摘要/Abstract
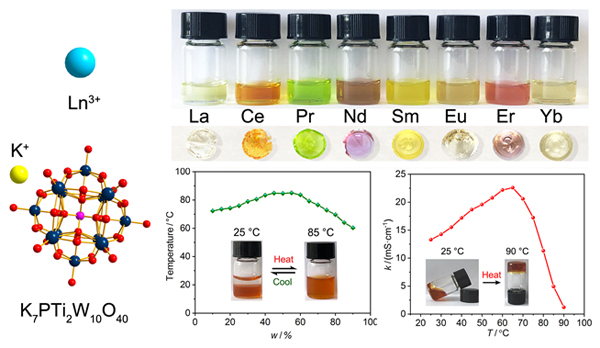
本工作报道了系列钛取代Keggin型钨磷酸稀土盐KLnH3PTi2W10O40•xH2O [Ln=La (1), Ce (2), Pr (3), Nd (4), Sm (5), Eu (6), Er (7), Yb (8)]的合成和性质. 这些无机离子型化合物在4~65 ℃范围表现为流动性很好的液态, 呈现离子液体行为. 微量的水是该系列离子液体组成中不可缺少的组分, 完全失去水后变为无定型的透明固体. 该系列离子液体在室温时具有良好的导电性, 电导率均高于10 mS•cm-1, 而且其电导率随温度发生明显的变化. 当从室温升高到65 ℃时, 含Ce的离子液体2电导率从13.3 mS•cm-1逐渐增大至22.6 mS•cm-1, 但从65 ℃升温至90 ℃时, 电导率明显降低. 研究还发现, 在室温环境下该系列离子液体与水均不互溶呈两相, 加热后混溶为均一相, 表现出上临界溶解温度(UCST)相行为. 据我们所知, 这种纯无机离子液体是非常少见的.
关键词: 钛取代Keggin型钨磷酸, 稀土, 无机离子液体, 电导率, 上临界溶解温度(UCST)相行为
Ionic liquids (ILs) usually refer to ionic compounds that are liquid at a temperature of 100 ℃ and below. As salts, ionic liquids are composed of ions. So by selecting the corresponding cations, anions or ion combinations, they can be designed for specific applications. Various new types of ionic liquids have been prepared and discovered. Polyoxometalates (POMs) are a kind of metal-oxygen cluster compounds with rich composition and diverse structure. Compared with other common inorganic anions (such as PO43-, SO42-, CO32-,etc.), polyoxometalate anions have the characteristics of large ion radius and low charge density, which are a good anion choice for constructing ionic liquids. Most of the currently reported polyoxometalate ionic liquids are composed of organic cations and polyoxometalate anions, while pure inorganic ionic liquids composed of inorganic cations and polyoxometalate anions are rarely reported. In this work, we report a series of lanthanide- containing compounds based on a Ti-substituted Keggin-type polyoxometalate prepared by ion exchange, KLnH3PTi2W10O40•xH2O [Ln=La (1), Ce (2), Pr (3), Nd (4), Sm (5), Eu (6), Er (7), Yb (8)]. They were characterized by inductively coupled plasma emission spectrometry (ICP), thermal gravimetric analysis (TG), Fourier transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (PXRD). Surprisingly, these inorganic ionic compounds behave as liquid with good fluidity in the temperature range of 4~65 ℃, and their conductivity are all higher than 10 mS•cm-1, showing ionic liquid behavior. Water is an indispensable component in their composition. After losing water, they become transparent and colored solids with amorphous structure. The study found that the conductivity of these ionic liquids change significantly with temperature. For example, when the temperature rises from room temperature to 65 ℃, the conductivity of Ce-containing ionic liquid gradually increases from 13.3 mS•cm-1 to 22.6 mS•cm-1. When the temperature rises from 65 ℃ to 90 ℃, the conductivity drops significantly, as low as 1.22 mS•cm-1 at 90 ℃. As far as we know, this kind of pure inorganic ionic liquid is very rare. The synthesis method of series ionic liquids is simple, easy to operate, and environmentally friendly. These properties make the series of ionic liquids have good machinability and potential applications in the fields of catalysis and phase separation.
Key words: Ti-substituted Keggin-type polyoxometalate, rare earth, inorganic ionic liquids, conductivity, upper critical solution temperature (UCST)
PDF全文下载地址:
点我下载PDF
