摘要/Abstract
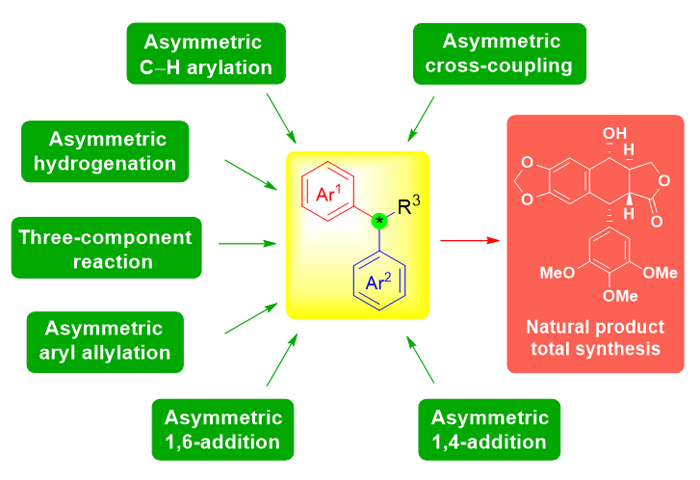
二芳基次甲基结构单元广泛存在于具有重要生理和药理活性的天然产物和药物当中. 同时, 该结构单元中所特有的二芳基次甲基立体中心的对映选择性构筑往往也是天然产物全合成的难点和挑战性所在. 因此, 引起了众多有机合成化学家的研究兴趣. 近年来, 该手性立体中心的构建方法发展迅速, 新方法和新反应的报道也层出不穷; 开发出来的一些高效催化剂, 展示出独特的催化活性和选择性. 本文根据反应类型的不同, 将其分为不对称共轭加成反应和不对称氢化反应等六类, 综述近十年来二芳基次甲基立体中心的不对称构建方法及相关方法在天然产物全合成中的应用. 最后, 从全合成的角度进一步总结和分析未来构建二芳基次甲基手性立体中心的发展趋势, 力求发展更加高效、避免贵金属的催化剂及环境友好型的新方法和新试剂.
关键词: 二芳基次甲基, 不对称合成, 天然产物, 共轭加成, 偶联反应
Diarylmethine structure units are widely present in natural products and pharmaceuticals with important physiological and pharmacological activities. The enantioselective control of its stereogenic center, which is the most unique in this structure unit, has often become a difficulty and challenge during the research of total synthesis of natural products. Therefore, this field has attracted great interest to many organometallic chemists and synthetic organic chemists. In recent years, the construction methods of this stereocenter developed rapidly, and new reactions and reagents have emerged one after another. Some highly efficient catalysts have been invented, exhibiting unique catalytic activity and selectivity. In this review, according to the difference of reaction types, these methods can be divided into six types, such as asymmetric conjugate addition reaction (asymmetric 1,4-addition reactions involving aryl boronic acids, asymmetric 1,4-addition reactions involving aryl borates, enantioselective organocatalytic 1,4-addition reactions, asymmetric 1,4-conjugate addition induced by Evans chiral imide and asymmetric 1,6-conjugate addition of para-quinone methides), asymmetric allylation or propargylation of aromatic rings, transition metal-catalyzed asymmetric cross-coupling reaction, transition metal-catalyzed asymmetric C―H bond activation and functionalization, three-component reactions for asymmetric synthesis of 1,1-diaryl alkanes, asymmetric hydrogenation reactions for 1,1-diaryl alkanes, etc. This review aims to collect and summarize the asymmetric construction methods of diarylmethine stereogenic centers, and their applications in the total synthesis of natural products in the past decade. Finally, from the perspective of total synthesis, we further summarize and analyze the future development trend for the construction of diarylmethine chiral stereogenic centers, and encourage young generation to develop new methods and reagents that can avoid use of precious metals/catalysts and therefore are more efficient and environmentally friendly. More importantly, we hope that the developments of these practical methodologies can be further applied to asymmetric total synthesis of natural products and medicines, and eventually solve the source problem of these “useful molecules” with potential medicinal value.
Key words: diarylmethine, asymmetric synthesis, natural product, conjugate addition, coupling reaction
PDF全文下载地址:
点我下载PDF
