸更多被用来合成与代谢相关的功能性蛋白而不是贮存蛋白[77,86-87]。
大气CO2浓度升高对作物籽实矿质元素浓度的影响也是关注的焦点问题。模型研究表明大气CO2浓度升高有利于增加植物根系生物量, 通过扩大根系所能控制土壤的体积实现对矿质元素的被动吸收能力增强[67]; 而FACE研究表明大气CO2浓度升高有利于增加松树(Pinus taeda)细根(直径 < 0.05 mm)的比例从而提高对矿质元素的主动吸收能力[88]。这两种变化都有利于植物对矿质元素的吸收。所以理论上大气CO2浓度升高后, 作物籽粒的矿质元素含量至少不会降低。目前最受到人们关注的是作物锌(Zn)和铁(Fe)元素浓度的变化。研究表明世界上至少有20亿人, 特别是未成年人面临Zn和Fe元素缺乏带来的健康风险, 包括阻碍发育、免疫力下降、增加儿童死亡率以及引起贫血造成孕妇死亡等[74,89-90]。Myers等[82]统计了世界不同地区关于作物籽粒Zn和Fe元素浓度对大气CO2浓度升高的响应, 其中包括64个小麦品种、31个水稻品种、25个大豆品种、4个玉米品种和4个高粱品种, 结果表明大气CO2浓度升高后Zn元素浓度在小麦、水稻、大豆、玉米和高粱籽粒中的变幅分别为-9.3%、-3.2%、-5.1%、-5.1%和-1.3%; Fe元素浓度在小麦、水稻、大豆、玉米和高粱籽粒中的变幅分别为-5.0%、-5.3%、-4.2%、-5.7%和+1.6%。所以, 当大气CO2浓度升高条件下, C3和C4作物籽粒中Zn和Fe元素浓度均有下降的趋势。Zhu等[91]利用FACE试验方法分析了亚洲18个主要水稻品种籽粒营养品质对大气CO2浓度升高(≈580 μmol mol-1)的响应。结果同样发现水稻籽粒中蛋白质含量、Zn和Fe元素浓度降低, 同时还发现水稻籽粒中维生素B1、B2、B5和B9浓度显著降低。随着大气CO2浓度不断升高, 收入较低且将水稻作为主要食物的国家面临严峻的国民健康问题, 受到影响的人口可能在6亿以上[91]。目前关于大气CO2浓度升高导致作物营养品质下降的机制还不是很清楚。有研究者用“稀释效应”解释这种作物营养品质下降的现象。在大气CO2浓度升高条件下作物对碳水化合物的积累速度大于其他化合物合成以及矿质元素的吸收速度, 最终使得其他化合物及矿质元素的浓度表现为下降的现象。但这种假说不能解释为什么部分元素(Zn和Fe等)浓度下降, 部分元素(Mg、S、Ca和P等)浓度升高, 稀释作用不应该具有选择性[77,84]。另外一种假说认为大气CO2浓度升高降低了气孔导度, 减小了蒸腾作用, 同时也会降低土壤质流作用(见2.1), 这会抑制植物对可移动元素的吸收[65,92]。但是这种假说仍然面临挑战, 很多研究报道大气CO2浓度升高条件下,可移动性元素氮浓度降低, 但仍然有可移动性元素如: 磷元素和钾元素浓度未发生变化甚至呈增加趋势[77,84,93]。此外, Pérez-López等[94]认为大气CO2浓度升高条件下, C3作物生长旺盛, 所以那些作为辅酶因子参与合成代谢反应的元素(如Ca、Mg和Mn)需求量增多, 而参与氧化还原反应的元素(Fe、Zn和Cu)的需求量相对少, 正是这种差异造成了不同元素浓度的变化不同。以上3种假说都不能完全解释大气CO2浓度升高对作物籽实品质的影响, 相关机制研究工作仍需进一步开展, 这不仅可以完善植物生理学, 还可以为高品质作物育种工作提供理论参考。
4 大气CO2浓度和温度升高的交互作用
全球人口不断增加使得粮食安全问题变得尤为突出。大气温度升高等不利于作物生产的环境因子变化给全球作物生产带来前所未有的挑战。根据IPCC (2013)预测, 到21世纪末全球地表平均气温将会增加1.8~4.0oC。大气温度升高所带来的负面作用会抵消大气CO2浓度升高对产量的促进。考虑到大气CO2浓度和温度升高的交互作用对作物生产的影响, 未来的粮食安全挑战将会更严峻。首先, 温度增加1.5oC以上时, 大气CO2浓度升高的“肥料效应”将会变得不确定[95]。模型研究表明, 在未来大气温度升高条件下(2050—2100年),东北亚和非洲的水稻产量均会有较大幅度的下降(15%~45%), 大气CO2浓度升高会部分抵消温升对产量的负面效应, 但程度受到作物品种和生产地区纬度的影响[96,97]。例如, 利用FACE研究表明, 大气CO2浓度升高到500 μmol mol-1,中国江苏地区水稻产量显著提高8%左右, 如果同时进行大气温度升高2oC处理, 大气CO2浓度升高所带来的肥料效应则完全消失[98]。但也有模型研究认为全球温度增高对水稻产量形成的不利影响只发生在中低纬度, 在高纬度地区水稻生产将受到大气温度和CO2浓度升高的双重促进作用[99]。玉米产量对大气温度升高的响应结果与水稻类似, 例如模型研究表明欧洲地区玉米产量在温升条件下有增加的趋势[100], 在美洲和非洲地区的研究表明大气温度每升高1oC玉米产量下降0.5 t hm-2 [101,102]。通过对历史大气温度和大豆产量耦合进行模型研究分析发现, 在大气温度高的年份大豆产量相对较低[103,104]。但温度对大豆产量形成的不利影响依然取决于不同地区的本底温度。美国中西部大豆主产区生长季的平均温度是22.5oC, 大气温度升高0.8oC该地区大豆的平均产量提高1.7%; 而在美国南部大豆主产区长季的平均温度是26.7oC, 大气温度升高0.8oC则该地区大豆平均产量下降2.4%[105]。在大田试验条件下升高大气CO2浓度到585 μmol mol-1同时升高大气温度3.5oC处理(eCO2+eT), 大豆的光合碳固定能力比只升高大气CO2浓度处理(eCO2)低10%左右。但这种处理间的差异在年际间变异很大, 变异来源于年际间平均温度和降水量的差别[106]。大豆光合作用对大气温度和CO2浓度升高交互作用的响应不是因为Vc, max和Jmax变化引起的, 而是由于大气温度和CO2浓度升高引起叶片gs下降, 最终导致光合碳固定能力的降低[106]。所以, 大气CO2浓度升高可以一定程度上抵消大气温度升高对作物产量形成的不利影响, 但程度取决于大气温度的增幅以及持续天数。在美国主要产区, 小麦、玉米和大豆生育期内经历大于30oC的天数分别为6.0、10.8和13.1 d, 未来高温程度和持续天数都呈增加趋势, 这会完全抵消掉大气CO2浓度升高对作物光合的促进作用[107]。前文讨论了大气CO2浓度升高可以提高作物的水分利用效率(见1.2), 降低蒸腾作用, 提高土壤含水量从而增加了作物在干旱条件下的生产能力。但是通过模型计算表明, 大气CO2浓度升高给作物带来的抗旱能力来抵消大气温度升高带来的干旱作用是微乎其微的[107]。此外, 大气CO2浓度升高后作物群体叶面积的增加会加剧冠层蒸腾作用, 最终作物的水分需求量并未表现出下降的趋势[108], 这也使得大气CO2浓度升高增强植物高温条件下抗旱能力假设失去了理论基础。但上述论断仅仅是针对雨养区或低纬度地区, 在一些本底温度较低的地区(如高纬度地区)和半干旱地区关于大气CO2浓度和温度升高对作物产量的影响仍然需要进一步研究。
5 研究展望
研究大气CO2浓度升高对植物(作物)生长影响的最主要目的是提高作物产量, 保障不断增加的全球人口粮食安全。如果我们可以完全发挥大气CO2浓度升高对作物特别是对C3作物产量的促进作用, 在2050年大气CO2浓度达到550 μmol mol-1时作物产量可以提高15%左右, 那么对于全球新增的近30亿人口来说意义重大[17]。作物产量和品质对气候变化的响应受到基因型差异的影响, 这是同种作物对气候变化的响应存在较大变异的基础。目前关于气候变化对作物产量形成影响的研究还没把这方面工作放到重要位置。历史上的作物品种选育工作很可能不自觉地利用了大气CO2浓度升高所带来的产量促进作用, 那么在较低大气CO2浓度下选育的年代久远的品种是否在未来气候条件下具有更大的增产潜能呢?能否在同种作物中找到某些更加适应高大气CO2浓度的优势基因?开展大气CO2浓度升高对植物(作物)生长发育影响的机理机制研究工作可以让我们了解作物高产的潜在可能, 但对于预测未来作物产量变化还远远不够。未来的作物生产受大气CO2浓度不断升高影响的同时还会受到大气温度不断攀升, 极端高温气候事件频发, 大尺度的降雨带迁移造成部分地区干旱(洪涝)加剧以及大气O3浓度不断增加等多因子的交互影响。应该针对我国不同地区气候变化特点, 开展多气候因子对作物生长发育的影响研究可以更加准确判断未来作物生产变化趋势。例如, 在本底温度较低的东北地区开展大气CO2浓度和温度升高的交互作用试验评估该地区水稻、玉米和大豆的增产潜能; 在西北干旱区开展大气CO2浓度升高和不同灌溉水平的交互作用试验为该地区未来节水农业发展提供理论参考; 在东部沿海地区不同季节O3浓度变化大的特点开展大气CO2浓度和O3浓度升高的交互试验评估环境变化对该区的作物产量和品质的影响。这些都是未来我们需要关注的研究方向。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 1]
DOI:10.1046/j.1469-8137.2002.00494.xURL [本文引用: 1]
DOI:10.1104/pp.109.144113URLPMID:19755541 [本文引用: 3]
URL [本文引用: 1]

