摘要/Abstract
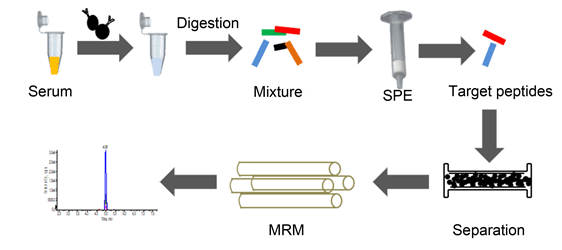
建立并优化了液相色谱串联质谱法定量血清肌钙蛋白I.免疫磁珠富集血清肌钙蛋白I,经变性、还原、乙酰化、胰酶消化、固相萃取纯化,采用SymmetryShieldC18柱分离,以含0.1%甲酸水溶液和含0.1%的甲酸乙腈溶液为流动相,梯度分离目标肽段,流速0.2 mL/min,温度35℃;使用电喷雾正离子模式扫描,在多反应监测模式下进行测定,目标肽段出峰时间约为4.95 min;方法的最低检测限为2.5 ng/mL,定量限为8.32 ng/mL,在(10~600)ng/mL范围内线性良好,相关系数为0.99;标准物质SRM2921偏倚为-7.94%~-6.49%;低、中、高样本总不精密度分别为6.43%、3.18%和2.75%;携带污染率为-0.47%~0.04%.本方法选择性高、重复性和准确度好、携带污染小,为进一步研究血清cTnI参考方法建立提供依据.
关键词: 液相色谱串联质谱, 肌钙蛋白I, 肽段, 定量, 免疫磁珠
Cardiac troponin I (cTnI) is one of the most popular biomarkers for the diagnosis of acute myocardial injury (AMI) in patients. In this study, a novel method was developed and optimized for quantification of cTnI in human serum by immunoaffinity enrichment combining isotope dilution liquid chromatography tandem mass spectrometry. cTnI was first captured from human serum with immunomagnetic beads conjugated with the monoclonal antibody, and then enzymatically hydrolyzed into peptides after a series of operation, including denaturation, reduction, acetylation, digestion and purification. Subsequently, enzymatic peptides were separated by passing through Symmetry Shield C18 column at a speed of 0.2 mL/min with 0.1% acid acetonitrile solution and 0.1% acid aqueous solution as mobile phases under gradient elution. A specific peptide, NITEIADLTQK, was selected and quantified. The qualitative analysis was achieved by three ion transitions under multiple reaction monitoring (MRM) when the isotopically labeled peptide, NITEIAD[(13C6,15N)L]TQK, was used as a reference. After the optimal conditional experiment, results showed that the surrogate peptide was separated out well at about 4.95 min with little interference. Ranging from 10 ng/mL to 600 ng/mL, a good linearity was shown with correlation coefficients all above 0.99. The limit of detection (LOD) and the limit of quantification (LOQ) were estimated to be 2.5 ng/mL, 8.32 ng/mL, respectively. This method also provided good accuracy (relative bias of three concentrations diluted from SRM2921 were between -7.94% and -6.49%) and repeatability (total relative standard deviations of three concentration were 6.43%, 3.18% and 2.75%, respectively). Carry-over rates were estimated between -0.47% and 0.04%. The novel assay successfully determined five specimens from AMI patients with concentrations from 16.38 ng/mL to 557.53 ng/mL. Our results demonstrate that this method can be applied for determination of serum cTnI in AMI patients with high selectivity, low carry-over rates, good repeatability and good accuracy, which helps to establish candidate reference measurement procedure of serum cTnI.
Key words: liquid chromatography tandem mass spectrometry, troponin I, surrogate peptides, quantification, immunomagnetic beads
PDF全文下载地址:
点我下载PDF
