摘要/Abstract
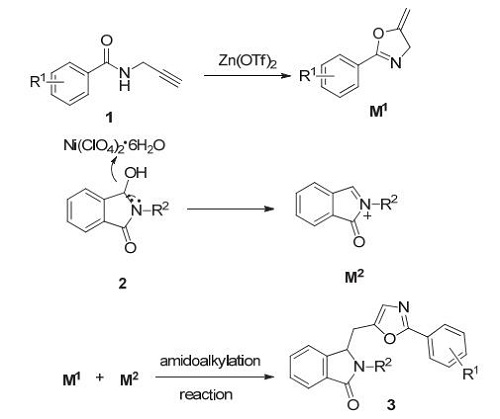
报道了一种新型的Zn/Ni双金属接力协同催化的串联反应,该方法通过Zn(OTf)2和Ni(ClO4)2·6H2O协同接力催化,一锅法进行分子内环异构化/分子间酰胺化反应构建噁唑衍生物.产物的形成主要是由Zn(OTf)2活化炔丙基酰胺的三键,发生分子内的环化反应构建噁唑啉中间体,由Ni(ClO4)2·6H2O催化3-羟基-2-苯甲基-异吲哚啉-1-酮类化合物形成酰亚胺离子,继而由噁唑啉中间体与酰亚胺离子发生分子间酰胺化反应实现了噁唑分子的合成.优化部分的对比实验证实Zn(OTf)2和Ni(ClO4)2·6H2O的存在对于该串联反应都是必须条件.大体而言,所有反应都是将各反应物和试剂一次性加入,在空气氛围下100℃加热进行反应.含有不同类型给电子取代基、含有不同富电子的芳环、含有不同吸电子取代基的炔丙基酰胺都可以顺利地和3-羟基-2-苯甲基-异吲哚啉-1-酮反应得到相应的噁唑衍生物,相比而言,含有吸电子基团的炔丙基酰胺比含有给电子基团或富电子的炔丙基酰胺所得到的产物的收率要低一些,这可能是因为含有吸电子基团的炔丙基酰胺所得到的噁唑啉中间体活性较低.3-羟基-2-苯基异吲哚啉-1-酮类化合物、3-羟基-2-苯甲基异吲哚啉-1-酮类化合物和3-羟基-2-苯乙基异吲哚啉-1-酮类化合物对反应同样表现出了良好的兼容性.该方法反应条件简单、原子经济性高、官能团兼容性好,对噁唑衍生物合成和酰亚胺离子形成具有重要的意义.
关键词: 接力催化, 环异构化, 酰亚胺离子, 酰胺化反应, 噁唑衍生物
Oxazole derivatives are widely found in natural products and pharmaceuticals with impressive biological properties, tremendous efforts have been devoted to the development of new methodologies and strategies to construct the oxazole rings. However, most of these reactions require harsh reaction conditions, limiting the wide application of these classical oxazole synthetic methods in organic synthesis. N-Acyliminium ions represent important electron deficient carbocations intermediates in organic synthesis because they provide various biologically important natural and unnatural products via C-C and C-heteroatom bondforming methodologies using an inter-or intramolecular path. The removal of a good leaving group at the α-position of amides or lactams usually generates N-acyliminium ions, which act as more electron-deficient carbocations toward nucleophiles. In this paper, a novel tandem metal relay catalytic system of Zn/Ni has been successfully developed. By using this unprecedented Zn(OTf)2/Ni(ClO4)2·6H2O bimetallic relay catalytic system, a variety of oxazole derivatives were obtained from easily available N-(propargyl)-arylamides and various γ-hydroxy lactams through intramolecular cycloisomerization/intermolecular amidoalkylation under mild conditions. The first step of the one-pot procedure is that Zn(OTf)2 acts as a π acid to activate the triple bond of N-(propargyl)-arylamides, and a subsequent intramolecular 5-exo-dig cyclization forms the oxazoline intermediate. Separately, Ni(ClO4)2·6H2O acts as Lewis acid to activate and facilitate the departure of 3-hydroxyl group to form the electrophilic acyliminium ions, which then in an intermolecular reaction is transformed to the oxazole derivatives in good to excellent yield. Control experiments in the optimization section disclose the fact that Zn(OTf)2 and Ni(ClO4)2·6H2O are both indispensable for this intramolecular cycloisomerization/intermolecular amidoalkylation reaction. Generally, the synthetic reactions run under air atmosphere by heating all the substrates and reagents in one-pot at 100℃. The N-(propargyl)-arylamide containing different types of electron-donating substituents, different electron-rich aromatic rings and different electron-withdrawing substituents can react with 3-hydroxy-2-benzyl-isoindolin-1-one to give the corresponding oxazole derivatives. In contrast, the propargyl amide containing an electron withdrawing group has a lower yield than the one using other propargyl amide, because the activity of the oxazoline intermediate obtained by the propargyl amide containing an electron withdrawing group is lower. 3-Hydroxy-2-phenylisoindoline-1-one, 3-hydroxy-2-phenylmethylisoindoline-1-one and 3-hydroxy-2-phenylethylisoindoline-1-one have also been found applicable to this reaction. The present method benefits from the distinctive features of simple reaction conditions, high atom economy and broad substrate tolerance. It is of great significance for the synthesis of oxazole derivatives and the formation of acyliminium ions.
Key words: relay catalysis, cycloisomerization, acyliminium ion, amidoalkylation reaction, oxazole derivatives
PDF全文下载地址:
点我下载PDF