利用开放式CO2浓度升高(Free Air Carbon dioxide Enrichment)系统平台,于冬小麦开花期、乳熟期对旗叶进行气体交换测量,根据光合模型计算光合参数,研究550μL.L-1CO2对冬小麦旗叶光合能力的影响。结果表明,无论是在冬小麦开花期还是乳熟期,FACE圈内的小麦叶片在短时间高CO2浓度下初始出现的光合速率增强逐渐减弱或消失,即FACE圈内的小麦叶片表现出对高CO2浓度的光合适应现象。低氮、常规施氮水平下均发现了小麦旗叶的光合适应现象,但是光合适应现象与施氮量没有显著的线性关系。另外,研究发现,FACE系统中,冬小麦旗叶的SPAD值和叶绿素含量降低,这可能是导致FACE系统中小麦叶片出现光合适应现象的原因。
[本文引用: 1]
DOI:10.1111/pce.1994.17.issue-5URL [本文引用: 2]
DOI:10.1073/pnas.0807043105URLPMID:18957552 [本文引用: 1]

Photorespiratory 2-phosphoglycolate (2PG) metabolism is essential for photosynthesis in higher plants but thought to be superfluous in cyanobacteria because of their ability to concentrate CO(2) internally and thereby inhibit photorespiration. Here, we show that 3 routes for 2PG metabolism are present in the model cyanobacterium Synechocystis sp. strain PCC 6803. In addition to the photorespiratory C2 cycle characterized in plants, this cyanobacterium also possesses the bacterial glycerate pathway and is able to completely decarboxylate glyoxylate via oxalate. A triple mutant with defects in all 3 routes of 2PG metabolism exhibited a high-CO(2)-requiring (HCR) phenotype. All these catabolic routes start with glyoxylate, which can be synthesized by 2 different forms of glycolate dehydrogenase (GlcD). Mutants defective in one or both GlcD proteins accumulated glycolate under high CO(2) level and the double mutant DeltaglcD1/DeltaglcD2 was unable to grow under low CO(2). The HCR phenotype of both the double and the triple mutant could not be attributed to a significantly reduced affinity to CO(2), such as in other cyanobacterial HCR mutants defective in the CO(2)-concentrating mechanism (CCM). These unexpected findings of an HCR phenotype in the presence of an active CCM indicate that 2PG metabolism is essential for the viability of all organisms that perform oxygenic photosynthesis, including cyanobacteria and C3 plants, at ambient CO(2) conditions. These data and phylogenetic analyses suggest cyanobacteria as the evolutionary origin not only of oxygenic photosynthesis but also of an ancient photorespiratory 2PG metabolism.
[本文引用: 1]
DOI:10.1007/BF00386231URLPMID:24306196 [本文引用: 1]

Various aspects of the biochemistry of photosynthetic carbon assimilation in C3 plants are integrated into a form compatible with studies of gas exchange in leaves. These aspects include the kinetic properties of ribulose bisphosphate carboxylase-oxygenase; the requirements of the photosynthetic carbon reduction and photorespiratory carbon oxidation cycles for reduced pyridine nucleotides; the dependence of electron transport on photon flux and the presence of a temperature dependent upper limit to electron transport. The measurements of gas exchange with which the model outputs may be compared include those of the temperature and partial pressure of CO2(p(CO2)) dependencies of quantum yield, the variation of compensation point with temperature and partial pressure of O2(p(O2)), the dependence of net CO2 assimilation rate on p(CO2) and irradiance, and the influence of p(CO2) and irradiance on the temperature dependence of assimilation rate.
DOI:10.1078/0176-1617-01229URLPMID:15202715 [本文引用: 1]

Increases in both atmospheric CO2 concentration ([CO2]) and ultraviolet-B (UV-B) radiation on the Earth's surface are features of current climate change patterns. An experiment was conducted in sunlit, controlled environment chambers known as Soil-Plant-Atmosphere-Research (SPAR) units to determine interactive effects of elevated [CO2] and UV-B radiation on leaf and canopy photosynthetic characteristics of cotton. Six treatments were comprised of two CO2 levels of 360 (ambient) and 720 (elevated) microL L(-1) and three levels of 0 (control), 8, and 16 kJ m(-2) d(-1) biologically effective UV-B radiation. Treatments were imposed for 66 days from crop emergence through three weeks after the first flower stage. Plants grown in elevated [CO2] had significantly greater leaf area, higher leaf and canopy net photosynthetic rates (PN), lower dark respiration rate (Rd), and lower light compensation point (LCP) than plants grown in ambient [CO2]. There was no difference in CO2 compensation point (gamma), maximum rate of Rubisco activity (Vcmax), or light-saturated rate of electron transport (Jmax) between ambient and elevated CO2 treatments. When plants were grown in 8 kJ m(-2) d(-1) UV-B radiation, most of the measured photosynthetic parameters did not differ from control plants. High UV-B (16 kJ) radiation, however, caused 47-50% smaller leaf area, 38-44% lower leaf PN, 72-74% lower Vcmax, and 61-66% lower Jmax compared to the control. There were no interactive effects of [CO2] and UV-B radiation on most of the photosynthetic parameters measured. From the results, it is concluded that decreased canopy photosynthesis due to enhanced UV-B radiation in cotton is associated with both smaller leaf area and lower leaf PN, and loss of Rubisco activity and electron transport are two major factors in UV-B inhibition of leaf PN.
DOI:10.1093/jxb/erg262URLPMID:14512377 [本文引用: 1]

The principles, equipment and procedures for measuring leaf and canopy gas exchange have been described previously as has chlorophyll fluorescence. Simultaneous measurement of the responses of leaf gas exchange and modulated chlorophyll fluorescence to light and CO2 concentration now provide a means to determine a wide range of key biochemical and biophysical limitations on photo synthesis in vivo. Here the mathematical frameworks and practical procedures for determining these parameters in vivo are consolidated. Leaf CO2 uptake (A) versus intercellular CO2 concentration (Ci) curves may now be routinely obtained from commercial gas exchange systems. The potential pitfalls, and means to avoid these, are examined. Calculation of in vivo maximum rates of ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) carboxylation (Vc,max), electron transport driving regeneration of RuBP (Jmax), and triose-phosphate utilization (VTPU) are explained; these three parameters are now widely assumed to represent the major limitations to light-saturated photosynthesis. Precision in determining these in intact leaves is improved by the simultaneous measurement of electron transport via modulated chlorophyll fluorescence. The A/Ci response also provides a simple practical method for quantifying the limitation that stomata impose on CO2 assimilation. Determining the rate of photorespiratory release of oxygen (Rl) has previously only been possible by isotopic methods, now, by combining gas exchange and fluorescence measurements, Rl may be determined simply and routinely in the field. The physical diffusion of CO2 from the intercellular air space to the site of Rubisco in C3 leaves has long been suspected of being a limitation on photosynthesis, but it has commonly been ignored because of the lack of a practical method for its determination. Again combining gas exchange and fluorescence provides a means to determine mesophyll conductance. This method is described and provides insights into the magnitude and basis of this limitation.
DOI:10.1007/s11099-017-0753-9URL [本文引用: 1]
DOI:10.1093/pcp/pci113URLPMID:15840641 [本文引用: 1]

Net photosynthetic rates (Pns) in leaves were compared between rice plants grown in ambient air control and free-air CO2 enrichment (FACE, about 200 micromol mol(-1) above ambient) treatment rings. When measured at the same CO2 concentration, the Pn of FACE leaves decreased significantly, indicating that photosynthetic acclimation to high CO2 occurs. Although stomatal conductance (Gs) in FACE leaves was markedly decreased, intercellular CO2 concentrations (Ci) were almost the same in FACE and ambient leaves, indicating that the photosynthetic acclimation is not caused by the decreased Gs. Furthermore, carboxylation efficiency and maximal Pn, both light and CO2-saturated Pn, were decreased in FACE leaves, as shown by the Pn-Ci curves. In addition, the soluble protein, Rubisco (ribulose-1,5-bisphosphate caboxylase/oxygenase), and its activase contents as well as the sucrose-phosphate synthase activity decreased significantly, while some soluble sugar, inorganic phosphate, chlorophyll and light-harvesting complex II (LHC II) contents increased in FACE leaves. It appears that the photosynthetic acclimation in rice leaves is related to both ribulose-1,5-bisphosphate (RuBP) carboxylation limitation and RuBP regeneration limitation.
DOI:10.1111/j.1365-3040.2007.01641.xURLPMID:17263773 [本文引用: 3]

This review summarizes current understanding of the mechanisms that underlie the response of photosynthesis and stomatal conductance to elevated carbon dioxide concentration ([CO2]), and examines how downstream processes and environmental constraints modulate these two fundamental responses. The results from free-air CO2 enrichment (FACE) experiments were summarized via meta-analysis to quantify the mean responses of stomatal and photosynthetic parameters to elevated [CO2]. Elevation of [CO2] in FACE experiments reduced stomatal conductance by 22%, yet, this reduction was not associated with a similar change in stomatal density. Elevated [CO2] stimulated light-saturated photosynthesis (Asat) in C3 plants grown in FACE by an average of 31%. However, the magnitude of the increase in Asat varied with functional group and environment. Functional groups with ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco)-limited photosynthesis at elevated [CO2] had greater potential for increases in Asat than those where photosynthesis became ribulose-1,5-bisphosphate (RubP)-limited at elevated [CO2]. Both nitrogen supply and sink capacity modulated the response of photosynthesis to elevated [CO2] through their impact on the acclimation of carboxylation capacity. Increased understanding of the molecular and biochemical mechanisms by which plants respond to elevated [CO2], and the feedback of environmental factors upon them, will improve our ability to predict ecosystem responses to rising [CO2] and increase our potential to adapt crops and managed ecosystems to future atmospheric [CO2].
[本文引用: 1]
DOI:10.1104/pp.86.1.200URLPMID:16665866 [本文引用: 1]

