摘要/Abstract
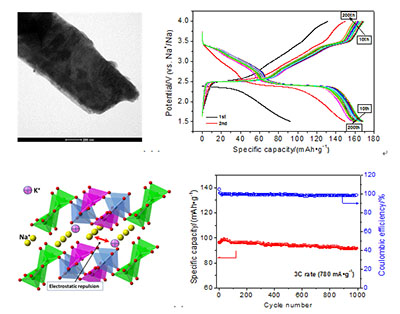
通过水热-热处理方法首次制备了K+离子掺杂的钒酸钠(Na5V12O32)正极材料,对样品进行了TEM、XRD和XPS表征,详细研究了钾掺杂量对样品的结构和储钠性能的影响规律.TEM照片显示,合成的材料具有纳米片形貌.XRD/XPS谱图分析表明,K+离子掺杂在钒酸钠晶体的层间.恒流充放电测结果显示,当1 mol Na5V12O32掺杂0.118 mol K+离子时,得到的Na5K0.118V12O32样品具有最佳的电化学性能:在1.5~4.0 V范围内,经过几次活化后,其于0.1C、0.2C、0.5C、1C、3C和10C倍率下的最大放电容量分别为169、160、148、132、98和69 mAh·g-1;3C循环1000次后容量保持率为93.0%.研究结果表明,层间掺杂的K+离子不仅扩大了Na5V12O32晶体的层间距,而且稳定了晶体的结构,从而显著改善了Na5V12O32材料的倍率性能和循环性能.研究结果证明,适量K+离子掺杂的Na5K0.118V12O32纳米片有望发展为一种新型钠离子电池正极材料.
关键词: 钠离子电池, 钒酸钠, 阳离子掺杂, 正极材料, 电化学性能
Na-ion batteries with lower cost than Li-ion batteries would be developed to large-scale energy-storage device to store solar and wind energies. However, large radius renders Na+ ions to insert into/extract out the layered transition metal oxides (LTMOs) sluggishly. To improve the intercalation dynamics of Na+ ions, the interlayer spacing of crystals has to be expanded for those LTMOs that are capable of fast lithiation and delithiation. Herein, a LTMO based on vanadium is firstly doped with larger K+ ions to expand the interlayer spacing to yield K+-doped sodium vanadate (Na5KxV12O32) cathode material by a hydrothermal method at 200℃ for 24 h followed by calcination at 500℃ for 3 h. The samples were characterized by scanning electron microscope (SEM)/transmission electron microscope (TEM), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) technologies. Effect of the doping amount of K+ on the structure and sodium-storage performance of the sample was studied in detail. The synthesized materials display nanoplate morphology viewing from the TEM images. K+ ions are doped into the interlayer of the sodium vanadate crystallites, which is proved by analysis of the XRD patterns and XPS spectra. Expanded interlayer spacing favors Na+ ions' intercalation and deintercalation between the[V3O8]- layers, which is testified by the chemical diffusion coefficient of cathodes, thus enhancing the rate capability. On the other hand, the chemically pre-intercalated K+ ions are pinned in the crystallites during insertion and extraction of Na+ ions and act as pillars to stabilize the layered structure, improving the cycliability of the cathode. However, excessive doping of K+ leads to a discounted rate capability of the cathode, suggesting an optimized amount of K+ doping into the crystal. The results from galvanostatic charge-discharge tests indicate that the obtained NVO(3K) sample, in which 0.118 mol of K+ ions are doped into per mol of Na5V12O32, presents the best electrochemical performance among the various samples. It can deliver the maximum capacities of 169, 160, 148, 132, 98 and 69 mAh·g-1 at the rates of 0.1C, 0.2C, 0.5C, 1C, 3C and 10C after activated for several times over the voltage window of 4.0~1.5 V (vs. Na+/Na), respectively. Even run at 3C rate, it can retain 93.0% of the maximum capacity after 1000 cycles, exhibiting excellent rate capability and stable cycliability. The results suggest that doping of K+ ions into the interlayer of crystallites can significantly improving the rate capability as well as cycling performance of the obtained Na5V12O32. Our investigation demonstrates that design of K+-doped sodium vanadate cathode materials is beneficial for harvesting superior performance, of which the Na5K0.118V12O32 nanoplates can be developed into a novel cathode material for sodium-ion batteries in the future.
Key words: sodium-ion battery, Na5V12O32, cation doping, cathode material, electrochemical performance
PDF全文下载地址:
点我下载PDF