Most studies on stomatal responses to CO(2) assume that guard cells respond only to intercellular CO(2) concentration and are insensitive to the CO(2) concentrations in the pore and outside the leaf. If stomata are sensitive to the CO(2) concentration at the surface of the leaf or in the stomatal pore, the stomatal response to intercellular CO(2) concentration will be incorrect for a ;normally' operating leaf (where ambient CO(2) concentration is a constant). In this study asymmetric CO(2) concentrations for the two surfaces of amphistomatous leaves were used to vary intercellular and leaf surface CO(2) concentrations independently in Xanthium strumarium L. and Helianthus annuus L. The response of stomata to intercellular CO(2) concentration when the concentration at the leaf surface was held constant was found to be the same as the response when the surface concentration was varied. In addition, stomata did not respond to changes in leaf surface CO(2) concentration when the intercellular concentration for that surface was held constant. It is concluded that stomata respond to intercellular CO(2) concentration and are insensitive to the CO(2) concentration at the surface of the leaf and in the stomatal pore.
DOI:10.1093/jxb/erp096URLPMID:19401412 [本文引用: 4]

Plant responses to the projected future levels of CO(2) were first characterized in short-term experiments lasting days to weeks. However, longer term acclimation responses to elevated CO(2) were subsequently discovered to be very important in determining plant and ecosystem function. Free-Air CO(2) Enrichment (FACE) experiments are the culmination of efforts to assess the impact of elevated CO(2) on plants over multiple seasons and, in the case of crops, over their entire lifetime. FACE has been used to expose vegetation to elevated concentrations of atmospheric CO(2) under completely open-air conditions for nearly two decades. This review describes some of the lessons learned from the long-term investment in these experiments. First, elevated CO(2) stimulates photosynthetic carbon gain and net primary production over the long term despite down-regulation of Rubisco activity. Second, elevated CO(2) improves nitrogen use efficiency and, third, decreases water use at both the leaf and canopy scale. Fourth, elevated CO(2) stimulates dark respiration via a transcriptional reprogramming of metabolism. Fifth, elevated CO(2) does not directly stimulate C(4) photosynthesis, but can indirectly stimulate carbon gain in times and places of drought. Finally, the stimulation of yield by elevated CO(2) in crop species is much smaller than expected. While many of these lessons have been most clearly demonstrated in crop systems, all of the lessons have important implications for natural systems.
DOI:10.1046/j.1469-8137.2001.00028.xURL [本文引用: 1]
DOI:10.1093/jxb/erg309URLPMID:14585828 [本文引用: 1]

The initial stimulation of photosynthesis observed on elevation of [CO2] in grasslands has been predicted to be a transient phenomenon constrained by the loss of photosynthetic capacity due to other limitations, notably nutrients and sinks for carbohydrates. Legumes might be expected partially to escape these feedbacks through symbiotic N2 fixation. The Free-Air Carbon dioxide Enrichment (FACE) experiment at Eschikon, Switzerland, has been the longest running investigation of the effects of open-air elevation of [CO2] on vegetation. The prediction of a long-term loss of photosynthetic capacity was tested by analysing photosynthesis in Trifolium repens L. (cv. Milkanova) in the spring and autumn of the eighth, ninth and tenth years of treatment. A high and low N treatment also allowed a test of the significance of exogenous N-supply in maintaining a stimulation of photosynthetic capacity in the long-term. Prior work in this Free Air CO2 Enrichment (FACE) experiment has revealed that elevated [CO2] increased both vegetative and reproductive growth of T. repens independent of N treatment. It is shown here that the photosynthetic response of T. repens was also independent of N fertilization under both current ambient and elevated (600 micro mol mol-1) [CO2]. There was a strong effect of season on photosynthesis, with light-saturated rates (Asat) 37% higher in spring than in autumn. Higher Asat in the spring was supported by higher maximum Rubisco carboxylation rates (Vc,max) and maximum rates of electron transport (Jmax) contributing to RuBP regeneration. Elevated [CO2] increased Asat by 37% when averaged across all measurement periods and both N fertilization levels, and decreased stomatal conductance by 25%. In spring, there was no effect of elevated [CO2] on photosynthetic capacity of leaves, but in autumn both Vc,max and Jmax were reduced by approximately 20% in elevated [CO2]. The results show that acclimation of photosynthetic capacity can occur in a nitrogen-fixing species, in the field where there are no artificial restrictions on sink capacity. However, even with acclimation there was a highly significant increase in photosynthesis at elevated [CO2].
DOI:10.1111/gcb.2017.23.issue-9URL [本文引用: 1]
DOI:10.1111/gcb.v25.12URL [本文引用: 1]
[本文引用: 1]
DOI:10.1007/s004420050472URLPMID:28307897 [本文引用: 1]

The eastern Colorado shortgrass steppe is dominated by the C4 grass, Bouteloua gracilis, but contains a mixture of C3 grasses as well, including Pascopyrum smithii. Although the ecology of this region has been extensively studied, there is little information on how increasing atmospheric CO2 will affect it. This growth chamber study investigated gas exchange, water relations, growth, and biomass and carbohydrate partitioning in B. gracilis and P. smithii grown under present ambient and elevated CO2 concentrations of 350 mul l(-1)and 700 mul l(-1), respectively, and two deficit irrigation regimes. The experiment was conducted in soil-packed columns planted to either species over a 2-month period under summer-like conditions and with no fertilizer additions. Our objective was to better understand how these species and the functional groups they represent will respond in future CO2-enriched environments. Leaf CO2 assimilation (A n), transpiration use efficiency (TUE, or A n/transpiration), plant growth, and whole-plant water use efficiency (WUE, or plant biomass production/water evapotranspired) of both species were greater at elevated CO2, although responses were more pronounced for P. smithii. Elevated CO2 enhanced photosynthesis, TUE, and growth in both species through higher soil water content (SWC) and leaf water potentials (Psi) and stimulation of photosynthesis. Consumptive water use was greater and TUE less for P. smithii than B. gracilis during early growth when soil water was more available. Declining SWC with time was associated with a steadily increased sequestering of total non-structural carbohydrates (TNCs), storage carbohydrates (primarily fructans for P. smithii) and biomass in belowground organs of P. smithii, but not B. gracilis. The root:shoot ratio of P. smithii also increased at elevated CO2, while the root:shoot ratio of B. gracilis was unresponsive to CO2. These partitioning responses may be the consequence of different ontogenetic strategies of a cool-season and warm-season grass entering a warm, dry summer period; the cool-season P. smithii responds by sequestering TNCs belowground in preparation for summer dormancy, while resource partitioning of the warm-season B. gracilis remains unaltered. One consequence of greater partitioning of resources into P. smithii belowground organs in the present study was maintenance of higher Psi and A n rates. This, along with differences in photosynthetic pathway, may have accounted for the greater responsiveness of P. smithii to CO2 enrichment compared to B. gracilis.
DOI:10.1071/PP97054URL [本文引用: 1]
DOI:10.1111/gcb.13473URL [本文引用: 1]
DOI:10.1071/PP9960053URL [本文引用: 1]
DOI:10.1046/j.1365-3040.2000.00609.xURL [本文引用: 1]
DOI:10.1007/s004250050660URLPMID:10592039 [本文引用: 1]

The effects of elevated CO(2) concentrations on the photochemistry, biochemistry and physiology of C(4) photosynthesis were studied in maize (Zea mays L.). Plants were grown at ambient (350 &mgr;L L(-1)) or ca. 3 times ambient (1100 &mgr;L L(-1)) CO(2) levels under high light conditions in a greenhouse for 30 d. Relative to plants grown at ambient CO(2) levels, plants grown under elevated CO(2) accumulated ca. 20% more biomass and 23% more leaf area. When measured at the CO(2) concentration of growth, mature leaves of high-CO(2)-grown plants had higher light-saturated rates of photosynthesis (ca. 15%), lower stomatal conductance (71%), higher water-use efficiency (225%) and higher dark respiration rates (100%). High-CO(2)-grown plants had lower carboxylation efficiencies (23%), measured under limiting CO(2), and lower leaf protein contents (22%). Activities of a number of C(3) and C(4) cycle enzymes decreased on a leaf-area basis in the high-CO(2)-grown plants by 5-30%, with NADP-malate dehydrogenase exhibiting the greatest decrease. In contrast, activities of fructose 1,6-bisphosphatase and ADP-glucose pyrophosphorylase increased significantly under elevated CO(2) condition (8% and 36%, respectively). These data show that the C(4) plant maize may benefit from elevated CO(2) through acclimation in the capacities of certain photosynthetic enzymes. The increased capacity to synthesize sucrose and starch, and to utilize these end-products of photosynthesis to produce extra energy by respiration, may contribute to the enhanced growth of maize under elevated CO(2).
DOI:10.1104/pp.105.073957URLPMID:16407441 [本文引用: 1]

While increasing temperatures and altered soil moisture arising from climate change in the next 50 years are projected to decrease yield of food crops, elevated CO2 concentration ([CO2]) is predicted to enhance yield and offset these detrimental factors. However, C4 photosynthesis is usually saturated at current [CO2] and theoretically should not be stimulated under elevated [CO2]. Nevertheless, some controlled environment studies have reported direct stimulation of C4 photosynthesis and productivity, as well as physiological acclimation, under elevated [CO2]. To test if these effects occur in the open air and within the Corn Belt, maize (Zea mays) was grown in ambient [CO2] (376 micromol mol(-1)) and elevated [CO2] (550 micromol mol(-1)) using Free-Air Concentration Enrichment technology. The 2004 season had ideal growing conditions in which the crop did not experience water stress. In the absence of water stress, growth at elevated [CO2] did not stimulate photosynthesis, biomass, or yield. Nor was there any CO2 effect on the activity of key photosynthetic enzymes, or metabolic markers of carbon and nitrogen status. Stomatal conductance was lower (-34%) and soil moisture was higher (up to 31%), consistent with reduced crop water use. The results provide unique field evidence that photosynthesis and production of maize may be unaffected by rising [CO2] in the absence of drought. This suggests that rising [CO2] may not provide the full dividend to North American maize production anticipated in projections of future global food supply.
DOI:10.1111/j.1399-3054.2012.01581.xURLPMID:22268610 [本文引用: 3]

In this study, we tested for the temporal occurrence of photosynthetic acclimation to elevated [CO(2)] in the flag leaf of two important cereal crops, rice and wheat. In order to characterize the temporal onset of acclimation and the basis for any observed decline in photosynthetic rate, we characterized net photosynthesis, g(s) , g(m) , C(i) /C(a) , C(i) /C(c) , V(cmax) , J(max) , cell wall thickness, content of Rubisco, cytochrome (Cyt) f, N, chlorophyll and carbohydrate, mRNA expression for rbcL and petA, activity for Rubisco, sucrose phosphate synthase (SPS) and sucrose synthase (SS) at full flag expansion, mid-anthesis and the late grain-filling stage. No acclimation was observed for either crop at full flag leaf expansion. However, at the mid-anthesis stage, photosynthetic acclimation in rice was associated with RuBP carboxylation and regeneration limitations, while wheat only had the carboxylation limitation. By grain maturation, the decline of Rubisco content and activity had contributed to RuBP carboxylation limitation of photosynthesis in both crops at elevated [CO(2)]; however, the sharp decrease of Rubisco enzyme activity played a more important role in wheat. Although an increase in non-structural carbohydrates did occur during these later stages, it was not consistently associated with changes in SPS and SS or photosynthetic acclimation. Rather, over time elevated [CO(2)] appeared to enhance the rate of N degradation and senescence so that by late-grain fill, photosynthetic acclimation to elevated [CO(2)] in the flag leaf of either species was complete. These data suggest that the basis for photosynthetic acclimation with elevated [CO(2)] may be more closely associated with enhanced rates of senescence, and, as a consequence, may be temporally dynamic, with significant species variation.
DOI:10.1111/pce.13206URLPMID:29611206 [本文引用: 1]

Rising atmospheric carbon dioxide concentration ([CO2 ]) significantly influences plant growth, development, and biomass. Increased photosynthesis rate, together with lower stomatal conductance, has been identified as the key factors that stimulate plant growth at elevated [CO2 ] (e[CO2 ]). However, variations in photosynthesis and stomatal conductance alone cannot fully explain the dynamic changes in plant growth. Stimulation of photosynthesis at e[CO2 ] is always associated with post-photosynthetic secondary metabolic processes that include carbon and nitrogen metabolism, cell cycle functions, and hormonal regulation. Most studies have focused on photosynthesis and stomatal conductance in response to e[CO2 ], despite the emerging evidence of e[CO2 ]'s role in moderating secondary metabolism in plants. In this review, we briefly discuss the effects of e[CO2 ] on photosynthesis and stomatal conductance and then focus on the changes in other cellular mechanisms and growth processes at e[CO2 ] in relation to plant growth and development. Finally, knowledge gaps in understanding plant growth responses to e[CO2 ] have been identified with the aim of improving crop productivity under a CO2 rich atmosphere.
DOI:10.1111/pce.1991.14.issue-8URL [本文引用: 1]
DOI:10.1111/j.1365-3040.1994.tb00312.xURLPMID:11537974 [本文引用: 1]

DOI:10.1046/j.1365-3040.1999.00432.xURL [本文引用: 1]
DOI:10.1016/s0014-5793(00)01873-1URLPMID:10940381 [本文引用: 1]

The influence of elevated atmospheric CO2 on transcript levels of photosynthetic genes was investigated in leaves of Nicotiana tabacum cv. SamsunNN and cv. Wisconsin38 plants. Plants were grown under ambient (400 ppm) and elevated (800/1,000 ppm) atmospheric CO2, and transcript levels were determined in leaves of different age. Down-regulation of photosynthetic gene transcripts was apparent in senesing leaves only. A correlation between transcript levels and leaf contents of soluble sugars could not be found. To investigate whether a shift in leaf ontogeny would be involved in the regulation of photosynthetic genes transgenic tobacco plants expressing either the gus or ipt gene under control of the senescence-specific SAG-12 promoter [Gan, S. and Amasino, R.M. (1995) Science 270, 1986-1988] were included in our studies. As expected SAG-12-driven GUS activity increased with leaf age. This increase of GUS activity was stimulated by elevated atmospheric CO2, accompanied by a loss of chlorophyll and the down-regulation of photosynthetic genes, verifying that high CO2 accelerates leaf ontogeny. Senescence as well as down-regulation of photosynthetic genes could be delayed by ipt expression. Levels of soluble sugars were indistinguishable from wild type or even slightly elevated in ipt transgenic plants. Therefore, sugar accumulation as a cause for down-regulation of photosynthetic genes under high CO2 can be excluded. It appears more likely that the high CO2-mediated decline in photosynthetic gene transcripts is due to a temporal shift in leaf ontogeny.
DOI:10.1071/FP08269URLPMID:32688647 [本文引用: 1]

It was anticipated that wheat net photosynthesis would rise under elevated CO2, and that this would alter the progress of senescence due to the unbalance of carbohydrates and nitrogen. Our study showed that ear carbon sink was limited, and sugar was accumulated, hexokinase activities and levels of phosphorylated sugar were increased within the flag leaves, grain nitrogen sink capacity was enhanced, and flag leaf senescence was accelerated under elevated CO2. However, if the ear of the main stem was covered, these responses to elevated CO2 were absent, and the senescence of flag leaf was not accelerated by elevated CO2. Thus, it appeared that elevated CO2 accelerated the rate of flag leaf senescence, depending on ear photosynthesis. The ears have far higher enhancement of net photosynthesis than flag leaves, and the role of the flag leaf relative to the ear was declined in supplying C assimilation to grain under elevated CO2. This indicates that as CO2 rises, the grain sink needs the N more than C assimilate from flag leaf, so the declining rates of N% and soluble proteins concentration were markedly accelerated under elevated CO2 conditions. This suggests that, the large increase in ear net photosynthesis accelerated grain filling, accelerated remobilising N within flag leaf as the result of the greater grain nitrogen sink capacity. In addition, as the result of grain carbon sink limitation, it limited the export of flag leaf sucrose and enhanced sugar cycling, which was the signal to accelerate leaf senescence. Hence, elevated CO2 subsequently accelerates senescence of flag leaf.
[本文引用: 1]
DOI:10.1046/j.1469-8137.2003.00850.xURL [本文引用: 1]
DOI:10.1111/j.1365-3040.2006.01503.xURLPMID:17080946 [本文引用: 1]

Poplar trees sustain close to the predicted increase in leaf photosynthesis when grown under long-term elevated CO2 concentration ([CO2]). To investigate the mechanisms underlying this response, carbohydrate accumulation and protein expression were determined over four seasons of growth. No increase in the levels of soluble carbohydrates was observed in the young expanding or mature sun leaves of the three poplar genotypes during this period. However, substantial increases in starch levels were observed in the mature leaves of all three poplar genotypes grown in elevated [CO2]. Despite the very high starch levels, no changes in the expression of photosynthetic Calvin cycle proteins, or in the starch biosynthetic enzyme ADP-glucose pyrophosphorylase (AGPase), were observed. This suggested that no long-term photosynthetic acclimation to CO2 occurred in these plants. Our data indicate that poplar trees are able to 'escape' from long-term, acclimatory down-regulation of photosynthesis through a high capacity for starch synthesis and carbon export. These findings show that these poplar genotypes are well suited to the elevated [CO2] conditions forecast for the middle of this century and may be particularly suited for planting for the long-term carbon sequestration into wood.
DOI:10.1093/jexbot/52.360.1383URLPMID:11457898 [本文引用: 1]

The concept that photosynthetic flux is influenced by the accumulation of photo-assimilate persisted for 100 years before receiving any strong experimental support. Precise analysis of the mechanisms of photosynthetic responses to sink activity required the development of a battery of appropriate molecular techniques and has benefited from contemporary interest in the effects of elevated CO2 on photosynthesis. Photosynthesis is one of the most highly integrated and regulated metabolic processes to maximize the use of available light, to minimize the damaging effects of excess light and to optimize the use of limiting carbon and nitrogen resources. Hypotheses of feedback regulation must take account of this integration. In the short term, departure from homeostasis can lead to redox signals, which cause rapid changes in the transcription of genes encoding photosystems I and II. End-product synthesis can exert short-term metabolic feedback control through Pi recycling. Beyond this, carbohydrate accumulation in leaves when there is an imbalance between source and sink at the whole plant level can lead to decreased expression of photosynthetic genes and accelerated leaf senescence. In a high CO2 world this may become a more prevalent feature of photosynthetic regulation. However, sink regulation of photosynthesis is highly dependent on the physiology of the rest of the plant. This physiological state regulates photosynthesis through signal transduction pathways that co-ordinate the plant carbon : nitrogen balance, which match photosynthetic capacity to growth and storage capacity and underpin and can override the direct short-term controls of photosynthesis by light and CO2. Photosynthate supply and phytohormones, particularly cytokinins, interact with nitrogen supply to control the expression of photosynthesis genes, the development of leaves and the whole plant nitrogen distribution, which provides the dominant basis for sink regulation of photosynthesis.
DOI:10.1111/gcb.2009.15.issue-1URL [本文引用: 1]
DOI:10.1016/j.agrformet.2003.09.002URL [本文引用: 1]
DOI:10.1104/pp.120.3.867URLPMID:10398723 [本文引用: 1]

The role of sucrose synthase (SS) in the fixation of N was examined in the rug4 mutant of pea (Pisum sativum L.) plants in which SS activity was severely reduced. When dependent on nodules for their N supply, the mutant plants were not viable and appeared to be incapable of effective N fixation, although nodule formation was essentially normal. In fact, N and C resources invested in nodules were much greater in mutant plants than in the wild-type (WT) plants. Low SS activity in nodules (present at only 10% of WT levels) resulted in lower amounts of total soluble protein and leghemoglobin and lower activities of several enzymes compared with WT nodules. Alkaline invertase activity was not increased to compensate for reduced SS activity. Leghemoglobin was present at less than 20% of WT values, so O2 flux may have been compromised. The two components of nitrogenase were present at normal levels in mutant nodules. However, only a trace of nitrogenase activity was detected in intact plants and none was found in isolated bacteroids. The results are discussed in relation to the role of SS in the provision of C substrates for N fixation and in the development of functional nodules.
DOI:10.1017/S096025850000057XURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/pce.1993.16.issue-1URL [本文引用: 1]
DOI:10.1104/pp.105.066233URLPMID:16183840 [本文引用: 1]

DOI:10.1016/0968-0004(79)90212-3URL [本文引用: 1]
DOI:10.1146/annurev.arplant.53.100301.135233URLPMID:12221984 [本文引用: 1]

Ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) catalyzes the first step in net photosynthetic CO2 assimilation and photorespiratory carbon oxidation. The enzyme is notoriously inefficient as a catalyst for the carboxylation of RuBP and is subject to competitive inhibition by O2, inactivation by loss of carbamylation, and dead-end inhibition by RuBP. These inadequacies make Rubisco rate limiting for photosynthesis and an obvious target for increasing agricultural productivity. Resolution of X-ray crystal structures and detailed analysis of divergent, mutant, and hybrid enzymes have increased our insight into the structure/function relationships of Rubisco. The interactions and associations relatively far from the Rubisco active site, including regulatory interactions with Rubisco activase, may present new approaches and strategies for understanding and ultimately improving this complex enzyme.
DOI:10.1146/annurev.arplant.48.1.609URLPMID:15012276 [本文引用: 2]

The primary effect of the response of plants to rising atmospheric CO2 (Ca) is to increase resource use efficiency. Elevated Ca reduces stomatal conductance and transpiration and improves water use efficiency, and at the same time it stimulates higher rates of photosynthesis and increases light-use efficiency. Acclimation of photosynthesis during long-term exposure to elevated Ca reduces key enzymes of the photosynthetic carbon reduction cycle, and this increases nutrient use efficiency. Improved soil-water balance, increased carbon uptake in the shade, greater carbon to nitrogen ratio, and reduced nutrient quality for insect and animal grazers are all possibilities that have been observed in field studies of the effects of elevated Ca. These effects have major consequences for agriculture and native ecosystems in a world of rising atmospheric Ca and climate change.
DOI:10.1046/j.1365-3040.1997.d01-10.xURL [本文引用: 1]
DOI:10.1104/pp.118.2.683URLPMID:9765554 [本文引用: 1]

Acclimation of photosynthesis to elevated CO2 has previously been shown to be more pronounced when N supply is poor. Is this a direct effect of N or an indirect effect of N by limiting the development of sinks for photoassimilate? This question was tested by growing a perennial ryegrass (Lolium perenne) in the field under elevated (60 Pa) and current (36 Pa) partial pressures of CO2 (pCO2) at low and high levels of N fertilization. Cutting of this herbage crop at 4- to 8-week intervals removed about 80% of the canopy, therefore decreasing the ratio of photosynthetic area to sinks for photoassimilate. Leaf photosynthesis, in vivo carboxylation capacity, carbohydrate, N, ribulose-1,5-bisphosphate carboxylase/oxygenase, sedoheptulose-1,7-bisphosphatase, and chloroplastic fructose-1, 6-bisphosphatase levels were determined for mature lamina during two consecutive summers. Just before the cut, when the canopy was relatively large, growth at elevated pCO2 and low N resulted in significant decreases in carboxylation capacity and the amount of ribulose-1,5-bisphosphate carboxylase/oxygenase protein. In high N there were no significant decreases in carboxylation capacity or proteins, but chloroplastic fructose-1,6-bisphosphatase protein levels increased significantly. Elevated pCO2 resulted in a marked and significant increase in leaf carbohydrate content at low N, but had no effect at high N. This acclimation at low N was absent after the harvest, when the canopy size was small. These results suggest that acclimation under low N is caused by limitation of sink development rather than being a direct effect of N supply on photosynthesis.
DOI:10.1016/j.fcr.2007.03.006URL [本文引用: 1]
DOI:10.1007/s10705-010-9379-zURL [本文引用: 1]
DOI:10.1016/j.pbi.2018.06.001URLPMID:29958824 [本文引用: 2]

Crops grown under elevated CO2 (eCO2) typically exhibit enhanced yields but at the same time decreased nutritional quality. The latter effect has often been explained as a growth dilution phenomenon, but this cannot be the only process involved since crop nutrient concentrations are decreased also when production is unaffected by eCO2. We review the current knowledge on eCO2 effects on crop nutritional quality with focus on the current understanding of the possible mechanisms and processes causing these effects. Emphasis is on crop nitrogen (N) and protein concentrations but effects on other nutrients and how they compare with those on N are also covered.
DOI:10.1016/j.scienta.2016.11.026URL [本文引用: 1]
DOI:10.1126/science.1114722URLPMID:16809532 [本文引用: 1]

Model projections suggest that although increased temperature and decreased soil moisture will act to reduce global crop yields by 2050, the direct fertilization effect of rising carbon dioxide concentration ([CO2]) will offset these losses. The CO2 fertilization factors used in models to project future yields were derived from enclosure studies conducted approximately 20 years ago. Free-air concentration enrichment (FACE) technology has now facilitated large-scale trials of the major grain crops at elevated [CO2] under fully open-air field conditions. In those trials, elevated [CO2] enhanced yield by approximately 50% less than in enclosure studies. This casts serious doubt on projections that rising [CO2] will fully offset losses due to climate change.
DOI:10.1111/j.1744-7909.2008.00754.xURL [本文引用: 2]

Plants grown under elevated atmospheric [CO2] typically have decreased tissue concentrations of N compared with plants grown under current ambient [CO2]. The physiological mechanisms responsible for this phenomenon have not been definitely established, although a considerable number of hypotheses have been advanced to account for it. In this review we discuss and critically evaluate these hypotheses. One contributing factor to the decreases in tissue N concentrations clearly is dilution of N by increased photosynthetic assimilation of C. In addition, studies on intact plants show strong evidence for a general decrease in the specific uptake rates (uptake per unit mass or length of root) of N by roots under elevated CO2, This decreased root uptake appears likely to be the result both of decreased N demand by shoots and of decreased ability of the soil-root system to supply N. The best-supported mechanism for decreased N supply is a decrease in transpiration-driven mass flow of N in soils due to decreased stomatal conductance at elevated CO2, although some evidence suggests that altered root system architecture may also play a role. There is also limited evidence suggesting that under elevated CO2, plants may exhibit increased rates of N loss through volatilization and/or root exudation, further contributing to lowering tissue N concentrations.
DOI:10.3389/fpls.2017.01546URLPMID:28959266 [本文引用: 1]

Nitrogen deficiency limits crop performance under elevated CO2 (eCO2), depending on the ability of plant N uptake. However, the dynamics and redistribution of N2 fixation, and fertilizer and soil N use in legumes under eCO2 have been little studied. Such an investigation is essential to improve the adaptability of legumes to climate change. We took advantage of genotype-specific responses of soybean to increased CO2 to test which N-uptake phenotypes are most strongly related to enhanced yield. Eight soybean cultivars were grown in open-top chambers with either 390 ppm (aCO2) or 550 ppm CO2 (eCO2). The plants were supplied with 100 mg N kg(-1) soil as (15)N-labeled calcium nitrate, and harvested at the initial seed-filling (R5) and full-mature (R8) stages. Increased yield in response to eCO2 correlated highly (r = 0.95) with an increase in symbiotically fixed N during the R5 to R8 stage. In contrast, eCO2 only led to small increases in the uptake of fertilizer-derived and soil-derived N during R5 to R8, and these increases did not correlate with enhanced yield. Elevated CO2 also decreased the proportion of seed N redistributed from shoot to seeds, and this decrease strongly correlated with increased yield. Moreover, the total N uptake was associated with increases in fixed-N per nodule in response to eCO2, but not with changes in nodule biomass, nodule density, or root length.
DOI:10.1080/03650340.2019.1575958URL [本文引用: 1]
DOI:10.1111/gcb.12938URL [本文引用: 1]
DOI:10.1111/ppl.1993.89.issue-3URL [本文引用: 1]
DOI:10.1111/pce.1995.18.issue-10URL [本文引用: 1]
DOI:10.1046/j.1365-3040.1999.00391.xURL [本文引用: 1]
DOI:10.1046/j.1365-2486.2001.00406.xURL [本文引用: 2]
DOI:10.1046/j.1365-3040.1999.00386.xURL [本文引用: 1]
DOI:10.1007/s004420000524URLPMID:28547443 [本文引用: 2]

Nutrients such as nitrogen (N) and phosphorus (P) often limit plant growth rate and production in natural and agricultural ecosystems. Limited availability of these nutrients is also a major factor influencing long-term plant and ecosystem responses to rising atmospheric CO2 levels, i.e., the commonly observed short-term increase in plant biomass may not be sustained over the long-term. Therefore, it is critical to obtain a mechanistic understanding of whether elevated CO2 can elicit compensatory adjustments such that acquisition capacity for minerals increases in concert with carbon (C) uptake. Compensatory adjustments such as increases in (a) root mycorrhizal infection, (b) root-to-shoot ratio and changes in root morphology and architecture, (c) root nutrient absorption capacity, and (d) nutrient-use efficiency can enable plants to meet an increased nutrient demand under high CO2. Here we examine the literature to assess the extent to which these mechanisms have been shown to respond to high CO2. The literature survey reveals no consistent pattern either in direction or magnitude of responses of these mechanisms to high CO2. This apparent lack of a pattern may represent variations in experimental protocol and/or interspecific differences. We found that in addressing nutrient uptake responses to high CO2 most investigators have examined these mechanisms in isolation. Because such mechanisms can potentially counterbalance one another, a more reliable prediction of elevated CO2 responses requires experimental designs that integrate all mechanisms simultaneously. Finally, we present a functional balance (FB) model as an example of how root system adjustments and nitrogen-use efficiency can be integrated to assess growth responses to high CO2. The FB model suggests that the mechanisms of increased N uptake highlighted here have different weights in determining overall plant responses to high CO2. For example, while changes in root-to-shoot biomass allocation, r, have a small effect on growth, adjustments in uptake rate per unit root mass, [Formula: see text], and photosynthetic N use efficiency, p*, have a significantly greater leverage on growth responses to elevated CO2 except when relative growth rate (RGR) reaches its developmental limit, maximum RGR (RGRmax).
[本文引用: 1]
DOI:10.1073/pnas.161279898URLPMID:11481499 [本文引用: 1]

The major
DOI:10.1016/j.soilbio.2016.11.018URL [本文引用: 1]
DOI:10.1641/0006-3568(2004)054[0731:PNLOER]2.0.CO;2URL [本文引用: 1]
DOI:10.1126/science.1224304URLPMID:22936776 [本文引用: 1]

The extent to which terrestrial ecosystems can sequester carbon to mitigate climate change is a matter of debate. The stimulation of arbuscular mycorrhizal fungi (AMF) by elevated atmospheric carbon dioxide (CO(2)) has been assumed to be a major mechanism facilitating soil carbon sequestration by increasing carbon inputs to soil and by protecting organic carbon from decomposition via aggregation. We present evidence from four independent microcosm and field experiments demonstrating that CO(2) enhancement of AMF results in considerable soil carbon losses. Our findings challenge the assumption that AMF protect against degradation of organic carbon in soil and raise questions about the current prediction of terrestrial ecosystem carbon balance under future climate-change scenarios.
DOI:10.1093/jxb/ers341URLPMID:23183255 [本文引用: 1]

Phosphorus (P) nutrition is always a key issue regarding plants responses to elevated CO(2). Yet it is unclear of how elevated CO(2) affects P uptake under different nitrogen (N) forms. This study investigated the influence of elevated CO(2) (800 microl l(-1)) on P uptake and utilization by Arabidopsis grown in pH-buffered phosphate (P)-deficient (0.5 microM) hydroponic culture supplying with 2mM nitrate (NO(3)(-)) or ammonium (NH(4)(+)). After 7 d treatment, elevated CO(2) enhanced the biomass production of both NO(3)(-)- and NH(4) (+)-fed plants but decreased the P amount absorbed per weight of roots and the P concentration in the shoots of plants supplied with NH(4)(+). In comparison, elevated CO(2) increased the amount of P absorbed per weight of roots, as well as the P concentration in plants and alleviated P deficiency-induced symptoms of plants supplied with NO(3)(-). Elevated CO(2) also increased the root/shoot ratio, total root surface area, and acid phosphatase activity, and enhanced the expression of genes or transcriptional factors involving in P uptake, allocation and remobilization in P deficient plants. Furthermore, elevated CO(2) increased the nitric oxide (NO) level in roots of NO(3)(-)-fed plants but decreased it in NH(4)(+)-fed plants. NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) inhibited plant P acquisition by roots under elevated CO(2). Considering all of these findings, this study concluded that a combination of elevated CO(2) and NO(3)(-) nutrition can induce a set of plant adaptive strategies to improve P status from P-deficient soluble sources and that NO may be a signalling molecule that controls these processes.
DOI:10.7554/eLife.03606URLPMID:25535795 [本文引用: 2]

Voltage-gated ion channels generate electrical currents that control muscle contraction, encode neuronal information, and trigger hormonal release. Tissue-specific expression of accessory (beta) subunits causes these channels to generate currents with distinct properties. In the heart, KCNQ1 voltage-gated potassium channels coassemble with KCNE1 beta-subunits to generate the IKs current (Barhanin et al., 1996; Sanguinetti et al., 1996), an important current for maintenance of stable heart rhythms. KCNE1 significantly modulates the gating, permeation, and pharmacology of KCNQ1 (Wrobel et al., 2012; Sun et al., 2012; Abbott, 2014). These changes are essential for the physiological role of IKs (Silva and Rudy, 2005); however, after 18 years of study, no coherent mechanism explaining how KCNE1 affects KCNQ1 has emerged. Here we provide evidence of such a mechanism, whereby, KCNE1 alters the state-dependent interactions that functionally couple the voltage-sensing domains (VSDs) to the pore.
DOI:10.1111/j.1469-8137.2012.04074.xURL [本文引用: 1]

We conducted the most extensive meta-analysis of plant and animal responses to elevated CO2 to date. We analysed > 5000 data points extracted from 270 papers published between 1979 and 2009. We examined the changes in 19 animal response variables to the main effect of elevated CO2. We found strong evidence for significant variation among arthropod orders and feeding guilds, including interactions in the direction of response. We also examined the main effects of elevated CO2 on: six plant growth and allocation responses, seven primary metabolite responses, eight secondary metabolite responses, and four physical defence responses. We examined these response variable changes under two-way and three-way interactions between CO2 and: soil nitrogen, ambient temperature, drought, light availability, photosynthetic pathway, reproductive system, plant growth rate, plant growth form, tissue type, and nitrogen fixation. In general we found smaller effect sizes for many response variables than have been previously reported. We also found that many of the oft-reported main effects of CO2 obscure the presence of significant two-and three-way interactions, which may help better explain the relationships between the response variables and elevated CO2.
DOI:10.1016/j.pbi.2013.02.015URLPMID:23529069 [本文引用: 1]

Oil produced in plant seeds is utilized as a major source of calories for human nutrition, as feedstocks for non-food uses such as soaps and polymers, and can serve as a high-energy biofuel. The biochemical pathways leading to oil (triacylglycerol) synthesis in seeds involve multiple subcellular organelles, requiring extensive lipid trafficking. Phosphatidylcholine plays a central role in these pathways as a substrate for acyl modifications and likely as a carrier for the trafficking of acyl groups between organelles and membrane subdomains. Although much has been clarified regarding the enzymes and pathways responsible for acyl-group flux, there are still major gaps in our understanding. These include the identity of several key enzymes, how flux between alternative pathways is controlled and the specialized cell biology leading to biogenesis of oil bodies that store up to 80% of carbon in seeds.
DOI:10.3389/fpls.2018.01413URLPMID:30386351 [本文引用: 6]

Although the effect of elevated CO2 (eCO2) on soybean yield has been well documented, few studies have addressed seed quality, particularly at the fresh edible (R6) and mature stages (R8). Under the current global scenario of increasing CO2 levels, this potentially threatens the nutritional content and quality of food crops. Using four soybean cultivars, we assessed the effects of eCO2 on the concentrations of crude protein, crude oil, and isoflavones and analyzed the changes in free amino acids, fatty acids, and mineral elements in seeds. At R6, eCO2 had no influence on soybean seed protein and oil concentrations. At R8, eCO2 significantly decreased seed protein concentration but increased seed oil concentration; it also significantly decreased total free amino acid concentration. However, at the same stage, the proportion of oleic acid (18:1) among fatty acids increased in response to eCO2 in the cultivars of Zhongke-maodou 2 (ZK-2) and Zhongke-maodou 3 (ZK-3), and a similar trend was found for linoleic acid (18:2) in Zhongke-maodou 1 (ZK-1) and Hei-maodou (HD). Total isoflavone concentrations increased significantly at both the R6 and R8 stages in response to eCO2. Compared with ambient CO2, the concentrations of K, Ca, Mg, P, and S increased significantly under eCO2 at R6, while the Fe concentration decreased significantly. The response of Zn and Mn concentrations to eCO2 varied among cultivars. At R8 and under eCO2, Mg, S, and Ca concentrations increased significantly, while Zn and Fe concentrations decreased significantly. These findings suggest that eCO2 is likely to benefit from the accumulation of seed fat and isoflavone but not from that of protein. In this study, the response of seed mineral nutrients to eCO2 varied between cultivars.
DOI:10.1104/pp.109.146282URLPMID:19783644 [本文引用: 1]
DOI:10.1186/s12870-016-0906-1URLPMID:27733139 [本文引用: 1]

BACKGROUND: The ability to modulate levels of individual fatty acids within soybean oil has potential to increase shelf-life and frying stability and to improve nutritional characteristics. Commodity soybean oil contains high levels of polyunsaturated linoleic and linolenic acid, which contribute to oxidative instability - a problem that has been addressed through partial hydrogenation. However, partial hydrogenation increases levels of trans-fatty acids, which have been associated with cardiovascular disease. Previously, we generated soybean lines with knockout mutations within fatty acid desaturase 2-1A (FAD2-1A) and FAD2-1B genes, resulting in oil with increased levels of monounsaturated oleic acid (18:1) and decreased levels of linoleic (18:2) and linolenic acid (18:3). Here, we stack mutations within FAD2-1A and FAD2-1B with mutations in fatty acid desaturase 3A (FAD3A) to further decrease levels of linolenic acid. Mutations were introduced into FAD3A by directly delivering TALENs into fad2-1a fad2-1b soybean plants. RESULTS: Oil from fad2-1a fad2-1b fad3a plants had significantly lower levels of linolenic acid (2.5 %), as compared to fad2-1a fad2-1b plants (4.7 %). Furthermore, oil had significantly lower levels of linoleic acid (2.7 % compared to 5.1 %) and significantly higher levels of oleic acid (82.2 % compared to 77.5 %). Transgene-free fad2-1a fad2-1b fad3a soybean lines were identified. CONCLUSIONS: The methods presented here provide an efficient means for using sequence-specific nucleases to stack quality traits in soybean. The resulting product comprised oleic acid levels above 80 % and linoleic and linolenic acid levels below 3 %.
URL [本文引用: 1]

在陕西省乾县典型调查的基础上,运用实证分析,对乾县奶牛产业化运作情况进行了分析,得出中国在发展农业产业化过程中存在的制度缺陷及主要发展瓶颈,提出建立合作组织、加快农村基础设施建设等具体措施,从而促进中国农业产业化健康发展。
[本文引用: 1]
DOI:10.1016/j.agee.2010.08.009URL [本文引用: 1]
DOI:10.1038/nature13179URL [本文引用: 2]

Dietary deficiencies of zinc and iron are a substantial global public health problem. An estimated two billion people suffer these deficiencies(1), causing a loss of 63 million life-years annually(2,3). Most of these people depend on C-3 grains and legumes as their primary dietary source of zinc and iron. Here we report that C-3 grains and legumes have lower concentrations of zinc and iron when grown under field conditions at the elevated atmospheric CO2 concentration predicted for the middle of this century. C-3 crops other than legumes also have lower concentrations of protein, whereas C-4 crops seem to be less affected. Differences between cultivars of a single crop suggest that breeding for decreased sensitivity to atmospheric CO2 concentration could partly address these new challenges to global health.
DOI:10.3390/plants8110465URL [本文引用: 1]
DOI:10.1016/j.scitotenv.2019.133784URLPMID:31756809 [本文引用: 3]

Elevated atmospheric CO2 concentration (eCO2) exerts significant influence on nutrient requirement in plant. The investigation of C:N:P ratios in major cropping soils is important for managing nutrient balance and maximizing their use efficiency in future farming systems. This study aimed to examine the effect of eCO2 on the C:N:P ratios in different plant parts among soybean cultivars. Twenty-four soybean cultivars were planted in open top chambers at two CO2 concentrations (390 and 550ppm) and sampled at the initial pod filling stage (R5) and the full maturity stage (R8). The C, N and P concentrations in root, stem, leaf and seed were determined. Elevated CO2 decreased the N concentrations in stem (-5.1%) and leaf (-3.2%) at R5, and in root (-24%), stem (-25%) and seed (-6.2%) at R8, resulting in a significant decrease of C:N ratio in the corresponding parts. The P concentration was significantly increased in root (6.0%), stem (7.9%) and leaf (16%) at R5, and in root (2.6%), stem (29%) and seed (16%) at R8 across 24 cultivars, leading to a decrease in the C:P ratio. Elevated CO2 significantly decreased the N:P ratio in root (-4.5%), stem (-12%) and leaf (-17%) at R5, and in root (-26%), stem (-57%) and seed (-22%) at R8. Furthermore, the response of C:N:P ratios to eCO2 varied greatly among soybean cultivars leading to significant CO2xcultivar interactions. Nitrogen, but not P was the limiting factor for the soybean plants grown in Mollisols under eCO2. The considerable variation in the C:N:P ratios among cultivars in response to eCO2 indicates a potential improvement in soybean adaptability to climate change via selection new cultivars. Cultivars SN22 and ZH4 that did not considerably altered the C:N and C:P ratios in response to eCO2 are likely the optimal genomes in soybean breeding programs for eCO2 adaption.
DOI:10.1017/S0007114512002413URLPMID:23107548 [本文引用: 1]

Indian diets derive almost 60 % of their protein from cereals with relatively low digestibility and quality. There have been several surveys of diets and protein intakes in India by the National Nutrition Monitoring Board (NNMB) over the last 25 years, in urban and rural, as well as in slum dwellers and tribal populations. Data of disadvantaged populations from slums, tribals and sedentary rural Indian populations show that the protein intake (mainly from cereals) is about 1 gm/kg/day. However, the protein intake looks less promising in terms of the protein digestibility corrected amino acid score (PDCAAS), using lysine as the first limiting amino acid, where all populations, particularly rural and tribal, appear to have an inadequate quality to their protein intake. The protein: energy (PE) ratio is a measure of dietary quality, and has been used in the 2007 WHO/FAO/UNU report to define reference requirement values with which the adequacy of diets can be evaluated in terms of a protein quality corrected PE ratio. It is likely that about one third of this sedentary rural population is at risk of not meeting their requirements. These levels of risk of deficiency are in a population with relatively low BMI populations, whose diets are also inadequate in fruits and vegetables. Therefore, while the burden of enhancing the quality of protein intake in rural India exists, the quality of the diet, in general, represents a challenge that must be met.
DOI:10.1093/jxb/eru320URL [本文引用: 1]

Amino acids play several critical roles in plants, from providing the building blocks of proteins to being essential metabolites interacting with many branches of metabolism. They are also important molecules that shuttle organic nitrogen through the plant. Because of this central role in nitrogen metabolism, amino acid biosynthesis, degradation, and transport are tightly regulated to meet demand in response to nitrogen and carbon availability. While much is known about the feedback regulation of the branched biosynthesis pathways by the amino acids themselves, the regulation mechanisms at the transcriptional, post-transcriptional, and protein levels remain to be identified. This review focuses mainly on the current state of our understanding of the regulation of the enzymes and transporters at the transcript level. Current results describing the effect of transcription factors and protein modifications lead to a fragmental picture that hints at multiple, complex levels of regulation that control and coordinate transport and enzyme activities. It also appears that amino acid metabolism, amino acid transport, and stress signal integration can influence each other in a so-far unpredictable fashion.
DOI:10.1007/s00425-003-1026-3URLPMID:12684787 [本文引用: 1]

Soybean ( Glycine max [L.] Merr.) seeds are rich in protein, most of which is contributed by the major storage proteins glycinin (11S globulin) and beta-conglycinin (7S globulin). Null mutations for each of the subunits of these storage proteins were integrated by crossbreeding to yield a soybean line that lacks both glycinin and beta-conglycinin components. In spite of the absence of these two major storage proteins, the mutant line grew and reproduced normally, and the nitrogen content of its dry seed was similar to that for wild-type cultivars. However, protein bodies appeared underdeveloped in the cotyledons of the integrated mutant line. Furthermore, whereas free amino acids contribute only 0.3-0.8% of the seed nitrogen content of wild-type varieties, they constituted 4.5-8.2% of the seed nitrogen content in the integrated mutant line, with arginine (Arg) being especially enriched in the mutant seeds. Seeds of the integrated mutant line thus appeared to compensate for the reduced nitrogen content in the form of glycinin and beta-conglycinin by accumulating free amino acids as well as by increasing the expression of certain other seed proteins. These results indicate that soybean seeds are able to store nitrogen mostly in the form of either proteins or free amino acids.
DOI:10.1111/nph.13123URLPMID:25348775 [本文引用: 1]

Predicting the response of fine roots to increased atmospheric CO(2) concentration has important implications for carbon (C) and nutrient cycling in forest ecosystems. Root architecture is known to play an important role in how trees acquire soil resources in changing environments. However, the effects of elevated CO(2) on the fine-root architecture of trees remain unclear. We investigated the architectural response of fine roots exposed to 14 yr of CO(2) enrichment and 6 yr of nitrogen (N) fertilization in a Pinus taeda (loblolly pine) forest. Root traits reflecting geometry, topology and uptake function were measured on intact fine-root branches removed from soil monoliths and the litter layer. CO(2) enrichment resulted in the development of a fine-root pool that was less dichotomous and more exploratory under N-limited conditions. The per cent mycorrhizal colonization did not differ among treatments, suggesting that root growth and acclimation to elevated CO(2) were quantitatively more important than increased mycorrhizal associations. Our findings emphasize the importance of architectural plasticity in response to environmental change and suggest that changes in root architecture may allow trees to effectively exploit larger volumes of soil, thereby pre-empting progressive nutrient limitations.
URL [本文引用: 1]
DOI:10.1177/156482650102200201URL [本文引用: 1]
DOI:10.1126/sciadv.aaq1012URLPMID:29806023 [本文引用: 2]

Declines of protein and minerals essential for humans, including iron and zinc, have been reported for crops in response to rising atmospheric carbon dioxide concentration, [CO2]. For the current century, estimates of the potential human health impact of these declines range from 138 million to 1.4 billion, depending on the nutrient. However, changes in plant-based vitamin content in response to [CO2] have not been elucidated. Inclusion of vitamin information would substantially improve estimates of health risks. Among crop species, rice is the primary food source for more than 2 billion people. We used multiyear, multilocation in situ FACE (free-air CO2 enrichment) experiments for 18 genetically diverse rice lines, including Japonica, Indica, and hybrids currently grown throughout Asia. We report for the first time the integrated nutritional impact of those changes (protein, micronutrients, and vitamins) for the 10 countries that consume the most rice as part of their daily caloric supply. Whereas our results confirm the declines in protein, iron, and zinc, we also find consistent declines in vitamins B1, B2, B5, and B9 and, conversely, an increase in vitamin E. A strong correlation between the impacts of elevated [CO2] on vitamin content based on the molecular fraction of nitrogen within the vitamin was observed. Finally, potential health risks associated with anticipated CO2-induced deficits of protein, minerals, and vitamins in rice were correlated to the lowest overall gross domestic product per capita for the highest rice-consuming countries, suggesting potential consequences for a global population of approximately 600 million.
[本文引用: 1]
DOI:10.1071/FP02007URLPMID:32689563 [本文引用: 1]

DOI:10.1016/j.envexpbot.2013.11.004URL [本文引用: 1]

The effects of elevated CO2 on the content of several nutrients in plants have been well studied, but few studies have investigated plant nutrient dynamics under future environmental conditions, which are expected to include elevated CO2 and elevated soil salt concentrations. This study investigated whether high salt and CO2 conditions, singly or in combination, might affect nutrient dynamics, and the underlying mechanisms. We measured macro- and micronutrient uptake and translocation rates, nutrient content and concentrations in whole seedlings and in each plant organ. We estimated whole-plant nutrient use efficiencies in barley subjected to 0, 80, 160, or 240 mM NaCl and grown at either 350 (ambient) or 700 (elevated) mu mol mol(-1) CO2. Under non-saline conditions, plants grown at elevated CO2 adjusted their root size and activity to change nutrient uptake and transport efficiency in response to the demand for a given nutrient. Under high saline conditions, salt stress reduced K, Ca, N, B, and S uptake rates and concentrations in tissues, which caused growth reduction. Nevertheless, barley had the ability to increase the selectivity of K over Na, and Ca over Na. Under combined conditions of salt stress and elevated CO2, barley seedlings were able to maintain higher uptake and translocation rates of almost all nutrients. This ability allowed the plants to adapt to higher demands under elevated CO2; they could grow more rapidly by allocating more C to root growth and by increasing active nutrient uptake and translocation. Our results indicated that salinity generally increased nutrient use efficiency under both CO2 conditions. However, we found no consistent evidence that nutrient use efficiency was affected by CO2 concentration, either under non-saline or saline conditions. (C) 2013 Elsevier B.V.
[本文引用: 1]
DOI:10.1111/gcb.2012.19.issue-2URL [本文引用: 1]
DOI:10.1111/gcb.2018.24.issue-3URL [本文引用: 1]
DOI:10.1016/j.agee.2016.01.028URL [本文引用: 1]
DOI:10.1104/pp.110.161349URLPMID:20921178 [本文引用: 1]
DOI:10.1016/j.agsy.2010.08.009URL [本文引用: 1]
DOI:10.1111/gcb.2014.20.issue-7URL [本文引用: 1]
DOI:10.3389/fpls.2016.01262URLPMID:27625660 [本文引用: 1]

West Africa is known to be particularly vulnerable to climate change due to high climate variability, high reliance on rain-fed agriculture, and limited economic and institutional capacity to respond to climate variability and change. In this context, better knowledge of how climate will change in West Africa and how such changes will impact crop productivity is crucial to inform policies that may counteract the adverse effects. This review paper provides a comprehensive overview of climate change impacts on agriculture in West Africa based on the recent scientific literature. West Africa is nowadays experiencing a rapid climate change, characterized by a widespread warming, a recovery of the monsoonal precipitation, and an increase in the occurrence of climate extremes. The observed climate tendencies are also projected to continue in the twenty-first century under moderate and high emission scenarios, although large uncertainties still affect simulations of the future West African climate, especially regarding the summer precipitation. However, despite diverging future projections of the monsoonal rainfall, which is essential for rain-fed agriculture, a robust evidence of yield loss in West Africa emerges. This yield loss is mainly driven by increased mean temperature while potential wetter or drier conditions as well as elevated CO2 concentrations can modulate this effect. Potential for adaptation is illustrated for major crops in West Africa through a selection of studies based on process-based crop models to adjust cropping systems (change in varieties, sowing dates and density, irrigation, fertilizer management) to future climate. Results of the cited studies are crop and region specific and no clear conclusions can be made regarding the most effective adaptation options. Further efforts are needed to improve modeling of the monsoon system and to better quantify the uncertainty in its changes under a warmer climate, in the response of the crops to such changes and in the potential for adaptation.
DOI:10.1088/1748-9326/2/1/014002URL [本文引用: 1]
DOI:10.1088/1748-9326/3/3/034003URL [本文引用: 1]
DOI:10.2134/agronj2010.0303URL [本文引用: 1]

Changes in temperature, CO2, and precipitation under the scenarios of climate change for the next 30 yr present a challenge to crop production. This review focuses on the impact of temperature, CO2, and ozone on agronomic crops and the implications for crop production. Understanding these implications for agricultural crops is critical for developing cropping systems resilient to stresses induced by climate change. There is variation among crops in their response to CO2, temperature, and precipitation changes and, with the regional differences in predicted climate, a situation is created in which the responses will be further complicated. For example, the temperature effects on soybean [Glycine max (L.) Merr.] could potentially cause yield reductions of 2.4% in the South but an increase of 1.7% in the Midwest. The frequency of years when temperatures exceed thresholds for damage during critical growth stages is likely to increase for some crops and regions. The increase in CO2 contributes significantly to enhanced plant growth and improved water use efficiency (WUE); however, there may be a downscaling of these positive impacts due to higher temperatures plants will experience during their growth cycle. A challenge is to understand the interactions of the changing climatic parameters because of the interactions among temperature, CO2, and precipitation on plant growth and development and also on the biotic stresses of weeds, insects, and diseases. Agronomists will have to consider the variations in temperature and precipitation as part of the production system if they are to ensure the food security required by an ever increasing population.
DOI:10.1104/pp.112.211938URL [本文引用: 2]

Extensive evidence shows that increasing carbon dioxide concentration ([CO2]) stimulates, and increasing temperature decreases, both net photosynthetic carbon assimilation (A) and biomass production for C-3 plants. However the [CO2]-induced stimulation in A is projected to increase further with warmer temperature. While the influence of increasing temperature and [CO2], independent of each other, on A and biomass production have been widely investigated, the interaction between these two major global changes has not been tested on field-grown crops. Here, the interactive effect of both elevated [CO2] (approximately 585 mu mol mol(-1)) and temperature (+3.5 degrees C) on soybean (Glycine max) A, biomass, and yield were tested over two growing seasons in the Temperature by Free-Air CO2 Enrichment experiment at the Soybean Free Air CO2 Enrichment facility. Measurements of A, stomatal conductance, and intercellular [CO2] were collected along with meteorological, water potential, and growth data. Elevated temperatures caused lower A, which was largely attributed to declines in stomatal conductance and intercellular [CO2] and led in turn to lower yields. Increasing both [CO2] and temperature stimulated A relative to elevated [CO2] alone on only two sampling days during 2009 and on no days in 2011. In 2011, the warmer of the two years, there were no observed increases in yield in the elevated temperature plots regardless of whether [CO2] was elevated. All treatments lowered the harvest index for soybean, although the effect of elevated [CO2] in 2011 was not statistically significant. These results provide a better understanding of the physiological responses of soybean to future climate change conditions and suggest that the potential is limited for elevated [CO2] to mitigate the influence of rising temperatures on photosynthesis, growth, and yields of C-3 crops.
DOI:10.1038/s41467-016-0009-6URLPMID:28232747 [本文引用: 2]

Cyclooxygenase-2 isozyme is a promising anti-inflammatory drug target, and overexpression of this enzyme is also associated with several cancers and neurodegenerative diseases. The amino-acid sequence and structural similarity between inducible cyclooxygenase-2 and housekeeping cyclooxygenase-1 isoforms present a significant challenge to design selective cyclooxygenase-2 inhibitors. Herein, we describe the use of the cyclooxygenase-2 active site as a reaction vessel for the in situ generation of its own highly specific inhibitors. Multi-component competitive-binding studies confirmed that the cyclooxygenase-2 isozyme can judiciously select most appropriate chemical building blocks from a pool of chemicals to build its own highly potent inhibitor. Herein, with the use of kinetic target-guided synthesis, also termed as in situ click chemistry, we describe the discovery of two highly potent and selective cyclooxygenase-2 isozyme inhibitors. The in vivo anti-inflammatory activity of these two novel small molecules is significantly higher than that of widely used selective cyclooxygenase-2 inhibitors.Traditional inflammation and pain relief drugs target both cyclooxygenase 1 and 2 (COX-1 and COX-2), causing severe side effects. Here, the authors use in situ click chemistry to develop COX-2 specific inhibitors with high in vivo anti-inflammatory activity.
DOI:10.1016/j.eja.2011.05.004URL [本文引用: 1]

The biomass and yield parameters were found to be significantly cultivar dependent. However, in all cultivars elevated temperature caused a significant reduction in yield parameters, while biomass was not affected significantly. Elevated [CO2] increased the vegetative biomass significantly, but seed yield was only significantly enhanced in one of the four cultivars studied. Increased [O-3] did not have significant effects on any of the cultivars. In general, the negative effects of a 5 degrees C temperature elevation on yield could not be compensated by elevated [CO2], when simultaneously applied in multifactor treatments. The evaluation of cultivar differences in productivity under elevated [CO2] in combination with increased temperatures and [O-3] is necessary to derive at a realistic prediction for the future food and biomass production and for the selection of cultivars providing an adaptation potential to environmental change. Our results suggest that future breeding of B. napus should be based on old cultivars, since more modern varieties seem to have lower potentials to respond to CO2 and thus counteract the detrimental effects of yield reducing environmental factors such as temperature and O-3. (C) 2011 Elsevier B.V.]]>
